Abstracts
Carbon nanotubes (CNTs) are one of the most promising materials in nanotechnology. The various synthesis, purification and postprocessing methods produce CNTs with diverse physical characteristics, appliable in many fields. Their extensive projected use makes it important to understand their potential harmful effects. Besides showing a notable range of results of some toxicology studies, this review concluded that: a) there are different types of CNTs; thus, they cannot be considered a uniform group of substances; and b) in environmental compartments, CNTs can be bioavailable to organisms. Their properties suggest a possible accumulation along the food chain and high persistence. In organisms, CNT absorption, distribution, metabolism, excretion and toxicity depend on the inherent physical and chemical characteristics (e.g., functionalization, coating, length and agglomeration state), influenced by external environmental conditions during CNT production, use, and disposal. Thus, characterized exposure scenarios could be useful in toxicology studies. However, upon reaching the lungs in enough quantity, CNTs produce a toxic response (time and dose-dependent). The risks to human health and environment should be identified for a successful introduction of CNTs in future applications.
Carbon nanotubes; Cytotoxicology; Environment; Human health; Nanotechnology
Os nanotubos de carbono(CNT)são um dos materiais mais promissores da nanotecnologia. Os métodos de síntese, purificação e pós-processamento produzem CNT com diversas características físicas e uso em várias áreas. A projeção de uso abrangente do CNT urge a compreensão de seus possíveis efeitos nocivos. Essa revisão mostra um leque de resultados de estudos toxicológicos e concluiu que: a) há diferentes tipos de CNT; portanto, não pode ser considerado um grupo uniforme de substâncias; e b) em compartimentos ambientais,o CNT pode ser biodisponível aos organismos. Suas propriedades sugerem possível acúmulo na cadeia alimentar e alta persistência. Em organismos, sua absorção, distribuição, metabolismo, excreção e toxicidade do dependem de características físicas e químicas inerentes (e.g., funcionalização, revestimento, comprimento e estado de aglomeração), influenciadas por condições ambientais externas durante a produção, uso e eliminação de CNT. Portanto, os cenários de exposição caracterizados podem ser úteis em estudos toxicológicos. Contudo, quando chega aos pulmões em quantidade suficiente, o CNT produz uma resposta tóxica (tempo e dose dependente). Os riscos à saúde humana e meio ambiente devem ser identificados para que o CNT possa ser usado com sucesso em futuras aplicações.
Nanotubos de carbono; Citotoxicologia; Meio ambiente; Saúde humana; Nanotecnologia
REVIEW REVISÃO
Reviewing the environmental and human health knowledge base of carbon nanotubes
Revisão sobre a base de conhecimento de saúde ambiental e humana dos nanotubos de carbono
Aasgeir HellandI; Peter WickII; Andreas KoehlerI; Kaspar SchmidIII; Claudia SomI
ITechnology and Society Lab, EMPA (Swiss Federal Laboratories for Materials Testing and Research), St. Gallen, Switzerland. Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland. asgeir.helland@empa.ch
IILaboratory for Biocompatible Materials, EMPA, St. Gallen, Switzerland
IIIInstitute for Occupational Health Sciences, Lausanne, Switzerland
ABSTRACT
Carbon nanotubes (CNTs) are one of the most promising materials in nanotechnology. The various synthesis, purification and postprocessing methods produce CNTs with diverse physical characteristics, appliable in many fields. Their extensive projected use makes it important to understand their potential harmful effects. Besides showing a notable range of results of some toxicology studies, this review concluded that: a) there are different types of CNTs; thus, they cannot be considered a uniform group of substances; and b) in environmental compartments, CNTs can be bioavailable to organisms. Their properties suggest a possible accumulation along the food chain and high persistence. In organisms, CNT absorption, distribution, metabolism, excretion and toxicity depend on the inherent physical and chemical characteristics (e.g., functionalization, coating, length and agglomeration state), influenced by external environmental conditions during CNT production, use, and disposal. Thus, characterized exposure scenarios could be useful in toxicology studies. However, upon reaching the lungs in enough quantity, CNTs produce a toxic response (time and dose-dependent). The risks to human health and environment should be identified for a successful introduction of CNTs in future applications.
Key words: Carbon nanotubes, Cytotoxicology, Environment, Human health, Nanotechnology
RESUMO
Os nanotubos de carbono(CNT)são um dos materiais mais promissores da nanotecnologia. Os métodos de síntese, purificação e pós-processamento produzem CNT com diversas características físicas e uso em várias áreas. A projeção de uso abrangente do CNT urge a compreensão de seus possíveis efeitos nocivos. Essa revisão mostra um leque de resultados de estudos toxicológicos e concluiu que: a) há diferentes tipos de CNT; portanto, não pode ser considerado um grupo uniforme de substâncias; e b) em compartimentos ambientais,o CNT pode ser biodisponível aos organismos. Suas propriedades sugerem possível acúmulo na cadeia alimentar e alta persistência. Em organismos, sua absorção, distribuição, metabolismo, excreção e toxicidade do dependem de características físicas e químicas inerentes (e.g., funcionalização, revestimento, comprimento e estado de aglomeração), influenciadas por condições ambientais externas durante a produção, uso e eliminação de CNT. Portanto, os cenários de exposição caracterizados podem ser úteis em estudos toxicológicos. Contudo, quando chega aos pulmões em quantidade suficiente, o CNT produz uma resposta tóxica (tempo e dose dependente). Os riscos à saúde humana e meio ambiente devem ser identificados para que o CNT possa ser usado com sucesso em futuras aplicações.
Key words: Nanotubos de carbono, Citotoxicologia, Meio ambiente, Saúde humana, Nanotecnologia
The worldwide funding devoted to nanotechnology research and development by governments, industry, and venture capitalists was estimated to be around US$9.6 billion in 20051. A large portion of this spending is still being allocated tothe development of nanoparticulate materials because of their many novel physical and chemical properties raising high expectations for a variety of applications. One of these new materials is carbon nanotubes (CNTs), which have commercial expectations in different manufacturing sectors.
