Abstracts
The advances in microsurgery procedures and the detailed knowledge of regeneration may contribute significantly to the improvement of the results of peripheral nerve repair. In the last years, several authors have used series of tissues and substances interposed between the stumps of the sectioned peripheral nerve, trying to stimulate the axon growth in the lesion site. Using the nerve entubulation technique, the author studied the effect of two neurokines, cardiotrophin-1 (CT-1) and oncostatin M (OsM), in the axonal growth and in the survival rate of sensory neurons of L-5 root ganglion after sciatic nerve lesion in C57BL/6J mice. The author used three groups of five animals that had their sciatic nerve sectioned and repaired with polyethylene tubular prostheses filled with cardiotrophin-1, oncostatin -M or cytochrome-C, dissolved in the collagen extract. The fourth group of three non-operated animals, were used the normal control group. Four weeks after surgery, the mice were sacrificed. The myelinated axons of the removed regenerating nerve cable were counted. The L-5 dorsal root ganglions were also dissected to count sensory neurons. Data were statistically analyzed, which allowed us conclude that both neurokines were effective in causing axonal sprouting, but they could not prevent sensory neuron death in L-5 dorsal root ganglion.
Os avanços das técnicas microcirúrgicas e o conhecimento detalhado do microambiente da regeneração podem contribuir significativamente na melhoria dos resultados das reparações nervosas periféricas. Nos últimos anos vários autores têm utilizado uma série de tecidos e substâncias interpostos entre os cotos de um nervo periférico seccionado, buscando estimular o crescimento axonal no local da lesão. Através da técnica de tubulização, os autores estudam o efeito de duas neurocinas, a cardiotrofina-1 (CT-1) e a oncostatina-M (OsM), no crescimento axonal e na sobrevida dos neurônios sensitivos nos gânglios da raiz dorsal de L5, após a lesão de nervos ciáticos em camundongos C57BL/6J. Utilizam 3 grupos de 7 animais que tiveram seus nervos seccionados e tubulizados com próteses de polietileno preenchidas com cardiotrofina-1, oncostatina-M e citocromo-C, associadas a um extrato de colágeno. Um quarto grupo de 3 animais, não operados, foi considerado por nós como grupo controle de normalidade. Após 4 semanas da cirurgia, os camundongos foram sacrificados, e realizada a contagem das fibras mielínicas nos cabos de regeneração retirados. Os gânglios das raizes dorsais de L5 também foram dissecados possibilitando a contagem dos neurônios sensitivos. Os dados foram analisados estatisticamente, permitindo concluir que as duas substâncias, utilizadas por nós, foram efetivas no estímulo ao brotamento axonal, porém, as mesmas não conseguiram impedir a morte dos neurônios sensitivos no gânglio da raiz dorsal de L5.
ARTIGO ORIGINAL
Comparative experimental trial on cardiotrophin-1 and oncostatin-m activity in the peripheral nerve regeneration
Sérgio Augusto M. da GamaI; Rames Mattar Jr.II; Ciro Ferreira da SilvaIII; Raquel Dias LainettiIV
IPós-graduando do IOT HC-FMUSP
IIChefe do grupo de Cirurgia da Mão do IOT HC-FMUSP
IIIProfessor Titular do Departamento de Histologia ICB-USP
IVPós-graduanda do ICB-USP
SUMMARY
The advances in microsurgery procedures and the detailed knowledge of regeneration may contribute significantly to the improvement of the results of peripheral nerve repair. In the last years, several authors have used series of tissues and substances interposed between the stumps of the sectioned peripheral nerve, trying to stimulate the axon growth in the lesion site.
Using the nerve entubulation technique, the author studied the effect of two neurokines, cardiotrophin-1 (CT-1) and oncostatin M (OsM), in the axonal growth and in the survival rate of sensory neurons of L-5 root ganglion after sciatic nerve lesion in C57BL/6J mice.
The author used three groups of five animals that had their sciatic nerve sectioned and repaired with polyethylene tubular prostheses filled with cardiotrophin-1, oncostatin -M or cytochrome-C, dissolved in the collagen extract.
The fourth group of three non-operated animals, were used the normal control group.
Four weeks after surgery, the mice were sacrificed. The myelinated axons of the removed regenerating nerve cable were counted. The L-5 dorsal root ganglions were also dissected to count sensory neurons.
Data were statistically analyzed, which allowed us conclude that both neurokines were effective in causing axonal sprouting, but they could not prevent sensory neuron death in L-5 dorsal root ganglion.
INTRODUCTION
The improvement of functional results of nerve regeneration is a major challenge to physicians and researchers.
The new advances in surgical techniques and devices have considerably changed the prognostic of peripheral nerve lesions over the last decades. However, it is not possible to obtain satisfactory results like the complete regeneration of a lesioned nerve.
Since the use of the microsurgical technique for the peripheral nerve regeneration by SMITH (1964) new and less aggressive methods have been searched in order to avoid the suture, even if using adequate microsurgery needles and threads.
The entubulation technique for the peripheral nerve regeneration consists of positioning the stumps of a sectioned nerve inside a tube. This technique allows analyze the activity of exogenous substances, which stimulate the regeneration between the extremities of a sectioned nerve with little manipulation of the stumps.
The best understanding of the physiologic process and biochemical regulation of the nerve regeneration may bring forward new perspectives.
The use of some substances that interfere in the nerve regeneration was described by a number of authors (MADISON et al., 1988; FIELDS et al., 1989; BAILEY et al., 1993; MUNSON et al., 1997; HO et al., 1998; SAINTS et al., 1999). Some benefits are well established, although there are no references upon their utilization in the clinical practice.
Among neurokines, which are substances with a typical proteinic chain, the cardiotrophin-1 (CT-1) and the oncostatin-M (OsM) have not been sufficiently tested with relationship to their activity in the nerve regeneration process.
As a hand surgeon we perform peripheral nerve reconstruction surgeries, and we feel stimulated to study nerve degeneration and regeneration and analyze the neurokines activity in enhancing nerve regeneration.
The main objective of this trial is to study the activities of cardiotrophin-1 (CT-1) and oncostatin-M (OsM) in C57BL/6J mice sciatic nerve regeneration, which is sectioned and immediately repaired through the entubulation technique.
