Abstracts
Experimentally, the authors demonstrate the standardization of the technique for captivating the motor evoked potential in rats through transcranial electric stimulus. Fifty Wistar rats, under anesthesia, were prepared according to the current rules of the Laboratory of Spinal Cord Trauma and Peripheric Nerves Studies of the Orthopaedics and Traumatology Institute of Hospital das Clínicas, Medical School of São Paulo. Average minimal latency of the upper limb responses was 2.5 ms and of the lower limb 6.5 ms. Average amplitude of the responses was 3.0 mV and 2.5 mV on upper and lower limbs, respectively. The authors conclude that the technique for obtaining motor evoked potential in rats, as presented in this study, is efficient for the analysis of the electrophysiological evolution of medullar lesions, unprecedent in our field, and it can be reproduced in a simple way, and presents quality and applicability standards similar to those seen in global literature.
Spinal cord Injuries; Rats; Wistar; Eletric stimulation
Os autores demonstram experimentalmente a padronização da técnica para obtenção do potencial evocado motor em ratos através da estimulação elétrica transcraniana. Foram utilizados 50 ratos Wistar devidamente anestesiados e preparados de acordo com as normas vigentes no Laboratório de Estudos do Traumatismo Raquimedular e Nervos Periféricos do Instituto de Ortopedia e Traumatologia do Hospital das Clínicas da Faculdade de Medicina de São Paulo. A latência mínima média das respostas dos membros superiores foi de 2,5 ms e de membros inferiores foi de 6,5 ms. A amplitude média das respostas foi de 3,0 mV e de 2,5 mV nos membros superiores e membros inferiores, respectivamente. Os autores concluem que a técnica para captação do potencial evocado motor em ratos apresentada neste estudo é eficaz na análise da evolução eletrofisiológica da lesão medular, inédita no nosso meio, podendo ser reproduzida de modo simples, além de apresentar padrões de qualidade e aplicabilidade semelhantes aos observados na literatura mundial.
Traumatismos da medula espinal; Ratos Wistar; Estimulação elétrica
ORIGINAL ARTICLE
Standardization of motor evoked potential captivation technique in rats through transcranial electric stimulus
Ricardo FerreiraI; Arnóbio Rocha OliveiraII; Tarcísio Eloy Pessoa de Barros FilhoIII
IAssociate Doctor to LETRAN Head of Neurophysiologic Monitoring
IIPhD student on the Post graduation Course, Orthopaedics and Traumatology Institute - Hospital das Clínicas Medical School, University of São Paulo
IIIChairman of the Orthopaedics and Traumatology Institute - Hospital das Clínicas Medical School, University of São Paulo
Correspondence Correspondence to Arnóbio Rocha Oliveira Departamento de Ortopedia e Traumatologia da Faculdade de Medicina da Universidade de São Paulo Curso de Pós-graduação e-mail: arnobior@terra.com.br
SUMMARY
Experimentally, the authors demonstrate the standardization of the technique for captivating the motor evoked potential in rats through transcranial electric stimulus. Fifty Wistar rats, under anesthesia, were prepared according to the current rules of the Laboratory for Studies on Spinal Cord Trauma and Peripheral Nerves of the Orthopaedics and Traumatology Institute, Hospital das Clínicas, Medical School of São Paulo. Average minimal latency of the upper limb responses was 2.5 ms and of the lower limb 6.5 ms. Average amplitude of the responses was 3.0 mV and 2.5 mV on upper and lower limbs, respectively. The authors conclude that the technique for obtaining motor evoked potential in rats, as presented in this study, is efficient for the analysis of the electrophysiological evolution of spinal cord injuries, unprecedented in our field, and can be reproduced in a simple way, presenting quality and applicability standards similar to those seen in global literature.
Keywords: Spinal cord Injuries; Wistar Rats; Electric stimulation.
INTRODUCTION
The search for appropriate knowledge on the mechanisms involved in spinal cord injuries has been strengthened in the last few years, justifying the increasingly production of experimental studies in the area of spinal cord regeneration(1).
Rats have been the animals mostly used in experimental models of spinal cord injuries(2), maybe due to the ease for obtaining and dealing with this kind of animal, as well as to the lower expenditures in research- intended financial resources.