However, epidemiologic studies of air pollution suggest that particulate matter has a strong association with cardiopulmonary diseases2. Research has shown that nanoparticles may enter the human body more easily and be more biologically active because of their larger surface area per mass unit compared with that of larger particles3. The prospective widespread use of engineered nanoparticles in consumer products may increase environmental, occupational, and public exposures dramatically. Consequently, different stakeholders have raised serious concerns regarding health effects of engineered nanoparticles4. Recent review articles on the toxicitypotential of nanoparticles5,3 conclude that the toxicity of nanoparticles depends on specific physiochemical and environmental factors. Thus, the toxic potential of each type of nanoparticle has to be evaluated individually.
Here we review the currently available literature on the potential risks of CNTs to human health and the environment. We also investigated the life cycle of CNTs, as release into different environmental compartments may occur at the production stages as well as at the product's use and disposal stages, which may directly or indirectly lead to human exposure.However, the published literature revealed many unanswered questions. Therefore, we also systematically interviewed seven leading world-class scientists and integrated their contemporary knowledge into this review (see Supplemental Material; http://www.ehponline.org/docs/2007/9652/suppl.pdf). This assisted us in identifying questions and developing recommendations. The scientists interviewed were key authors or project leaders who have investigated and reported the potential impacts of CNTs on human health or environment. Through this combined approach we are able to present an updated and contemporary knowledge base for scientific discussion.
In this review, we use the term "carbon nanotubes" when addressing the generalaspects of the material, which includes singlewalled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs). Here "multi" is defined as two or more walls. The terms "SWCNTs" and "MWCNTs" are used in a specified manner.
Exposure to carbon nanotube material
Exposure in occupational settings
Procedures for the handling of CNTs can result in aerosol release of these materials into the surroundings6. MWCNT aerosols generally have diameters between 20 nm to > 200 nm, lengths from 1,000 nm to > 106 nm, and different shapes (straight, partly rigid, bent, curled, and partly flexible) that may appear single or in clumps or ropes7.
Only one published study has investigated the potential for SWCNTs to become airborne. A laboratory study by Maynard et al.6 investigating the physical nature of the aerosol formed during mechanical agitation was complemented by a field study of SWCNT release during handling of unrefined SWCNTs. The authors found that sufficient agitation of unrefined SWCNT material can release fine particles into the air, but the concentrationsgenerated while handling the material in the field were very low (< 53 µg/m3). The laboratory study also revealed that different SWCNT production methods produced different types of aerosols. The laser ablation process generated a more compact aerosol that was difficult to break down into smaller particles, whereas the HiPCO (high-pressure carbon monoxide) process generated a more extended material that was easier to break down into smaller particles and appeared to lead to higher airborne concentrations.
Maynard et al.6 also found glove deposits of SWCNTs during handling that were estimated at between 0.2 and 6 mg per hand and thus concluded that large SWCNT containing clumps had the propensity to become airborne and could remain so for long periods. This may cause dermal exposure and health risks even in less well-protected areas. Maynard et al.6 noted that production volume was very small (research facility) and that workers took great care to reduce product loss during handling of the material. However, CNTs contain catalyst metals such as nickel, which is associated with increased risks of cancerin the nose region8.
Cleaning operations can also lead to emissions. The cleaning of the production chambers is performed usually using solvents or water, tissues, brushes, and sponges that are discarded after cleaning9. This waste carries CNTs into the waste stream, thereby possibly becoming a source of release into the environment.
Exposure through environmental media
Exposure through environmental media is highly relevant for several reasons: a) The widespread applications envisioned for CNTs may lead to substantial production volumes, and consequently to increased emissions into the environmental compartments air, groundwater, and soil. b) The physical and chemical processes in the environmental compartments may alter the properties of CNTs, for example, abiotic factors such as ultraviolet light may alter the coatings of CNTs as observed with fullerenes10 and quantum dots11. Consequently, this may also change the behavior of CNTs in the environment and thus influence their environmental fate and impact. c) CNTs are possibly one of the least biodegradable man-made materials ever devised12. They are totally insoluble in water in pristine form12 and are lipophilic by nature13. It is generally known that biopersistent and lipophilic chemicals may accumulate along the food chain; therefore, such a scenario should also be evaluated with CNTs. In aqueous environments, SWCNTs clump together to form aggregates in the micrometer range; these aggregates do not change in size distribution with increasing salinity or temperature14. However, the aggregation differs with pH changes in water14 and postsynthesis treatment of the SWCNTs with, for example, acid or surfactants15. Both these studies found that pristine nanotubes formed stable aggregates,whereas acid-treated nanotube suspension showed greater dispersion variability over time, yielding looser structures at large-length scales and more compact structures at small-length scales. The addition of a surfactant to CNTs resulted in a hydrophilic interface at the tip of the nanotubes that significantly enhanced nanotube dispersion. In laboratory assessments designed to assess the potential migration innatural porous media, SWCNTs have been shown to have mobility and deposition behaviors different from those of other nanoparticles16, 17. SWCNTs functionalized to facilitate dispersion in water displayed the highest mobility together with water-soluble fullerol, whereas colloidal C60, anatase titanium dioxide (TiO2), and ferroxane were among the least mobile of the nanomaterials evaluated.
The large surface area of CNTs may cause other molecules to adhere and potentially pick up pollutants and transport these throughout the environment18. Several studies have investigated different carbon nanomaterials as superior sorbents of organic pollutants, metals, fluoride, and radionuclide 243-americium [243Am(III)]19-21. Yang et al.21 found a high adsorption capacity of polycyclic aromatic hydrocarbons (PAHs) with different types of CNTs (MW and SW). This finding indicates a potential effect on the fate of PAHs upon their release into the environment. The adsorption capacity was found with the order SWCNTs > MWCNTs > C60 and seemed to be related tothe surface area, micropore volume, and the volume ratios of mesopore to micropore.