MATERIAL AND METHOD
1- Material
Fifteen male C57BL/6J mice, aged 8 weeks at the surgery were used. Animals were divided into 3 experimental groups in accordance with the substance utilized inside the tubular prosthesis. A group formed by 3 non-operated animals was considered as a control-group.
The humane and lyophilized CT-1 and OsM were obtained in Peprothec Company (USA). The cytochrome-c, a substance used as control in growth factor studies, was obtained in Sigma (USA).
All the substances above were given with an extract of collagen (Vitrogen, Collagen Corporation, Palo Alto, CA, USA). By contacting the animal body temperature, the extract of collagen becomes a gel, which avoid the leakage of the substances placed inside the tubular prostheses.
We divided the 3 groups composed of 5 operated animals as the following: the first group had its prostheses filled out with collagen solution plus CT-1, the second group, with collagen solution plus cytochrome-c, and the third group, with collagen plus OsM.
2- Method
2 a - Surgical technique
Surgical procedures were performed under general anesthesia using Avertin (tribromoethanol 500 mg and 2-methyl-2-butanol 250 mg, plus distilled water 19.5 ml) at a dose of 0.02 ml/g which was given intraperitoneally. Then, mice left thigh were shaven and they were positioned for sciatic nerve dissection. Under microscopic vision (Zeiss OPM 240F) the posterolateral musculature and the sciatic nerve were exposed through an incision parallel to the femur (Figure 1).
The nerve was sectioned in the medium portion of the thigh. After the retraction of proximal and distal segments, they were fixated through a suture (mononylon 10-0 thread, a 75mm needle - Ethicon) into a tube of polyethylene (6-mm length x 0.76-mm internal diameter x 1.22-mm external diameter). The distance between both nerve stumps was of 4 mm (Figure 2).
Completions of prostheses with substances were done with the aid of a Hamilton syringe (10ml) and under microscopic vision in order to avoid air bubbles generation inside the prostheses (Figure 3).
The substances were used in the liquid form about 4o C temperature. To avoid the leakage of this preparation, Vaseline was used in the distal extremity of the tube. After 20 minutes of the implant, the solution formed a gel. The muscular layer was sutured, and the skin was closed with the aid of 7.5-mm surgical staples (Figure 4).
2b - Postoperative:
Following this, animals were placed in individual cages where 12-hour alternating cycles (clear x darkness) occurred during every postoperative period, and under appropriate feeding.
2c - Euthanasia and perfusion
After 4 weeks, animals were anesthetized for the laparotomy, which was performed through a longitudinal incision made by iris scissors. A heparin solution 0.2-ml (5000 UI/ml) was injected in the spleen (Figure 5).
2d - Histological analysis:
The polyethylene tubular prostheses with the regenerated nerve segments were dissected and removed and they were placed in the same solution of fixation about 4o C for 1 day. Then the material was post-fixed in osmium tetroxide solution (2% sodium phosphate buffer 0.1 M and pH 7.3) for 2 hours about 4o C. It was dehydrated using ethyl alcohol, cleared up in propylene oxide and included in Epoxy resin. Traverse sections of 1-mm thickness were made of the medium part of the regenerate cables. Toluidine blue 0.5% was used to stain them.
After the perfusion period, the spinal cord of the animals was opened for removing the L-5 spinal ganglion. The ganglion was included in historesin, and serially cut in sections of 5mm of thickness. Then it was stained through toluidine blue/fuchsin solution. Sensory neuron cell bodies of L-5 ganglion that had an apparent nucleolus were counted through a light microscope (Zeiss) 25 x. The count was made in 1 among 10 cuts of the series.
The myelinated fibers of the regenerate cables underwent a quantitative analysis. Sections of 1 mm of thickness were analyzed through a light microscope (Zeiss), which was connected to a clear camera (Zeiss) and a PC. The interaction with the image observed by the operator was made through a digitized table (Summagraphics) connected to the computer. It was used the CARP program (Biographics Inc. USA) based on the operating system UNIX.
This quantitative analysis followed the following steps: a) the system used for analysis of the images was gauged according to the different increases of the microscope lenses. b) Using a lens of low increase, cut contours to be studied were traced. c) The system automatically divided the contours in fields and stored them in the computer memory. d) Using the cursor of the table through the clear camera and lens of 100X myelin fibers were marked by the observer. e) The operator signalized the end of the demarcation of all fibers of a certain field. f) When completing the last field, the computer made the sum of the marked fibers, followed by their plottage in the monitor screen. This system avoided the double count of fibers of the observed field.
STATISTICAL ANALYSIS
The statistical analysis of the quantitative data obtained from each one of the experimental situations was performed according to Newman-Keuls method, using a computer program named "Primer of Biostatistics" (version 1.0 / 1988).
The significance level was p <0,05.
RESULTS
QUALITATIVE DATA
Four weeks after the tubular prostheses implant, a milky-white structure named regeneration cable (RC), with a proximal-distal decreasing diameter between nerve stumps, was noted in all animals.
In all the RC extension a net of blood vessels was observed. A yellowish liquid filled out the space between the external surface of RC and the internal wall of the tubular prosthesis.
Traverse sections of the RC medium portion, which were observed to the light microscope, showed that animals that had the prostheses filled out with collagen and CT-1 or OsM presented a larger diameter of RC than animals that were implanted with collagen and cytochrome-C. Through the same sections, RC presented groups of axons organized in small fascicles, which is a characteristic of the compartment process, typical of the peripheral nerve regeneration. Their own perineurium surrounded these fascicles. Several of these united groups were surrounded for an epineurium.
In traverse sections of sciatic nerves of non-operated animals, it was possible to distinguish their four fascicles to form tibial, fibular, sural nerves and the cutaneous branch.
QUANTITATIVE DATA
After 4 weeks from entubulation, the total number of RC myelinated fibers of the 3 groups of operated animals and the sciatic nerves of the 3 non-operated mice were counted and data obtained are in the Table 1 and Graph 1.
Results from statistical analysis by Newman-Keuls method showed that the number of myelinated axons in RC of the animals who were implanted with collagen plus OsM (2086 ± 102) and collagen plus CT-1 (2040 ± 56) was significantly larger (p<0.05) in comparison to animals implanted with collagen plus cytochrome-C (1449±102). Otherwise, this number was significantly smaller (p<0.05) in comparison to the group of non-operated animals (3257 ± 59). Graph 1.
