Abstracts
An experimental model of spinal cord injury at a precise and reproducible site is an extremely important tool for studying new therapies in spinal cord injuries. OBJECTIVES: To develop an experimental model of spinal cord injury in rats that is able to produce a complete injury (paraplegia) and placing a system enabling agents access close to injury site in order to test local therapeutic agents. METHODS: Fifteen Wistar rats were submitted to surgical transection of the spine, performed by using scissors at the level of T-13 to L-3 vertebral bodies, and, at the end of the procedure, to the insertion of a subdermal catheter intended to enable local therapeutic agents’ access to injury site. RESULTS: An experimental model of paraplegia was consistently developed by adding a supplementary catheter for local therapeutic agents’ access to injury site. CONCLUSION: An animal model of spinal cord injury and a system for local therapeutic agents’ access can be reproduced for the study of different modifiers of the regenerative response in a model of rats with spinal cord injury.
Spinal cord; Paraplegia; Rats
Um modelo experimental de lesão raquimedular com localização precisa e reproduzível é uma ferramenta extremamente importante para o estudo de novas terapias em lesões raquimedulares. OBJETIVOS: Desenvolver um modelo experimental de lesão raquimedular em ratos que produza lesão completa (paraplegia) com o posicionamento de um sistema que permita o acesso de agentes próximo ao local da lesão para testar agentes terapêuticos locais. MÉTODOS: Quinze ratos Wistar foram submetidos à transecção cirúrgica da medula espinhal, realizada com o uso de tesoura ao nível dos corpos vertebrais de T-13 a L-3 e, ao final do procedimento, à implantação de um cateter subcutâneo para o acesso de agentes terapêuticos locais ao local da lesão. RESULTADOS: Um modelo experimental de paraplegia foi consistentemente desenvolvido com a adição suplementar de um cateter para o acesso de agentes terapêuticos locais ao local da lesão. CONCLUSÃO: Um modelo animal de lesão raquimedular e um sistema para o acesso de agentes terapêuticos locais pode ser reproduzido para o estudo de diferentes modificadores da resposta regenerativa em um modelo de ratos com lesão raquimedular.
Medula espinhal; Paraplegia; Ratos
ORIGINAL ARTICLE
Experimental model of spinal cord injury in rats with a device for local therapeutic agents access
Jefferson Braga-SilvaI; Daniel GehlenII; Javier A.RomanIV; Denise Cantarelli MachadoIII; Jaderson Costa da CostaVI; Manuel FaúndezIV; Victor Vieira OrsiV; Rafael BragaII
IFull Professor of Hand Surgery, UNIFESP
IIMedicine student
IIIBiologist
IVOrthopaedic Doctor Traumatologist
VPlastic Surgeon
VINeurologist Doctor
Correspondences to Correspondences to: Jefferson BragaSilva MD, PhD Contact: Jefferson Braga–Silva Av. Ipiranga 6690 Centro Clínico PUCRS, Sala 216 CEP 90610-000, Porto Alegre, Brasil Telefone: 55 51 3320 50 40 Fax: 55 51 3320 50 39 Email: jeffmao@terra.com.br
SUMMARY
An experimental model of spinal cord injury at a precise and reproducible site is an extremely important tool for studying new therapies in spinal cord injuries.
OBJECTIVES: To develop an experimental model of spinal cord injury in rats that is able to produce a complete injury (paraplegia) and placing a system enabling agents access close to injury site in order to test local therapeutic agents.
METHODS: Fifteen Wistar rats were submitted to surgical transection of the spine, performed by using scissors at the level of T-13 to L-3 vertebral bodies, and, at the end of the procedure, to the insertion of a subdermal catheter intended to enable local therapeutic agents access to injury site.
RESULTS: An experimental model of paraplegia was consistently developed by adding a supplementary catheter for local therapeutic agents access to injury site.
CONCLUSION: An animal model of spinal cord injury and a system for local therapeutic agents access can be reproduced for the study of different modifiers of the regenerative response in a model of rats with spinal cord injury.
Keywords: Spinal cord; Paraplegia; Rats
INTRODUCTION
There are a vast number of experimental studies primarily targeting the creation of a nervous injury in animals that could allow researchers to test new techniques and reach to new findings that could ultimately lead to changes on the natural history of this condition.