Oberdörster et al.22, in a preliminary study, investigated the ingestion of SWCNTs by the suspension-feeding worm Caenorhabditis elegans. SWCNTs moved through the digestive tract and were not absorbed by the animal. However, even if SWCNTs did stay in the digestive tract, these materials could move up the food chain, as these worms and other organisms are consumed by benthivores. SWCNTs have also been shown to be bioavailable to aquatic organisms, as both water-solubilized (wrapped with a synthetic peptide) and unsolubilized SWCNTs were detected in the fecal material collected from the digestive tract in the exposed fish23. For the water-solubilized SWCNT-exposed fish, clumps of SWCNTs were also found on the gill, but similar clumps were not visible in unsolubilized SWCNT-exposed fish. However, the fish mistook the unsolubilized SWCNTs (floating on top of the water) for food and ingested them22. Furthermore, because pristine CNTs are lipophilic, there is concern that they might be taken up by microbial communities and roots22 and, consequently, accumulate in plant tissues.
Carbonaceous nanoparticles, including MWCNTs, can also be formed by natural processes24 and anthropogenic combustion processes25. Although these MWCNTs can be prime suspects in the pathogenesis of cardiopulmonary diseases induced by fine particulate matter, there are physical differences between combustion-generated and manufactured MWCNTs26. These MWCNT structures may therefore be less important when the impacts of engineered CNTs are being assessed, as the studies to date suggest that when the properties of CNTs are altered by engineering, changes in the environmental fate of and human exposure to CNTs occur through the different environmental media.
Human health
In vivo studies
Pulmonary toxicity
The firstin vivo study of fullerene soot containing CNTs did not find any inflammation in the respiratory tract of guinea pigs or change of pulmonary function 4 weeks after exposure27, but it is possible that the method applied in this study (not examining lung pathology) did not reflect the actual toxicity of the material. The followup study with analysis 90 days postexposure of six different types of MWCNTs administered in individual doses (12.5 mg) to guinea pigs found small differences between the types of MWCNTs28,29. However, for all types of MWCNTs there were multifocal granulomas observed around the materials, inflammatory reactions of terminal and respiratory bronchioles, and in some animals, mild fibrosis in alveolar septa.
SWCNT soot generated by the laser ablation method and intratracheally instilled in rats produced transient inflammation, cell injury effects, and a subsequent non-dosedependent series of multifocal granulomas30. In comparison, equal doses of quartz produced sustained pulmonary inflammation, cytotoxicity, and fibrosis in a dose-dependent fashion. The authors questioned whether the failed observation of a dose-dependent relationship between SWCNT exposure and formation of the granulomas could be the result of the method of instillation and suggested that it should be clarifiedin an inhalation toxicity study.
In an intratracheal instillation study of mice, three types of SWCNTs were investigated12two types made bythe HiPCO method and one by the electric arc method. The results from all three types showed that regardless of the amount of metal impurities, dose-dependent lung lesions were characterized chiefly by interstitial granulomas. The study also showed that both SWCNTs and ultrafine carbon black were taken up by alveolar macrophages, but that the effects of these materials were different. Macrophages containing carbon black were homogeneously distributed over the alveolar space, but macrophages containing SWCNTs clustered to form granulomas in centrilobular locations. In comparison, quartz induced mild to moderate pulmonary inflammation, which was considered less severe than that induced with SWCNTs.
The consequent agglomeration of CNTs into nanoropes31 makes it difficult to manipulate and administer these particles to experimental animals without forming nonrespirable particles that can lead to mechanical blockage of the airways, as observed in other studies28,30. Therefore, Muller et al.32 compared the pulmonary toxicity of ground and unground MWCNTs in rats, using asbestos (Rhodesian chrysotile) and carbon black as references. The length of the individual MWCNT was reduced from 5.9 to 0.7 µm as aresult of the grinding procedure. The distribution in the lungs after intratracheal instillation was different for the two types. Agglomerates of intact MWCNTs remained in the largest airways, whereas ground MWCNTs were much better dispersed on the lung tissue surface. Regarding toxicity, the study found that after 60 days there were indications of a higher degree of pulmonary inflammation with ground MWCNTs than that found with intact MWCNT-treated animals. The induced effects were dose dependent [bronchoalveolar lavage fluid (BALF) lactate dehydrogenase (LDH) activity and protein content]. Histologic and biochemical analyses demonstrated a fibrotic reaction (granulomas) in the bronchi with unground MWCNTs and in the alveolar space or interstitial tissue with ground MWCNTs. In summary, the findings of Muller et al.32 indicate that asbestos-ground MWCNTs and unground MWCNTs induce at least partially similar effects [inflammation, fibrotic reaction, and increased tumor necrosis factor (TNF)-aproduction], whereas carbon black showed atransient inflammation with elevated TNF-a. The authors therefore concluded that these data point to a specific toxicity related to the unique properties of CNTs.
In a pharyngeal aspiration study of mice by Shvedova et al.33, the effects of HiPCOproduced SWCNTs purified to a carbon content > 99% were investigated. The SWCNT aggregate depositions were correlated with granulomatous inflammation, whereas interstitial fibrosis with alveolar wall thickening was observed to be greater at 60 days than at 28 days postexposure in lung regions distant from the SWCNT aggregates. SWCNTs were compared with equal doses of nanoparticulated carbon black and fine crystalline silica, which did not induce granulomas and alveolar wall thickening and caused significantly less inflammation. Furthermore, the authors investigated whether exposure caused damage to pulmonary cells. This was confirmed by an increased number of alveolar type II cells (type II cells replicate after alveolar type I cell death). Exposure to SWCNTs also resulted in accumulation of an oxidative stress biomarker (4-hydroxynonenal) 1 day after exposure and also in a time- and dose-dependent depletion of a major antioxidant, glutathione, which was most severe 1 day postexposure. The rapid and dose-dependent fibrogenic response in regions of the lungs distant from SWCNT aggregates and in the absence of persistent inflammation was a unique finding in this study.