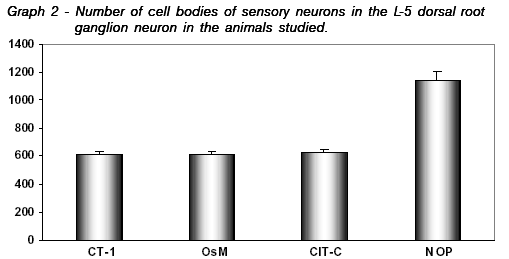
After 4 weeks, the number of cell bodies of sensory neurons in L-5 dorsal root ganglion neuron in operated and non-operated animals is showed in Table 2 and Graph 2.
After the statistical analysis, the comparison between the number of cellular bodies of the 4 groups demonstrated no significant difference (p>0.05) between animals implanted with collagen plus CT-1 (634±29) and collagen plus OsM (694±4) and collagen plus cytochrome-C (657±18). The three groups showed a number of cell bodies significantly smaller (p <0.05) than the non-operated control group (1141±64).
DISCUSSION
Changes in proximal and distal portions of a sectioned nerve have been hardly studied over the last decades.
Several authors studied the axonal sprouting in myelinated fibers and the participation of growth cones in this process (RAMON Y CAJAL, 1928; HOLMES; YOUNG, 1942; you GO, 1996; MANTHORPE et al., 1983; BIXBY et al. 1988).
Many researchers named proximal changes of a sectioned peripheral nerve as "retrograde effect" or "axonal reaction", including the chromatolysis (NISSL, 1892 apud RAMON Y CAJAL, 1928; GRANT; ALDSKOGIUS, 1967; LIEBERMAN, 1971; SUNDERLAND, 1978; FIORI et al., 1988; KREUTZBERG, 1995). Simultaneously, we observed that metabolic changes occurred and favored the proteinic production for cellular restructuring, in detriment to the synaptic transmission (LIEBERMAN, 1971; KREUTZBERG, 1995).
The neuronal reaction to its axonal lesion can vary from a retrograde degeneration up to cellular death (RAMON Y CAJAL, 1928; HYDÉN, 1960; LIEBERMAN 1971, 1974; KERR et al., 1972; FAWCETT; KEYNES, 1990; KREUTZBERG, 1995; GROVES, 1997)
Similarly to the proximal portion of the sectioned nerve, the distal portion also underwent well-defined changes. WALLER (1850) apud GREEN (1988) was the first to report the distal portion changes of a sectioned nerve, describing the granular degeneration of the myelin sheath and the disappearance of axons distally to the lesion, which he named "Wallerian degeneration". (RAMON Y CAJAL, 1928. SUNDERLAND; BRADLEY, 1950).
Several trials have contributed to the best knowledge of the changes in the distal portion of the lesioned nerve. Macrophage phagocytic activity of fragmented myelin and degenerate axons, promoting the proliferation of Schwann cells, and producing important cytokines in nerve regeneration were emphasized (HOLMES; YOUNG, 1942; LUBINSKA, 1982; BEUCHE; FRIEDE, 1984; PERRY et al., 1987).
It is very important to remind SUNDERLAND (1978) and LUNDBORG (1987) reports about the endoneurial tube formation, through which there will be the growth axonal.
Schwann cells produced in the distal stump of the lesioned nerve and stimulated by the axonal membrane, myelin fragments and macrophages is another indispensable element in nerve regeneration. They are responsible for degrading and phagocytozing the myelin and producing neurotrophic factors (SATINSKY et al., 1964; SALZER; BUNGE, 1980; HALL, 1986; HEUMANN et al., 1987; FAWCETT; KEYNES, 1990; SENDTNER et al., 1992; DE VRIES, 1993; REYNOLDS; WOOLF, 1993). Schwann cells are prolonged and connected longitudinally forming an image similar to a pearl necklace, named "bands of Büngner" (SUNDERLAND; BRADLEY, 1950; BUNGE, 1980). They act as guide for myelinated and non-myelinated fibers to reach their target organs (SON; THOMPSON, 1995).
In spite of several researches on changes in the extremities of a peripheral sectioned nerve, the biochemical regulation of this process is not totally cleared.
Several studies reported the difficult nerve regeneration after a peripheral axotomy, characterized by the decrease of the regenerate fiber diameter, formation of a thinner myelin sheath, and subsequent decrease of the conduction speed (SCHRODER, 1972; FIELDS;ELLISMAN, 1986 a,b).
Although described by RANSON in 1909, we agreed with the authors (ALDSKOGIUS et al., 1992; SUZUKI et al., 1993) who affirm that there are several doubts on cellular death and its causes. Our findings are in accordance with those from the literature i.e., the count of sensory neuron nucleolus shows a cellular loss up to 25% in lumbar dorsal root ganglions (YGGE; ALDSKOGIUS, 1984; RICH et al., 1989; HIMES; TESSLER, 1989; LISS et al. 1994).
The great challenge is to stimulate the axonal sprouting after a nerve section and prevent cellular death in order to achieve better functional restoration, mainly sensitive.
We believe in the strong indications that sensitive regeneration is worse than motor regeneration (GORDON; STEIN, 1982; SCHMALBRUCH, 1987; LAINETTI et al. 1995; SENDTNER et al., 1997; LJUNGBERG et al., 1999), although MADORSKY et al. (1998) do not agree with this statement. After a peripheral nerve lesion, both motor and sensory neurons are deprived of neurotrophic factors from target organs. The survival of nerve cells depends on these factors. However, motor neurons produce their specific factor CNTF immediately from Schwann cells, and are able to survive in spite of the axonal lesion (DOBREA et al., 1992). Sensory neurons are more susceptible to the degeneration after the lesion, once their synthesis of neurotrophic factors from Schwann cells (NGF and BDNF) is delayed. The death of the sensory neurons, which are dependent from these factors, is inevitable (LAINETTI et al., 1995).
We addressed our trial for analyzing the sensory neuron changes after a section. Through the addition of exogenous substances, CT-1 and OsM, we tried to stimulate the axonal regeneration and avoid the neuronal death in dorsal root ganglions.