An experimental model of spinal cord injury should have standardized and reproducible characteristics. Aiming to compare the effects of different therapeutic agents, an access port system near injury site is proposed here, using a catheter enabling an easy access to it in order to indroduce local agents that are able to modify cicatritial response after trauma.
In this study we developed a model presenting both characteristics: reproducible injury level and magnitude, as well as the appropriate placement of a system enabling the introduction of therapeutic agents at the injury site, after surgery.
MATERIALS AND METHODS
Preparation of the animals
In total, fifteen Wistar rats of both genders, weighting between 250g and 300 g, at the age of two months, have been used in this study. Ration and water were provided ad libitum during peroperative period.
Two hours before surgery, intraperitoneal Ciprofloxacin (FloxenTM 2mg/ml, Cellofarm, Carapina, Brazil) was administered to the animal (10ml/kg of body weight) for antibiotic prophylaxis purposes.
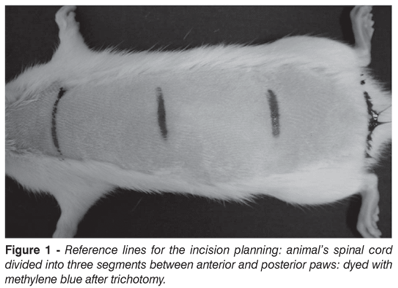
The animals were sedated with 0.3 ml for each 100g of body weight with a solution containing 20% Chlorpromazin (ClorpromazTM, 5 mg/ml ) and 80% Ketamin (Ketamin-S(+),50 mg/ml), via intramuscular injection. The animals were positioned at pronation, with stretched anterior and posterior legs, and trichotomized. After routine disinfection procedure, a longitudinal incision of approximately 2.5 cm was performed. Incision site plane is shown on Figure 1; we regarded as reference two horizontal lines drawed perpendicularly to the vertebral axis, one crossing at the upper limbs level and the other crossing the lower limbs level. Then, the space between these two lines was divided into three equal extension portions: an upper portion, a medial portion and a lower portion. The incision was performed at 1 cm below the union site between upper and medial segments. By using this technique, we addressed the animals spinal cord between T-13 and L3, as shown on X-ray image (Figure 2).
Surgical Procedure
After skin incision, dissection by planes was performed on the spinous process, dettaching the spinotrapezium muscle from bone (Fig. 3), and the vertebral layer was resected, exposing distal spinal cord meninges. Then, a cross-sectional transection of the spinal cord was performed with scissors. This procedure frequently caused meninges to bleed, being handled with compression of the affected portion with wet surgical gauze.
The subcutaneous implant of a 22G radiopaque catheter (JelcoTM Johnson&Johnson, Lenneke Marelaan, Belgium) covered with an intermitent latex-free membrane (IN 400, B|Braun S.A, São Gonçalo, Brazil) was performed, connecting the exposed cord and the subcutaneous area on lumbar region, using paravertebral muscle tissue. The distal portion of the catheter was kept between paravertebral muscle tissue and the proximal portion was covered with an intermittent latex-free membrane and kept beneath the skin, remaining fully covered by skin. At last, muscle and skin layers were closed by planes with suture (Figures 3 and 4).
All surgical procedure steps have been performed according to ethical procedures for the use of animals in laboratory experiments.
RESULTS
All animals lost mobility below injury level and became paraplegic. Then, after surgery and post-anesthetic recovery, a full loss of spontaneous mobility was seen both in the lower paws and in the tail, as well as loss of nervous response to pain, carefully tested by pinching. After a long-term follow-up (more than 3 months), no spontaneous motor or sensitive recovery was observed. There was no motor or sensitive loss on the upper limbs or head, allowing the animal to move and feed. No serious respiratory, digestive or urinary dysfunction was found, although we havent specifically tested it.
DISCUSSION
Spinal cord injuries represent one of the worst aggressions the human body can suffer, resulting in physical, psychological and emotional disability. One of the major causes of physical disability in human beings is the spinal cord injury, which account for approximately 10,000 new acute palsy cases every year in the USA, 1,000 of these in children(1).