Initially, Shvedova et al.33 associated the interstitial fibrosis with deposition of dispersed SWCNT structures and pointed out that the mechanism for this response differs from the classic fibrogenic particle response in that it is not driven by chronic inflammation and chronic activation of alveolar macrophages. Additionally, the authors found that if pathogenic bacteria were inhaled by mice in combination with SWCNTs, bacterial clearance from the lungs was significantly slower. This indicates that SWCNTs, in addition to their primary effects, may also diminish general resistance to pathogenic attacks.
Dermal toxicity
There is only one published study in vivoon the dermal toxicity of fullerene soot containing CNTs34. Forty volunteers were subjected to a patch test, and four albino rabbits were subjected to an eye test. The study found no evidence of the induction of any response; thus, the authors concluded that soot containing CNTs is not associated with any risks34. However, information is insufficient on CNT material characterization and the study design for adequate validation of these results.
Translocation
There are some contradictory findings on the kinetics of water-soluble functionalized and radioactively labeled CNTs in the body. Wang et al.35 showed that when hydroxylated SWCNTs with radioactive iodine-125 atoms were injected into mice, the SWCNTs behaved like small molecules, passed easily through a number of compartments, accumulated especially in bone, and were distributed throughout the whole mouse body except the brain. Eighty percent of the total dosing of the SWCNTs was excreted by the feces and urine after 11 days. In contrast, Singh et al.36 found no accumulation of chelated diethylentriaminepentaacetic and indium-111-labeled SWCNTs or MWCNTs in mice after 24 hr. The biological behavior of functionalized (f)-CNTs is therefore comparable to small molecules. They are removed from the blood through the renal excretion route. No toxic side effects or mortalities were observed, and the excreted f-SWCNTs and f-MWCNTs were intact36. Both studies are valid only for functionalized tubes and cannot be interpolated for non-functionalized tubes because of their hydrophobic nature. The difference in the distribution in bone is probably because the functionalization of the tubes was not the same, which influenced behavior in the organism.
Histopathologic analysis ex vivo
Sato et al.37 investigated the influence of different MWCNT lengths on the cytotoxicity of MWCNTs in the human THP-1 leukemia cell line in vitroand in subcutaneous tissue of rat in vivo. In a long-term assay in vivo, the authors observed 4 weeks after surgery that an increased inflammatory response was established only by the 825-nm MWCNTs, as indicated by the formation of granulation tissue.Most of the 220-nm MWCNTs were observed in phagocytes, with many of these recognizable in lysosomes. Conversely, the 825-nm MWCNTs were also observed in the intercellular space.
In vitro studies
The methodology of how the toxicity of CNTs is evaluated depends strongly on how CNTs are administered to the cells (i.e., homogenously dispersed or not, with or without surfactant, type and concentration of the surfactant). Furthermore, CNTs contain contaminants that may be bioactive per se. Often the exact methodology and CNT characteristics are not described, which makes it difficult to compare available data.
Pulmonary cytotoxicity
In an in vivostudy performed with nanoparticles such as TiO2, Geiser et al.38 showed that these particles could be taken up by the lung, passed through the air-blood barrier, and translocated into the bloodstream. This pathway cannot be excluded for CNTs and may be supported at the single-cell level. SWCNTs suspended in Pluronic F108 (a nonionic surfactant) were incorporated by peritoneal macrophage-like cells using near-infrared fluorescence microscopy. Cherukuri et al.39 found that 1.5 µg of 7.3 µg/mL SWCNTs were taken up within 24 hr by macrophage cell cultures grown in 24-well culture dishes. The fastest reaction evoked by SWCNTs in macrophages was an oxidative stress that occurred within hours40. The improved properties of nanomaterials also resulted in a catalytic activity. This catalytic activity may contribute to a new aggressive form of longterm toxicity. Oxidative stress is an imbalance between the production of reactive oxidizing species (ROS) and their degradation by antioxidants. The intracellular equilibrium may be disturbed by the presence and/or uptake of nanomaterials. The concentration of ROS may be increased by the particle itself or by the disturbance of the ROS degradation pathway. Both cause an additional production of ROS, which interacts uncontrollably with the cell membrane, DNA, and/or other cell compounds, severely damaging these cell compounds. According to Muller et al.32 and as described in previous sections, the adverse effects of MWCNTs depend on the length of the material used in vivo. Similar to their in vivostudy, the authors found that long untreated tubes using Triton X-100 as the vehicle evoked an LDH release of nearly 20% at a concentration of 100 µg MWCNTs/one 24-well of a 24-well plate, whereas the short ground MWCNTs induced a dose-dependent LDH release up to 35% greater than the corresponding vehicle (Triton X-100) treatment. This suggests that also in vitrothe short MWCNTs are more toxic than the long ones. Interestingly, TNF-a was induced in vivo (measured in BALF) as well as in vitro(measured in preactivated peritoneal macrophages),indicating that TNF-a could be a reliable marker for future in vitrostudies.
A comparative in vitrocytotoxicity study of several manufactured nanoparticles and nanotubes revealed that aggregated SWCNTs/ ropes, MWCNT raw material, and MWCNTaggregated tubes suspended in 5 mg/mL dimethylsulfoxide (DMSO) affected cell viability in murine lung alveolar macrophages at nearly similar concentrations41. Compared with different oxide particles of differing quality such as aluminum oxide, zirconium oxide, or ferric oxide, the SWCNTs and MWCNTs expressed comparable median effective concentration (EC50) values (micrograms per milligrams), which is analogous to the same values obtained with asbestos used in the same studies as reference material41,42. However, it is speculative to generalize these results in terms of health risks to humans, as the presented comparison study is based only on short-term effects during a 48-hr period. The physiologic relevance of these results remains to be determined. In addition to the size, shape, and novel physical and chemical properties, the degree and type of agglomeration are important factors in the cytotoxicologic assessment of CNTs. Well-dispersed SWCNTs were less cytotoxic than micrometer-sized agglomerates fof SWCNTs43. To determine which carbonaceous nanomaterials have the most severe effect, comparison studies were conducted with the same biological model system. Jia et al.20 compared SWCNTs, MWCNTs, and C60 fullerenes suspended in cell culture medium and found that the cytotoxicity is apparently based on SWCNTs > MWCNT > quartz > C60. Fiorito et al.19 in a second study on murine and humanmacrophages claimed that graphite had the most severe effect, followed by SWCNTs and C60 fullerenes.