We agreed with FUNAKOSHI et al. (1998) that one of the major difficulties in nerve regeneration study are the complex trophic needs from either sensory or motor neurons, which are not supplied by a single neurotrophic factor.
Since the first report about a microsurgery of peripheral nerves (SMITH, 1964), the technical and material advances over the last decades make nerve stump regeneration less aggressive. The entubulation technique has been improved.
We opted for this technique because it is less traumatic. Also, it allows fibers growth, avoids the invasion of cicatricial tissue and facilitates the morphology, biochemistry and physiology study of the micro-environment between nerve stumps after the administration of exogenous substances or interposition of different materials (DA SILVA, 1987; FIELDS et al., 1989; LANGONE, 1991; SAUCER, 1994; LAINETTI, 1995). This can be significant in improving results after peripheral nerve regeneration.
The first references on entubulated nerve lesion come from last century and numberless of subsequent experimental models searched for inert, flexible, and bioabsorbable materials, without causing fibrosis, ischemia, or inflammation and allowing growth factors accumulation (FIELDS et al. 1989).
As many other authors (GARRITY, 1955; DA SILVA; LANGONE, 1989; LANGONE, 1991; MARQUES, 1992; PEAR TREE, 1993, 1998), we chose polyethylene tubes due to their availability in our laboratory.
The effect of exogenous substances administered on the place of a nerve lesion, or materials placed between nerve stumps (like facilitative elements of the regeneration) are attracting researchers' attention. (CHIU et al., 1982; RICH et al., 1987; MADISON et al., 1988; FIELDS et al., 1989; BAILEY et al., 1993; ANSSELIN et al., 1997; FRANCEL et al., 1997; KAKINOKI et al., 1997; MUNSON et al., 1997; DI BENEDETTO, 1998).
In our trial we used two neurokines, cardiotrophin-1 (CT-1) and OsM. They have a similar molecular structure, a common receiving unit (gp 130), and both are found in larger amount in the peripheral nervous system rather than in central nervous system (MOSLEY et al., 1996). CNTF, LIF, GPA and both interleukin-6 and interleukin-11 (IL-6/IL-11) are also part of this group (HOPKINS; ROTHWELL, 1995).
Previous studies (ADLER et al., 1979; GEARING et al., 1987; YAMAMORI et al., 1989; RICHARDSON, 1994; PEREIRA, 1998; THOMPSON; MAJITHIA, 1998) demonstrated that CNTF and LIF stimulated nerve regeneration after their administration on lesion of adult mice sciatic nerves.
Since its identification (PENNICA et al., 1995), several actions of CT-1 on the hematopoietic system, liver, kidneys or on the induction of cardiomyocytes hypertrophy in vitro (JIN et al., 1996; PENNICA et al., 1996) have been described. However, there is no reference in the literature regarding its action on lesioned peripheral nerves (SENDTNER et al., 1997). A recent trial proved that this substance is involved in the regulation of sensory neurons survival in newborn mice, which suggest that it can be important against the post-traumatic degeneration (HORTON et al., 1998; THIER et al., 1999).
ZARLING et al. (1986) made the first publication on OsM. They isolated it from cells of a lymphoma histiocytic. In 1989, MALIK and collaborators recognized the OsM as the cytokine that regulates several mammal cellular cultures growth.
We did not find any reference in the literature on the action of CT-1 and OsM in peripheral nerve regeneration process.
Such substances might be produced in "target organs" and liberated through neuronal cells by retrograde pathway, promoting regeneration after an axotomy (PURVES, 1986; PATTERSON; NAWA, 1992; THOMPSON et al., 1997).
We chose C57BL/6J mouse as the experimentation animal, because it is available in our laboratory. Also, it is easy to manipulate, and has low body weight (30 g), which allows lower expense of material. In mice it was demonstrated that, after a peripheral lesion, the nerve regeneration (mainly sensory nerve regeneration) is decreased, which allows study the activity of exogenous substances on the section area of the nerve (LAINETTI et al. 1995).
Only one paw was operated because we observed that animals underwent bilateral denervation have difficulty in locomotion. Proper feeding is limited, which can lead to autophagia of the denervated paw and subsequent infection and death.
Between nerve stumps, a solution of collagen plus CT-1 or OsM was interposed. By contacting the animal body temperature, the extract of collagen becomes a gel, which avoid the leakage of the substances placed inside the tubular prostheses. To avoid the leakage of this preparation, Vaseline was used in the distal extremity of the tube. The control group received collagen plus cytochrome-C, a substance with molecular weight similar to neurotrophin, but without neurotrophic activity.
The mice from our groups were placed in individual cages where 12-hour alternating cycles (clear x darkness) occurred during every postoperative period to avoid hormonal changes that might influence the final result.
In spite of discordance (KIYOTANI et al., 1996; SUZUKI et al, 1999), several trials studied the axonal growth through empty tubes and concluded that 10 mm was the maximum distance to achieve an appropriate regeneration (BRUNELLI et al., 1994; DEN DUNNEN et al. 1996; FRANCEL et al., 1997). The use of exogenous substances inside the tubes allowed nerve regeneration using distances up to 25 mm (MADISON et al., 1988; BAILEY et al., 1993; ANSSELIN et al., 1997; KAKINOKI et al., 1997; DI BENEDETTO, 1998; HO et al., 1998). This aspect proved that the manipulation of the microenvironment could stimulate the regeneration. Thus, the 4-mm distance between nerve stumps seemed adequate as our pattern, since it is a space that can be easily crossed by axons in regeneration.
Euthanasia was performed after 4 weeks postoperatively to observe the presence of a cylindrical vascular structure joining proximal and distal stumps (named for us as regeneration cable - RC). Between the regeneration cable and the tube wall, a space filled out by a fluid similar to plasma was noted (LUNDBORG et al. 1982; WILLIAMS et al. 1983; DA SILVA, 1987; LANGONE, 1991).
The L-5 dorsal root ganglion was chosen because it is significant for sciatic nerve formation (SWETT et al., 1991).