Functional recovery after spinal cord injury in rats may present different functional patterns depending on the severity of trauma and on the treatment given. The record of this recovery may be done through various behavioral tests in those animals(3,4,5,6), anatomicopathological studies of the affected spinal cord segment (7,8) and electrophysiological methods(2,7,9,10,11,12).
There is no report in local literature concerning the study on the motor evoked potential in rats. To date, the evaluation of spinal cord injuries recovery in rats described in our environment has been performed only through clinical tests on motor function and histological tests(13,14).
According to literature(2), many electrophysiological methods have been developed for monitoring neurological recovery in rats. Some authors used stimulating screws directly attached to the skull, which remained inserted up to the end of the experimental study. In other studies, stimulating microelectrodes have been placed, directly attached to cerebral cortex; such procedures are technically different and more complex when compared to the transcranial stimulus model proposed in this study, of which needle electrodes are only inserted into animals' scalp.
OBJECTIVES
The objective of this study is to demonstrate the technique for obtaining motor evoked potential in rats, through transcranial electrical stimulus, in a simple, economic and highly reproducible way for any experimental research laboratory.
MATERIALS AND METHODS
This study has been developed at the Laboratory for Studies on Spinal Cord Trauma and Peripheral Nerves (LETRAN) and at the Laboratory of Microsurgery of the Orthopaedics and Traumatology Institute - Hospital das Clínicas - Medical School, University of São Paulo (IOT - FMUSP), since 06/30/2003. Fifty male Wistar rats weighing 360g in average, and with average body temperature of 28º C, duly anesthetized with 55 75 mg/ kg of intraperitoneal Pentobarbital, associated to 55 75 mg/kg of intramuscular Ketamina.
Materials required for the procedure of obtaining motor evoked potential consist of a 4-channel electromyography instrument, 2 needle monopolar electrodes corkscrew EO401 type from Neuromedical Supplies for transcranial stimulation, 1 needle monopolar electrode to be used as grounding and four pairs of needle monopolar electrodes to be used on the capture of motor responses in UULL and LLLL.
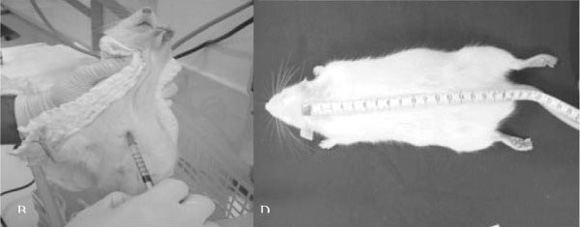
Below, the steps for obtaining the motor evoked potential in rats will be described. Rats' weight (Figure 1A) is used both for calculating anesthetic dosage to be applied and for maintaining weight uniformity of the animals used in the experiment. Anesthesia (Figure 1B) intraperitoneal, with 55 - 75 mg/Kg of intraperitoneal Pentobarbital associated to 55 - 75 mg/Kg of intramuscular Ketamina. After animals were anesthetized, Trichotomy (Figure 1C) of the skull region was performed, in order to enable the insertion of needle electrodes into the scalp. Skull-tail length (Figure 1D) of the animal was measured between the occipital region and the point where tail basis begins. This datum shall be used for comparing latencies seen in different rats. Rats' temperature (Figure 1E) is measured on lower limb with a digital thermometer. The capture (Figure 1F Figure 3) of muscular responses is performed by placing pairs of needle monopolar electrodes (capturer and reference), with determined and fixed interelectrodes distance for UULL and LLLL capture, inserted on proximal and anterior musculature of the UULL and LLLL. Grounding (Figure 3) electrode is placed on lumbar region through a needle monopolar electrode. Transcranial electric stimulation (Figure 2 and Figure 3) is performed by placing two corkscrew-type needle electrodes into the scalp on frontal region (anode) and occipital region (cathode) at the interhemispheric line, for simultaneous bilateral stimulus. After electrodes are placed in the rats, the equipment (Figure 4A) is turned on and the impedance (Figure 4B) of those electrodes is checked in order to prove their good adaptability so as to obtain sharper, safer and reliable responses. Equipment calibration is performed in two aspects of muscular responses capture: scan: 20ms- window, sensitiveness: 2 mV/div., low-frequency filter: 10 Hz and high frequency filter: 10 Khz and the transcranial electric stimulation through a single stimulus, 0.2ms long. Stimulus intensity considered as supramaximal.