Dermal cytotoxicity
To emphasize the dermal toxicity of CNTs, Monteiro-Riviereet al.44 exposed human epidermal keratinocytes to MWCNTs that were produced without further purification processes. These particles were taken up by the keratinocytes in vacuoles where MWCNTs retained their structure, as demonstrated by transmission electron microscopy analysis. The cell viability parameters were reduced by 400 µg/mL MWCNTs to as much as 20% after a 24-hr exposure. The expression of interleukin (IL)-8 was increased up to 6 times (400 µg MWCNTs/mL) in a dose-dependent manner after that period compared with the corresponding control cell cultures44. A detailed characterization of the molecular mechanism evoked by exposure of human skin fibroblast cells to 0.6 and 0.06 µg MWCNTs/mL was evaluated by gene microarray analysis. This analysis showed that MWCNTs induced cell cycle arrest and increased apoptosis and necrosis. Of the genes evaluated, 216 genes changed their expressionlevels after treatment with MWCNTs at 0.6 mg/mL. The most significant gene categories were Golgi vesicle transport, protein metabolism, secretory pathway, fatty acid biosynthesis G1/S transition of mitotic cell cycle, and cell homeostasis. The fact that these cellular processes were affected by the presence of MWCNTs indicates that this stress has a remarkable influence on cell performance45. Shvedova et al.46 exposed human keratinocytes to HiPCO-produced SWCNT material containing 30% by weight of iron. One of the first reactions on SWCNT treatment, which occurred 18 hr after incubation, was an increased oxidative stress, as indicated by the presence of free radicals. The decrease ofthe total antioxidant reserve and the reduction of vitamin E as well as the increase of the lipid peroxidation products compared with the control cell culture support strongly the presence and the damage of the oxidative stress within the cell cultures. Shvedova et al.46 claimed that this oxidative stress was the reason for the reduced cell viability. In contrast to the work presented previously in which CNTs were suspended by sonification in the presence of a solvent or cell culture medium, Sayes et al.47 functionalized purified SWCNTs before dispersing themin the cell culture medium. They produced SWCNTphenylsulfate, SWCNTphenylsodium sulfate (SO3H), and six mixed SWCNTcarbonsulfate and SWCNTphenylSO3H samples with ratios of 18, 41, and 80, respectively. According to the authors only water-dispersible SWCNTs were of considerable interest for biological applications. The suspensions obtained were used to treat human dermal fibroblast cultures. The pristine unmodified SWCNTs evoked a rate of 80%cell death at a concentration of 20 µg/mL, whereas functionalized SWCNTphenylSO3H induced < 5% cell death at a concentrationof 2,000 µg/mL. Cytotoxicity was alsostrongly dependent on the type of sidewall groups: SWCNTphenylSO3H was less toxic than the SWCNT withphenyl-(COOH)2 group. The general conclusion reached by Sayes et al.47 was that an inverse relationship exists between the toxic potential and the degree of side wall functionalization. This is an important issue for further applications of CNTs in nanomedicine. The intensive skin cellCNT interaction studies emphasize that penetration of CNTs is still not understood.
In vitro studies with neurons
Polym-aminobenzene sulfonic acid and ethylenediamine functionalized MWCNTs (MWCNTPABS; MWCNTEN) coatings were used to coat glass cover slips as substrates for neuronal growth. To achieve this coating, the tubes were suspended in water and glass cover slips were coated with the suspension. After the water was evaporated, hippocampal neurons were seeded on the cover slips. After 3 days of incubation, the number of branches per neuron, total neurite length, and the number of neurites per neuron of the cultivated neurons were increased on functionalized MWCNTs but not on pristine MWCNTs. However, it must be noted that the performance of the neurons grown on polyethyleneimine (positive control) was better than that of neurons grown on MWCNT-coated surfaces. Additionally, Hu et al.48 claimed that the surface charge of the modified MWCNT can be used to control neurite outgrowth. An increase in neuronal outgrowth and branching was achieved by the functionalization of SWCNTs with polyethyleneimine (SWCNTPEI) compared with the modified MWCNTs49. The authors described the morphology of these nerve cells in detail, but the functional proof, namely, in how far these cells exhibited spontaneous action potential and/or were able to bestimulated, is still lacking. All these studies reflected a positive effect of SWCNTs on neurons, but compared with other cytotoxicologic assessments, these tubes were immobilized on glass. A similar study was conducted by Liopo et al.50, who assessed growth and attachment of the neuroblastoma glioma NG108, a model neuronal cell, on unmodified SWCNT substrates and/or substrates from SWCNTs modified with 4-benzoic acid or 4-tertbutylphenyl functional groups using a simple functionalization method. Liopo et al.50 found that SWCNT films support cell growth but at a reduced level compared with that of tissue culturetreated polystyrene. Therefore, all studies mentioned here investigated in principle the degree to which nanostructured surfaces with chemical characteristics of CNTs affect the outgrowth of nerve cells and not the toxicity of suspended CNTs.
In vitro cytotoxicity studies in different cell types
For human embryo kidney (HEK293) cells it was reported that in 0.5% DMSO, suspended SWCNTs are able to inhibit cell proliferation and to decrease cell adhesive ability in a dose- and time-dependent manner51. Additionally, these HEK293 cells exhibit an active response to SWCNTs such as the secretion of a 20- to 30-kDa protein with unknown function. After SWCNT treatment, further observations were made that were analogous to those in the reports in the previous sections. These included observations on cell cycle arrest in the G1 phase, up- or down-regulation of cell cycleassociated genes, and formation of apoptosis/necrosis. The degree to which the presence of the DMSO affects the cell surface and cell performance is unclear in the present study.