In the RC and L-5 ganglion histological analysis, we used the methodology described by TOME, MIRA (1981), which consists of fixing the material in glutaraldehyde and osmium tetraoxide 2%, followed by the inclusion in resin Epoxy. Following, sheets are prepared using sections of 2 m of thickness, which are stained using methylene blue, and observed to the microscope.
After 4 weeks from tube implant, all animals underwent surgery exhibited RC.
In animals where CT-1 and OsM were used, regeneration cables of larger diameter and larger amount of myelinated axons were observed in relation to groups whose prostheses were filled out with cytochrome-c, showing the role of both substances in the axonal sprouting.
Analyzing the number of sensory neurons in L-5 dorsal root ganglions, we verified that it was statistically lower in the groups where neurokines were used in relation to control groups non-operated. No significant difference in relation to the group whose tubes were filled out with cytochrome-c was observed. We believed that these results confirm the low efficacy of CT-1 and OsM in sensory neuron survival.
Our expectation from this work is to use the entubulation technique to reduce sutures aggression to nerve stumps, and, simultaneously, to administer exogenous substances to the humane sectioned nerve. This procedure can improve the prognostic of these lesions and allow the repair of losses without using grafts. It is probable that the future of the peripheral nerve repair will depend on the understanding of the biochemical phenomena that regulate the survival of the cell and the process of nerve regeneration. Several substances, as neurokines, participate in this regulation. The study of the physiologic mechanisms is fundamental for the scientific progress in this field. The comprehension of CT-1 and OsM usage in the treatment of lesioned peripheral nerves is contributing to recover patients with this type of lesion.
CONCLUSIONS
1. The topic administration of cardiotrophin-1 and oncostatin-M enhances the peripheral nerve regeneration. This aspect is expressed by the number of myelinated fibers in the cable of regeneration of sciatic nerves of mice underwent lesion and reconstruction by the entubulation technique.
2. The administration of cardiotrophin-1 and oncostatin -M to peripheral nerves does not avoid the death of sensory neurons underwent axotomy.
REFERENCES
This trial was performed at IOT, HC-FMUSP and at ICB-USP
- 1) ADLER, R.; LANDA, K.B.; MANTHORPE, M.; VARON, S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science, v. 204, p. 1434-6, 1979.
- 2) ALDSKOGIUS, H.; ARVIDSSON, J.; GRANT , G. Axotomy-induced changes in primary sensory neurons. Sensory Neurons. Oxford University Press, p. 363-83, New York, 1992.
- 3) ANSSELIN, A.D.; FINK, T.; DAVEY, D.F. Peripheral nerve regeneration through nerve guides seeded with adult Schwann cells. Neuropathol. Appl. Neurobiol., v. 23, p. 387-98, 1997.
- 4) BAILEY, S.B.; EICHLER, M.E.; VILLADIEGO, A.; RICH, K.M. The influence of fibronectin and laminin during Schwann cell migration and peripheral nerve regeneration through silicon chambers. J. Neurocytol., v. 22, p. 176-84, 1993.
- 5) BEUCHE, W.; FRIEDE, R.L. The role of non-resident cells in Wallerian degeneration. J. Neurocytol., v. 13 , p. 767-96, 1984.
- 6) BIXBY, J.L.; LILIEN, J.; REICHARDT, L.F. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J. Cell Biol., v. 107, p. 353-61, 1988.
- 7) BRUNELLI, G.A.; VIGASIO, A.; BRUNELLI, G.R. Different conduits in peripheral nerve surgery. Microsurgery, v. 15, p. 176-8, 1994.
- 8) BUNGE, R.P. Some observations on the role of the Schwann cells in peripheral nerve regeneration. In McCARROLL, J. ed. Nerve repair and regeneration _ Its clinical and experimental basis. St. Louis, Mosby, 1980. p. 58-64.
- 9) CHIU, D.T.W.; JANECKA, I.; KRIZEK, T.J. Autogenous vein graft as a conduit for nerve regeneration. Surgery, v. 91, p. 226-33, 1982.
- 10) DA SILVA, C.F. Estudo experimental da regeneração de nervos no interior de próteses tubulares. São Paulo, 1987. 58p. Tese (Livre-Docência) _ Instituto de Ciências Biomédicas, Universidade de São Paulo.
- 11) DA SILVA, C.F.; LANGONE, F. Addition of nerve growth factor to the interior of a tubular prosthesis increases sensory neuron regeneration in vivo. Braz. J. Med. Biol. Res., v. 22, p. 691-4, 1989.
- 12) DE VRIES, G.H. Schwann cell proliferation. In: DYCK, P.J.; THOMAS, P.K.; GRIFFIN, J.W.; LOW, P.D.; PODUSLO, J., ed. Peripheral Neuropathy. Philadelphia, W.B. Saunders, 1993. p. 290-8.
- 13) DEN DUNNEN, W.F.; VAN DER LEI, B.; SCHAKENRAAD, J.M.; STOKROOS, J.; BLAAUW, E.; BARTELS, H.; PENNINGS, A.J.; ROBINSON, P.H. Poly (DL-lactide-epsilon-caprolactone) nerve guides perform better than autologous nerve grafts. Microsurgery, v. 17, p. 348-57, 1996.
- 14) DI BENEDETTO, G.; ZURA, G.; MAZZUCHELLI, R.; SANTINELLI, A.; SCARPELLI, M.; BERTANI, A. Nerve regeneration through a combined autologous conduit (vein plus acellular muscle grafts). Biomaterials, v. 19, p. 173-81, 1998.
- 15) DOBREA, G.M.; UNNERSTALL, J.R.; RAO, M.S. The expression of CNTF message and immunore-activity in the central and peripheral nervous system of the rat. Devl. Brain Res., v. 66, p. 209-19, 1992.
- 16) FAWCETT, J.W.; KEYNES, R.J. Peripheral nerve regeneration. Ann.Ver. Neurosci., v. 13, p. 43-60, 1990.
- 17) FIELDS, R.D.; ELLISMAN, M.H. Axons regenerated through silicone tube splices. I. Conduction properties. Exp. Neurol., v. 92, p. 48-60, 1986a.
- 18) FIELDS, R.D.; ELLISMAN, M.H. Axons regenerated through silicone tube splices. II. Functional morphology. Exp. Neurol., v. 92, p. 61-74, 1986b.