Several studies have been developed in an attempt to understand the mechanisms involved both in the injury itself and the repair following a spinal cord injury. In the last decade, new biotechnological concepts emerged, such as bioengineering, which brought new perspectives with the use of biological agents (e.g., stem cells) to promote healing on different tissues. The therapeutic potential of those new technologies has already been recognized; however, their true role in medical practice still needs to be established. Therefore, for the purpose of studying new strategies for nervous repair, suitable experimental models are largely desirable.
Throughout the evolution of the studies addressing spinal cord injury and repair, several experiments in animals were developed. The first attempts to obtain paraplegic animals or other spinal cord injury models were achieved by sequentially hitting animals back or by dropping the animals from a certain height.
Allen, in 1911, was the pioneer in terms of experimental studies intended to create a standardized animal model of spinal cord injury. His method employed some loads thrown though a tube placed directly on animals previously exposed spinal cord.
In 1936, Amako and Freeman still used the contusion model constituted of load throw devices in their researches. Eidelberg, in 1976, created a spinal cord injury model in rats caused by direct epidural compression. Tarlov, in 1953, published a model in which a dog had its spinal cord injured by an inflated ballon inside spinal channel. New techniques were developed and improved, such as, for example, spinal cord stabilization and precise distribution of strengths involved on impact, the use of mechanisms able to measure the strength to which an animals spinal cord is exposed(2), as well as the invention of pneumatic impact mechanisms based on the recommendations described by Allen in the early twentieth century(3-5).
It is important to emphasize that all models described in literature are standardized both in terms of mechanism and of trauma strength magnitude, being equal to all animals in a study; however, these do not assure contusions of similar magnitude in all subjects in a single study.
In an animal model of paraplegia, we seek to accurately and methodologically reproduce an injury, so that it could be used in different studies enabling a comparison between different analyses and a correlation with characteristics present in human beings with similar nervous damage.
Among the uncountable models and ways to create a paraplegic animal, some are distinguished by their successful attempts in creating analogous clinical features to the ones found in human paraplegia. Nevertheless, in the present study, similarities with real trauma were not immediately addressed. This study aimed to systematically lead the animal to paraplegia, and to develop a device that could provide different therapeutical agents access to the injury site.
CONCLUSION
Once tests and correlations with other animal models of spinal cord injury were performed, we concluded that the present technique enables to know which structures of the rat will be influenced by spinal cord transection.
The technique of making an incision on a pre-determined site promotes, with consistent safety, paraplegia in these animals. After a number of tests, we concluded that the absence of a precise location could lead us to uncertain results, such as anterior paws palsy, quadriplegia, shock or death. The antibiotic therapy used during preoperative period intended to prevent a potential bacterial translocation from the bowel, as suggested by Liu et al(6), which could contribute to the development of endotoxemia.
The most relevant factor in the present study is catheter implantation, enabling the rapid and safe introduction of any preparation directly on injury site, at the desired amount, as many times required, without the need to submit the animal to further incisions or surgeries.
REFERENCES
Received in: 04/24/06, approved in: 05/10/07
Hand Surgery and Reconstructive Microsurgery Service, Pontíficia Universidade Católica do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
Biomedics Research Institute, Pontíficia Universidade Católica do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
- 1. Carlson GD, Gorden C. Current developments in spinal cord injury research. Spine J 2002; 2(2):116-28.
- 2. Yeo SJ, Hwang SN, Park SW, et al. Development of a rat model of graded contusive spinal cord injury using a pneumatic impact device J Korean Med Sci 2004: 19: 574-80.
- 3. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection Exp Neurol 1996; 139(2):244-56.
- 4. Brown KM, Wolfe BB, Wrathall JR. Rapid functional recovery after spinal cord injury in young rats J Neurotrauma 2005; 22(5):559-74.
- 5. Kaufman S. Animal Models of Spinal Cord Injury, Perspective on Medical Research, 1990,Vol 2. Avalaible from: http://www.curedisease.com/Perspectives/vol_2_1990/Contents.html
- 6. Liu J, An H, Jiang D, et al. Study of bacterial translocation from gut after paraplegia caused by spinal cord injury in rats. Spine. 2004; 15;29(2):164-9.
Correspondences to:
Publication Dates
-
Publication in this collection
04 Sept 2007 -
Date of issue
2007
History
-
Received
24 Apr 2006 -
Accepted
10 May 2007