As described previously, the surface chemistry strongly affects the toxic potential of CNTs. For example, the cell viability of human T lymphocytes was decreased to 80% by exposure to 400 µg/mL of oxidized MWCNTs (oxidized by refluxing the MWCNTs in concentrated nitric acid). The reduction in cell viability was explained by the increase of apoptotic cells after the treatment52. In comparison pristine MWCNTs reduced viability by up to 40% and carbon black by only 15% at the same concentration. Interestingly, the oxidized MWCNTs appeared to be shorter and straighter. Several studies were conducted in which SWCNTs were modified by adding various biological molecules such as phospholipids conjugated with polyethylene glycol (PEG) folic acid, PEGNH2, PEGSSDNA, etc.53-55. In all the studies the water dispersibility increased and the tubes were incorporated in HL60 and HeLa cells. Depending on the modification of the SWCNTs, these tubes could act as multifunctional biological transporters, for example, DNA or siRNA56-58. Unfortunately, no quantitative measurement was performed to allow a statement and comparison of these modified SWCNTs regarding the uptake rate and induced toxicity.
Carbon nanotubes have also been proposed by Price et al.59 as a possible new orthopedic/ dental implant surface material because of their unique mechanical, electrical, and cytocompatibility properties. Cell viability and number of human osteoblast CRL-11372 were determined after 3, 6, 11, and 24 hr of incubation by ethidium homodimer and calcein AM staining. The authors used conventional fibers (diameter > 100 nm) and nanoscaled fibers (diameter < 100 nm). The fibers agglomerated within 1 week in cell culture media to ropes of about 340 nm in diameter in the case of conventional fibers and about 670 nm in the case of nanoscaled fibers. The nanoscaled carbon fibers appeared to influence osteoblast viability less than their conventional dimensioned counterparts. The suspended carbon fiber agglomerates were taken up by the osteoblasts and were incorporated in lysosomal vacuoles59. This future application of carbon nanotubes as implant material is in itself a promising issue, but one of the key questions is do carbon nanotubes influence the differentiation of osteoblast progenitor cells and to what degree is the formation and activation of osteoblasts affected? In conclusion, CNTs were taken up by different cell types and evoked diverse effects in the cells. A first and fast reaction is the formation of free radicals (oxidative stress), which has been suggested as a key factor in further cell reactions [e.g., Nelet al.5]. Several cell biological effects and changes in gene expression patterns were described, but because of the different CNT material and suspension procedures used, quantitative and comparison statements on the cytotoxicity of CNTs in different cell types and tissue are nearly impossible. Focused studies on inhalation exposures are needed to evaluate in vitro studies on their reliability and to build up the epidemiologic databases.
Discussion
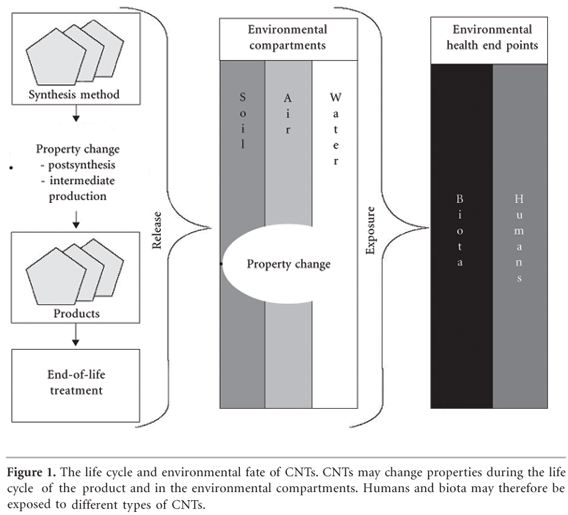
In the present review, we have investigated the state of knowledge regarding the impact of CNTs on human health and the environment. At the time of the review, there were approximately 50 studies focusing on the effects of CNTs on human health and environment, with the majority of them in vitrostudies (Supplemental Material, Tables S4 and S5; http://www.ehponline.org/docs/2007/9652/ suppl.pdf). Environmental impacts in particular have been investigated poorly. One of the findings of the expert interviews was that the current impact assessments have been conducted without any overall research strategy (Supplemental Material). Acknowledging this, the experts have called for a better coordination of research, which could provide interdisciplinary and complementary results. There are different types of CNTs produced in and applied to products with a variety of physical and chemical properties and potential exposure routes. The knowledge base suggests that altering these properties induces different effects on environmental health. Additionally, these properties may change during the life cycle of a product containing CNTs, as external physical and chemical influences differ at each stage. Therefore CNTs may cause different environmental healtheffects depending on the life cycle of the product and fate of the product in the environment. Tracing the fate of CNTs from each stage of the CNT product life cycle, as illustrated in Figure 1, may be an effective orientation for prioritizing research.
CNT material
The particular properties of CNTs depend on the particular production process used (Supplemental Material; http://www.ehponline.org/docs/2007/9652/ suppl.pdf). After synthesis the raw material contains nanoparticulate impurities that influence the toxicity of the species. Postsynthesis treatment alters various properties of CNTs such as length, purity, degree of aggregation, wall structure (doping), and surface functionalization. These properties are thought to determine toxicologic-relevant factors such as particle size, mobility in the environment, chemical reactivity, persistence, and bioavailability. Hence, the impacts of engineered CNTs are likely to differ from those of naturally occurring CNTs and may additionally depend on the technical application and circumstances of release.
Exposure
Emissions of CNTs may, depending on the application, occur at all stages in the product life cycle: synthesis, production of intermediates, further processing, product use, and disposal. From the production sites, emission of CNTs into surrounding air depends on process control, handling procedures (yielding, bottling, packing), cleaning, safety installations and procedures (in case of leakages and accidents), and waste conditioning. These procedures may also lead to release of CNTs into the wastewater. Waste items such as contaminated hand gloves, packaging, or worn filter pads could disperse CNTs in cases of sufficient agitation. The CNTs released from the different stages may show very different characteristics such as length, surface properties, attachments, and agglomeration size. The variety of effects observed in the studies seems to result from the characteristics of the particular CNTs tested. It is therefore essential to scrutinize whether CNTs used in these studies are of the same type as the CNTs that organisms may be exposed to under real environmental conditions. A critical investigation of the types of exposures to CNTs before using CNTs in studies seems crucial for the information value of the study. Therefore, focusing on characterized exposure scenarios could be very useful when conducting toxicologic studies.