- 19) FIELDS, R.D.; LE BEAU, J.M.; LONGO, F.M.; ELLISMAN, M.H. Nerve regeneration *through artificial tubular implants. Prog. Neurobiol., v. 33, p. 87-134, 1989.
- 20) FIORI, M.G.; DI GREGORIO F.; FABRIS, M.; MARINI, P.; TRIBAN, C.; TOFFANO, G. Processes of peripheral nerve and neuromuscular repair. Ann. Inst. Super. Sanita, v. 24, p. 143-58, 1988.
- 21) FRANCEL, P.C.; FRANCEL, T.J.; MACKINNON, S.E.; HERTL, C. Enhancing nerve regeneration across a silicone tube conduit by using interposed short-segment nerve grafts. J. Neurosurg., v. 87, p. 887-92, 1997.
- 22) FUNAKOSHI, H.; RISLING, M.; CARLSTEDT, T.; LENDAHL, U.; TIMMUSK, T.; METSIS, M.; YAMAMOTO, Y.; IBAÑEZ, C.F. Targeted expression of a multifunctional chimeric neurotrophin in the lesioned sciatic nerve accelerates regeneration of sensory and motor axons. Proc. Natl. Acad. Sci., v. 95, p. 5269-74, 1998.
- 23) GARRITY, R.W. The use of plastic and rubber tubing in the management of irreparable nerve injuries. Neurological Surg., v. 6, p. 517-20, 1955.
- 24) GEARING, D.P.; GOUGH, N.M.; KING, J.A.; HILTON, D.J.; NICOLA, N.A.; SIMPSON, R.J.; NICE, E.C.; KELSO, A.; METCALF, D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J., v. 6, p. 3995-4002, 1987.
- 25) GORDON, T.; STEIN, R.B. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. J. Physiol., v. 323, p. 307-23, 1982.
- 26) GRANT, G.; ALDSKOGIUS, H. Silver impregnation of degenerating dentrites, cells and axons central to axonal transections. A nauta study on the hipoglossal nerve in kittens. Exp. Brain Res., v. 3, p. 150-62, 1967.
- 27) GROVES, M.J.; CHRISTOPHERSON, T.; GIOMETTO, B.; SCARAVILLI, F. Axotomy_induced apoptosis in adult rat primary sensory neurons. Journal of Neurocytology, v. 26, p. 615-24, 1997.
- 28) HALL, S.M. Regeneration in cellular and acellular autografts in the peripheral nervous system. Neuropathol. Appl. Neurobiol., v. 12, p. 27-46, 1986.
- 29) HEUMANN, R.; KORSHING, S.; BANDTLOW, C.; THOENEN, H. Changes of nerve growth factor synthesis in non-neuronal cells in reponse to sciatic nerve transection. J. Cell Biol., v. 104, p. 1623-31, 1987.
- 30) HIMES, B.T.; TESSLER, A. Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J. Comp. Neurol., v. 284, p. 215-30, 1989.
- 31) HO, P.R.; COAN, G.M.; CHENG, E.T.; NIELL, C.; TARN, D.M.; ZHOU H., SIERRA, D.; TERRIS, D.J. Repair with collagen tubules linked with brain derived neurotrophic factor and ciliary neurotrophic factor in a rat sciatic injury model. Arch. Otolaryngol Head Neck Surg., v. 124, p. 761-6, 1998.
- 32) HOLMES, W.; YOUNG, J.Z. Nerve regeneration after or immediate delayed suture. J. Anat. (Lond.), v. 77, p. 63-96, 1942.
- 33) HOPKINS, S.J.; ROTHWELL, N.J. Cytokines and the nervous system I: expression and recognition. TINS, v. 18, p. 83-8, 1995.
- 34) HORTON, A.R.; BARLETT, P.F.; PENNICA, D.; DAVIES, A.M. Cytokines promote survival of mouse cranial sensory neurones at different developmental stages. Eur. J. Neurosci., v. 10, p. 673-9, 1998.
- 35) HYDÉN, H. Cell, In: BRACHET, J.; MIRSKY, A.E., ed. New York, Academic Press, 1960. p. 215-323.
- 36) IDE, C. Peripheral nerve regeneration. Neurosci. Res., v. 25, p. 101-21, 1996.
- 37) JIN, H.; YANG, R.; KELLER, G.A.; RYAN, A.; KO, A.; FINKLE, D.; SWANSON, T.A.; LI, W.; PENNICA, D.; WOOD, W.I.; PAONI, N.F. In vivo effects of cardiotrophin-1. Cytokine, v 8, p. 920-6, 1996.
- 38) KAKINOKI, R.; NISHIJIMA, N.; UEBA, Y.; OKA, M.; YAMAMURO, T.; NAKAMURA, T. Nerve regeneration over a 25mm gap in rat sciatic nerves using tubes containing blood vessels: the possibility of clinical application. Int. Orthop., v. 21, p. 332-6, 1997.
- 39) KERR, J.F.R.; WYLLIE, A.H.; CURRIE, A.R. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. British J. of Cancer, v. 26, p. 239-57, 1972.
- 40) KIYOTANI, T.; TERAMACHI, M.; TAKIMOTO, Y.; NAKAMURA, T.; SHIMIZU, Y.; ENDO, K. Nerve regeneration across a 25-mm gap bridged by a poliglicolic acid-collagen tube: a histological and electrophysiological evaluation of regenerated nerves. Brain Research, v. 740, p. 66-74, 1996.
- 41) KREUTZBERG, G.W. Reaction of the neuronal cell body to axonal damage. In: WAXMAN, S.G., KOCSIS, J.D. AND STYS, P.K. ed. The Axon: structure, function and pathofisiology. New York, Oxford University Press, 1995. p. 355-74.
- 42) LAINETTI, R.D.; PEREIRA, F.C.; DA-SILVA, C.F. Reduced sensory neuron regeneration by C57BL/6J mice. Braz. J. Med. Biol. Res., v. 28, p.781-5, 1995.
- 43) LANGONE, F. Estudo ultra-estrutural e morfométrico de nervos regenerados no interior de próteses tubulares. São Paulo, 1991. 201p (Tese de doutorado _ Instituto de Ciências Biomédicas da Univ. de São Paulo).