RESULTS
On Figure 5, some records of the motor evoked potential in rats are shown. Minimal latency measured on UULL responses was 2.5ms and on LLLL was 6.5ms. UULL responses average amplitude was 3.0mV and LLLL responses average amplitude was 2.5mV.
DISCUSSION
At the current knowledge level about spinal cord injury recovery, experimental studies are crucial to understand the mechanisms involved in injuries genesis, as well as to obtain functional improvement after spinal cord trauma. This kind of trauma is usually permanent and may cause devastating sequels for the individual, which reflects in family life and in the society in which he/ she exists.
In our environment, the study of neurological recovery in rats after spinal cord injury can also be evaluated with the motor evoked potential, in addition to clinical and histological tests that have already been proven in previous studies (13,14).
The method presented in this study makes the achievement of motor evoked potential in rates simpler. In literature, we did not find studies detailing existent techniques in a step-by-step fashion. Thus, we justify the conduction of this study, and also describe a feasible new evaluation method for neurological function in the increasing number of experiments performed in our environment.
CONCLUSIONS
We believe that the technique for capturing the motor evoked potential in rats as presented in this study is a safe method for analyzing the electrophysiological evolution of the spinal cord injury, and can be reproduced in a simple way, in any research laboratory, in addition to present quality and applicability standards similar to those seen in worldwide literature.
REFERENCES
Laboratory of Microsurgery, Orthopaedics and Traumatology Institute of the Hospital das Clínicas - Medical School, University of São Paulo (IOT - FMUSP).
- 1. Barros Filho TEP. Tratamento medicamentoso no traumatismo raquimedular. Rev Bras Ortop 2000; 35:143-6.
- 2. Schlag MG, Hopf R, Redl H. Serial recording of sensory, corticomotor, and brainstem-derived motor evoked potentials in rat. Somatosens Mot Res 2001; 18:106-16.
- 3. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12:1-21.
- 4. Bresnahan JC, Beattie MS, Noyes DH. A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Exp Neurol 1987;95:548-70.
- 5. Little JW, Harris RM, Solhberg RC. Locomotor recovery following subtotal spinal cord lesions in a rat model. Neurosci Lett 1988; 87:89-94.
- 6. Saruhashi Y, Young W. Effect of mianserin on locomotory function after thoracic spinal cord hemisection in rats. Exp Neurol 1994; 129:207-16.
- 7. Dimar JR, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine1999; 24:1623-33.
- 8. Fehlings MG, Nashmi R. Assessment of axonal dysfunction in an in vitro model of acute compressive injury to adult rat spinal cord axons. Brain Res1995; 677:291-9.
- 9. Fehlings MG, Tator CH, Linden RD, Piper R. Motor and somatosensory evoked potentials recorded from the rat. Eletroenceph Clin Neurophysiol 1988; 69:65-78.
- 10. Jou IM. Effects of core body temperature on changes in spinal somatosensory-evoked potential in acute spinal cord compression injury. An experimental study in the rat. Spine 2000; 25:1878-83.
- 11. Nascimento AC, Bartels M, Loew F. Acute changes in somatosensory evoked otentials following graded experimental spinal cord compression. Surg Neurol 1986; 25:62-6.
- 12. Zileli M, Schramm J. Motor versus somatosensory evoked potential changes fter acute experimental spinal cord injury in rats. Acta Neurochir 1991; 108:140-7.
- 13. Tebet MA, Barros Filho TEP, Machado IR et al. Efeito da metilprednisolona na esão medular em ratos: análise funcional e histológica. Acta Ortop Bras 2003; 11: 80-6.
- 14. Vialle E, Vialle LRG, Rasera E et al. Avaliação da recuperação motora em ratos submetidos a lesão medular experimental. Rev Bras Ortop 2002; 37:83-8.
Publication Dates
-
Publication in this collection
01 Nov 2005 -
Date of issue
2005
History
-
Accepted
05 May 2005 -
Received
16 July 2004