Environmental compartments
The fate of CNTs in environmental compartments may differ depending on their specific properties such as surface chemistry, electrical properties, and oxidative potential. Studies show that functionalization and state of aggregation are likely to influence the behavior in water and soil. It would therefore be useful to characterize the CNT type and form that could be released into the environmental compartments to assess the fate of CNTs. However, the physical and chemical conditions of the different environmental compartments (such as redox potential, pH, temperature, ultraviolet light, or synergistic effects with toxins) are also likely to alter the properties of CNTs or their functional attachments, and thus their environmental fate. Other molecules or particles stick easily to the surface of CNTs and may alter the the behavior of CNTs. Consequently, pollutants may be transported in the environmental compartments. Therefore, the question arises as to whether and to what extent it would be possible to foresee and control the effects caused by different CNT material properties in the environment.
Environmental health end points
Once CNTs are released into environmental compartments, preliminary studies have shown CNTs to be bioavailable to different organisms in addition to being biopersistent. One can therefore not exclude the possibility that CNTs may accumulate along the food chain. These studies emphasize a further need to study ecotoxicity as well as longer time-scale impacts covering bioaccumulation, biopersistency, andnegative effects on reproduction. Once taken up by humans or other species, CNTs may cause oxidative stress, inflammation, cell damage, adverse effects on cell performance, and, in a long-term perspective, pathological effects like granulomas, fibrosis, and wall thickening. These effects have been observed time and dose dependently in the majority of toxicology studies. However, a remarkable range of results has been observed in toxicology studies (Supplemental Material, Tables S4 and S5; http://www.ehponline.org/docs/2007/9652/ suppl.pdf). Comparing these different studies is difficult because different CNT species and cell model systems have been employed. An indepth characterization of the CNTs employed is crucial for interpretation of cytotoxicologic and toxicologic assessments. The contradictions in some of the results obtained could also originate from the types and degrees of agglomeration of the CNTs. In addition, the surfactants or surface coating may have a significant influence on the CNTcell interaction, an effect that remained unaccounted for in several studies.
Strategies to enhance the explanatory power of future toxicology studies should comprise some standardization of the CNT material, test procedures, and cell models/organisms employed. To that end, experts recommended using SWCNTs from arc discharge synthesis, as these SWCNTs show relatively good purity and structural uniformity (Supplemental Material; http://www.ehponline.org/docs/2007/9652/suppl.pdf). In addition, a set oftoxic equivalency factors (TEFs) would be useful for comparing the toxicologic effects of different types of CNTs. TEFs have been developed to compare the toxicity of substance classes such as PAHs, which comprise a wide range of compounds60. The establishment of TEFs for CNTs would need to consider nanospecific properties of the material (e.g., particle size, aspect ratio, agglomeration state) and would require investigation of mechanisms and doseresponse dependency on the cellular level. To what extent CNTs can enter the bloodstream, for example, through the alveolar passage, and whether organisms are able to eliminate pristine CNTs remain unexplained. To date, no biodegradation mechanisms for CNTs that support elimination from the organism have been investigated. However, agglomeration and immobilization of CNTs within tissue have been observed, for example, in lung tissue. Some studies have shown that functionalization may, in addition to influencing CNT mobility, also influence the degree of toxicity. Functionalization may therefore be a key parameter for controlling the impact of CNTs on human health and the environment. The important considerations discussed above combine different scientific disciplines ranging from materials science to biology. Integrating nanotoxicology with a life-cycle perspective will therefore be a prerequisite for the development of nanotechnology-based applications in a safe and responsible manner.
Acknowledgments
We thank A Bruinink, V Colvin, A Maynard, H Krug, and J Wöörle-Knirsch for their comments on this paper. We acknowledge the funding within the project "Nanorisk" from the Swiss Federal Office for the Environment (FOEN), the Federal Office of Public Health (BAG), the Federal Office for Professional Education and Technology (OPET-CTI), and EMPA Materials Science and Technology.
* This article was originally published by the journal Environmental Health Perspectives 115:11251131 (2007). doi:10.1289/ehp.9652 available via http://dx.doi.org/ [Online 10 May 2007] and is part of the scientific collaboration between Rev C S Col and EHP. Supplemental material is available online (http://www.ehponline.org/docs/2007/9652/suppl.pdf). The authors declare they have no competing financial interests. Received 25 August 2006; accepted 10 May 2007.
- 1. Lux Resarch Inc. 2006. The Nanotech Report. 4th ed. [accessed 13 July 2006]. Available from: http://luxresearchinc.com/pdf/TNR4_TOC.pdf
- 2. Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004; 109(1):71-77.
- 3. Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 2005; 113:823-839.
- 4. Helland A, Kastenholz H, Thidell A, Arnfalk P, Deppert K. Nanoparticulate materials and regulatory policy in Europe: an analysis of stakeholder perspectives. J Nanopart Res 2006; 8(5):709-719.
- 5. Nel A, Xia T, Määdler L, Li N. Toxic potential of materials at the nanolevel. Science 2006; 311:622-627.
- 6. Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A 2004; 67(1):87-108.
- 7. Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrester G, Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 2006; 92(1):5-22.
- 8. Feron VJ, Arts JHE, Kuper CF, Slootweg PJ, Woutersen RA. Health risks associated with inhaled nasal toxicants. Crit Rev Toxicol 2002; 31(3):313-347.
-
9VDI. Technological Analysis: Industrial Application of NanomaterialsùChances and Risks Düsseldorf: VDI (Verein Deutscher Ingenieure), Future Technologies Division of VDI Technologiezentrum GmbH; 2004.
- 10. Kamat JP, Devasagayam TPA, Priyadarsini KI, Mohan H, Mittal JP. Oxidative damage induced by the fullerene C60 on photosensitization in rat liver microsomes. Chem-Biol Interact 1998; 114(3):145-159.
- 11. Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 2004; 4(1):11-18.
- 12. Lam C-W, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci 2004; 77(1):126-134.