- 44) LIEBERMAN, A.R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int. Ver. Neurobiol., v. 14, p. 49-124, 1971.
- 45) LISS, A.G.; EKENSTAM, F.W.; WIBERG, M. Cell loss in sensory ganglia after peripheral nerve injury. An anatomical tracer study using lectin-coupled horseradish peroxidase in cats. Scand. J. Plast. Reconstr. Surg. Hand Surg., v. 28, p. 117-88, 1994.
- 46) LJUNGBERG, C.; NOVIKOV, L.; KELLERTH, J.O.; EBENDAL, T.; WIBERG, M. The neurotrophins NGF and NT-3 reduce sensory neuronal loss in adult rat after peripheral nerve lesion. Neurosci. Lett., v. 262, p. 29-32, 1999.
- 47) LUBINSKA, L. Patterns of Wallerian degeneration of myelinated *fibres in short and long peripheral stumps and in isolated segments of rat phrenic nerve. Interpretation of the role of axoplasmic flow of the trophic factor. Brain Res. , v. 233, p. 227-40, 1982.
- 48) LUNDBORG, G.; GELBERMAN, R.H., LONGO, F.M. In vivo regeneration *of cut nerves encased in silicone tubes. J. Neuropat. Exp. Neurology, v. 41, p. 412-22, 1982.
- 49) LUNDBORG, G. Nerve regeneration and repair. Acta Orthop. Scand., v. 58, p. 145-69, 1987.
- 50) MADISON, R.D.; DA SILVA, C.F.; DIKKES, P. Entubulation repair with protein additives increases the maximum nerve gap distance successfully bridged with tubular prostheses. Brain Res., v. 447, p. 325-34, 1988.
- 51) MADORSKY, S.J.; SWETT, J.E.; CRUMLEY, R.L. Motor versus sensory neuron regeneration trough collagen tubules. Plast. Reconstr. Surg., v. 102, p. 430-6, 1998.
- 52) MALIK, N.; KALLESTAD, J.C.; GUNDERSON, N.L.; AUSTIN, S.D.; NEUBAUER, M.G.; OCHS, V.; MARQUARDT, H.; ZARLING, J.M.; SHOYAB, M.; WEI, C.M. Molecular cloning, sequence analysis and functional expression of a novel growth regulator, oncostatin M. Mol. Cell. Biol., v. 9, p. 2847-53, 1989.
- 53) MANTHORPE, M.; ENGVALL, E.; RUOSLAHTI, E.; LONGO, F.M.; DAVIS, G.E.; VARON, S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J. Cell Biol., v. 97, p. 1882-90, 1983.
- 54) MARQUES, M.J. Estudo ultra-estrutural da junção neuromuscular após regeneração nervosa no interior de próteses tubulares. São Paulo, 1992. 140p. Tese (Doutorado) _ Instituto de Ciências Biomédicas, Universidade de São Paulo.
- 55) MOSLEY, B.; DE IMUS, C.; FRIEND, D.; BOIANI, N.; THOMA, B.; PARK, L.S.; COSMAN, D. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J. Biol. Chem., v. 271, p. 32635-43, 1996.
- 56) MUNSON, J.B.; SHELTON, D.L.; MCMAHON, S.B. Adult mammalian sensory and motor neurons: roles of endogenous neurotrophins and rescue by exogenous neurotrophins after axotomy. J. Neurosci., v. 17, n. 1, p. 470-6, 1997.
- 57) NISSL, F. Uber die veranderungender ganglienzellen am fazialiskern der kaninchen nach ausreissung der nerven. Allg. Z. Psychiat., v. 48, p. 197-8, 1892 apud RAMON Y CAJAL, S. Histology of the nervous system. Oxford University Press, 1928.
- 58) PATTERSON, P.H.; NAWA, H. Neuronal differentiation factor/cytokines and synaptic plasticity. Cell, v. 72, p. 123-37, 1992. Supplement.
- 59) PENNICA, D.; KING, K.L.; SHAW, K.J.; LUIS, E.; RULLAMAS, J.; LUOH, S.M.; DARBONNE, W.C.; KNUTZON, D.S.; YEN, R.; CHIEN, K.R. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hipertrophy. Proc. Natl. Acad. Sci., v. 92, p. 1142-6, 1995.
- 60) PENNICA, D.; WOOD, W.I.; CHIEN, K.R. Cardiotrophin-1: a multifunctional cytokine that signals via LIF receptor-gp 130 dependent pathways. Cytokine Growth Factor, v. 1, n. 1, p. 81-91, 1996.
- 61) PEREIRA, F.C. Estudo experimental e quantitativo da reinervação muscular após regeneração de nervos no interior de próteses tubulares. São Paulo, 1993. 135p. Dissertação (Mestrado) _ Instituto de Ciências Biomédicas, Universidade de São Paulo.
- 62) PEREIRA, F.C. Ações das neurocinas CNTF e IL-6 exógenas na regeneração nervosa periférica. São Paulo, 1998. 79p. Tese (Doutorado) _ Instituto de Psicologia, Universidade de São Paulo.
- 63) PERRY, V.H.; BROWN, M.C.; GORDON, S. The macrophage response to central and peripheral nerve injury. J. Exp. Med., v. 165, p. 1218-23, 1987.
- 64) PIRES, R.S. Padrão morfométrico de nervos regenerados no interior de próteses tubulares e influência do fator de crescimento nervoso (NGF) exógeno. São Paulo, 1994. P. 171p. Dissertação (Mestrado) _ Instituto de Psicologia, Universidade de São Paulo.
- 65) PURVES, D. The trophic theory of neural connections. Trends in Neurosci., v. 9, p. 486-9, 1986.
- 66) RAMON Y CAJAL, S. Degeneration and regeneration of the nervous system. London. Oxford. University Press., p. 459, 1928.
- 67) RANSON, S.W. Alterations in the spinal ganglion cells following neurotomy. J. Comp. Neurol., v. 19, p. 125-53, 1909 apud SCHMALBRUCH, H., 1987, p. 323.
- 68) REYNOLDS, M.L.; WOOLF, C.J. Reciprocal Schwann cell-axon interaction. Curr. Op. Neurobiol., v. 3, p. 683-93, 1993.
- 69) RICH, K.M.; LUSZCZYNSKI, J.R.; OSBORNE, P.A.; RICHARDSON, P.M. Nerve growth factor protects adult sensory neurons from cell death and atrophy caused by nerve injury. J. Neurocytol., v. 16, p. 261-8, 1987.
- 70) RICH, K.M.; DISCH, S.P.; EICHLER, M.E. The influence of regeneration and nerve growth factor on the neuronal cell body reaction to injury. J. Neurocytol., v. 18, p. 569-76, 1989.
- 71) RICHARDSON, P.M. Ciliary neurotrophic factor: a review. Pharmacol. Ther., v. 63 , p. 187-98, 1994.
- 72) SALZER, J.L.; BUNGE, R.P. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J. Cell. Biol., v. 84, p. 739-52, 1980.
- 73) SANTOS, X.; RODRIGO, J.; HONTANILLA, B.; BILBAO G. Regeneration of the motor of the rat sciatic nerve with local administration of neurotrophic growth factor in silicone chambers. J. Reconstr. Microsurg., v. 15, p. 207-13, 1999.
- 74) SATINSKY, D.; PEPE, F.A.; LIU, C.N. The neurilemma cell in peripheral nerve degeneration and regeneration. Expl. Neurol., v. 9, p. 441-51, 1964.
- 75) SCHMALBRUCH, H. Loss of sensory neurons after sciatic nerve section in the rat. Anat. Rec., v. 219, p. 323-9, 1987.
- 76) SCHRODER, J.M. Altered ratio between axon diameter and mielyn sheat thickness in regenerated nerve fibers. Brain Res., v. 45, p. 49-65, 1972.
- 77) SENDTNER, M.; GÖTZ, R.; HOLTMANN, B.; THOENEN, H. Endogenous ciliary neurotrophic factor is a lesion factor for axotomized motoneurons in adult mice. J. Neurosci., v. 17, p. 6999-7006, 1997.
- 78) SENDTNER, M.; STOCKLI, K.A.; THOENEN, H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J. Cell Biol., v. 118, p. 139-48, 1992.
- 79) SMITH, J.W. Microsurgery of peripheral nerves. Plast. Reconst. Surg., v. 33, p. 317-329, 1964.
- 80) SON, Y.L.; THOMPSON, W.J. Schwann cell processes guide regeneration of peripheral axons. Neuron, v. 14, p. 125-32, 1995.
- 81) SUNDERLAND, S. Degeneration of the axon and associated changes. In: Ed. S. Sunderland 2 ed. Nerves and nerve injuries. Edinburgh, Churchil Livingstone, 1978. p. 82-107.
- 82) SUNDERLAND, S.; BRADLEY, K.C. Endoneural tube shrinkage in the distal segment of a severed nerve. J. Comp. Neurol., v. 93, p. 411-20, 1950.
- 83) SUZUKI, H.; OYANAGI, K.; TAKAHASHI, H.; KONO, M.; YOKOYAMA, M.; IKUTA, F. A quantitative pathological investigation of the cervical cord, roots and ganglia after long-term amputation of the unilateral upper arm. Acta Neuropathol., v. 85, p. 666-73, 1993.
- 84) SUZUKI, Y.; TANIHARA, M.; OHNISHI, K.; SUZUKI, K.; ENDO, K.; NISHIMURA, Y. Cat peripheral nerve regeneration across 50 mm gap repaired with a novel nerve guide composed of freeze-dried alginate gel. Neurosci. Lett. v. 259, p. 75-8, 1999.
- 85) SWETT, J.E.; TORIGOE, Y.; ELIE, V.R.; BOURASSA, C.M.; MILLER, P.G. Sensory neurons of the rat sciatic nerve. Exp. Neurobiol., v. 114, p. 82-103, 1991.
- 86) THIER, M.; HALL, M.; HEATH, J.K.; PENNICA, D.; WEIS, J. Trophic effects of cardiotrophin-1 and interleukin-11 on rat dorsal root ganglion neurons in vitro. Brain Res. Mol., v. 64, p. 80-4, 1999.
- 87) THOMPSON, S.W.; MAJITHIA, A.A. Leukemia inhibitory factor induces sympathetic sprouting in intact dorsal root ganglia in the adult rat in vivo. J. Physiol., v. 506 , p. 809-16, 1998.
- 88) THOMPSON, S.W.; VERNALIS, A.B.; HEATH, J.K.; PRIESTLEY, J.V. Leukaemia inhibitory factor is retrogradely transported by a distinct population of adult rat sensory neurons: co-localization with trkA and other neurochemical markers. Eur. J. Neurosci., v. 9, p. 1244-51, 1997.
- 89) TOME, F.M.S.; MIRA, J.C. Contribution of the morphological technique to the study on peripheral nerve lesions in man and experimental animals. Int. J. Microsurg., v. 3, p. 152-7, 1981.
- 90) WALLER, A.V. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibers. Philos. Trans. R. Soc. London, B., v. 140, p. 423-9, 1850 apud GREEN, D.P. Operative Hand Surgery, Churchill Livingstone, 1988, p. 1373.
- 91) WILLIAMS, L.R.; LONGO, F.M.; POWELL, H.C.; LUNDBORG, G.; VARON, S. Spatial - temporal progress of peripheral nerve regeneration within a silicone chambers: Parameters for a bioassay. J. Comp. Neurol., v. 218, p. 460-70, 1983.
- 92) YAMAMORI, T.; FUKADA, K.; AEBERSOLD, R.; KORSCHING, S.; FANN, M.J.; PATTERSON, P.H. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science, v. 15, p. 1412-6, 1989.
- 93) YGGE, J.; ALDSKOGIUS, H. Intercostal nerve transection and its effect on the dorsal root ganglion. A quantitative study on thoracic ganglion cell numbers and sizes in the rat. Exp. Brain Res., v. 55, p. 402-8, 1984.
- 94) ZARLING, J.M.; SHOYAB, M.; MARQUARDT, H.; HANSON, M.B.; LIOUBIN, M.N.; TODARO, G.J. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc. Natl. Sci., v. 83, p. 9739-43, 1986.
Publication Dates
-
Publication in this collection
22 May 2007 -
Date of issue
June 2000