- 13. Wu Y, Hudson J, Lu Q, Moore J, Mount A, Rao A, Alexov E, Ke PC. Coating single-walled carbon nanotubes with phospholipids. J Phys Chem B 2006; 110(6):2475-2478.
- 14. Cheng H, Cheng J. The aggregation of single-walled carbon nanotubes in fresh water and sea water [Abstract]. Off J Soc Toxicol 2005; 84(S1):9.
- 15. Chen Q, Saltiel C, Manickavasagam S, Schadler LS, Siegel RW, Yang H. Aggregation behaviour of single-walled carbon nanotubes in dilute aqueous suspension. J Colloid Interface Sci 2004; 280:91-97.
- 16. Lecoanet HF, Bottero J-Y, Wiesner MR. Laboratory assessment of the mobility of nanomaterials in porous media. Environ Sci Technol 2004; 38(19):5164-5169.
- 17. Lecoanet HF, Wiesner MR. Velocity effects on fullerene and oxide nanoparticle deposition in porous media. Environ Sci Technol 2004; 38(16):4377-4382.
- 18. Kleiner K, Hogan J. How safe is nanotech? New Scientist 2003; 177:14-15.
- 19. Fiorito S, Serafino A, Andreola F, Bernier P. Effects of fullerenes and single-wall carbon nanotubes on murine and human macrophages. Carbon 2006; 44:1100-1105.
- 20. Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: single-wall nanotube,multi-wall nanotube, and fullerene. Environ Sci Technol 2005; 39(5):1378-1383.
- 21. Yang K, Zhu L, Xing B. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ Sci Technol 2006; 40(6):1855-1861.
- 22. Oberdörster E, Zhu S, Blickley TM, McClellan-Green P, Haasch ML. Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C60) on aquatic organisms. Carbon 2006; 44:1112-1120.
- 23. Oberdörster E, Ortiz-Acevedo A, Xie H, Pantano P, Baughman RH, Musselman IH, Draper RK. Exposure of fathead minnow to fullerene and single-walled carbon nanotubes [Abstract]. Off J Soc Toxicol 2005; 84(S1):325.
- 24. Velasco-Santos C, Martinez-Hernandez AL, Consultchi A, Rodriguez R, Castano VM. Naturally produced carbon nanotubes. Chem Phys Lett 2003; 373(34):272-276.
- 25. Murr LE, Bang JJ, Esquivel EV, Guerrero PA, Lopez DA. Carbon nanotubes, nanocrystal forms, and complex nanoparticle aggregates in common fuel-gas combustion sources and the ambient air. J Nanopart Res 2004; 6(2):241-251.
- 26. Lam C-W, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol 2006; 36:189-217.
- 27. Huczko A, Lange A, Calko E, Grubek-Jaworska H, Droszcz P. Physiological testing of carbon nanotubes: are they asbestos-like? Fullerene Sci Tech 2001; 9(2):251-254.
- 28. Grubek-Jaworska H, Nejman P, Czuminska K, Przybylowski T, Huczko A, Lange H, Bystrzejewski M, Baranowski P, Chazan R. Preliminary results on the pathogenic effects of intratracheal exposure to onedimensional nanocarbons. Carbon 2006; 44(6):1057-1063.
- 29. Huczko A, Lange H, Bystrzejewski M, Baranowski P, Grubek-Jaworska H, Nejman P, Przybylowski T, Czuminska K, Glapinski J, Walton DRM, Kroto HW. Pulmonary toxicity of 1-D nanocarbon materials. Fuller Nanotub Car N 2005; 13:141-145.
- 30. Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci 2004; 77:117-125.
- 31. Tagmatarchis N, Prato M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions.J Mater Chem 2004; 14(4):437-439.
- 32. Muller J, Huaux F, Moreau N, Misson P, Heilier J-F, Delos M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol 2005; 207(3):221-231.
- 33. Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich Alla I, Tyurina YY , Gorelik O, Arepalli S , Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol-Lung C 2005; 289(5):L698-L708.
- 34. Huczko A, Lange A. Carbon nanotubes: experimental evidence for a null risk of skin irritation and allergy. Fullerene Sci Tech 2001; 9(2):247-250.
- 35. Wang H, Wang J, Deng X, Sun H, Shi Z, Gu Z, Liu Y, Zhao Y. Biodistribution of carbon single-wall carbon nanotubes inmice. J Nanosci Nanotech 2004; 4(8):1019-1023.
- 36. Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA 2006; 103(9):3357-3362.
- 37. Sato Y, Yokoyama A, Shibata K, Akimoto Y, Ogino S, Nodasaka Y, Kohgo T , Tamura K, Akasaka T, Uo M, Motomiya K, Jeyadevan B, Ishiguro M, Hatakeyama R, Watari F, Tohji K. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-I in vitro and subcutaneous tissue of rats in vivo. Mol Biosyst 2005; 1(2):176-182.
- 38. Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect 2005; 113:1555-1560.
- 39. Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J Am Chem Soc 2004; 126(48):15638-15639.
- 40. Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, Kisin ER, Schwegler-Berry D, Mercer R, Castranova V, Shvedova AA. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett 2006; 165(1):88-100.
- 41. Murr LE, Garza KM, Soto KF, Carrasco A, Powell TG, Ramirez DA, Guerrero PA, Lopez DA, Venzor J 3rd. Cytotoxicity assessment of some carbon nanotubesand related carbon nanoparticle aggregates and the implications for anthropogenic carbon nanotube aggregatesin the environment. Int J Environ Res Public Health 2005; 2(1):31-42.
- 42. Soto KF, Carrasco A, Powell TG, Garza KM, Murr LE. Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy.J Nanopart Res2005; 7:145-169.
- 43. Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett 2007; 168(2):121-131.
- 44. Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactionswith human epidermal keratinocytes. Toxicol Lett 2005; 155:377-384.
- 45. Ding LH, Stilwell J, Zhang TT, Elboudwarej O, Jiang HJ, Selegue JP, Cooke PA, Gray JW, Chen FQF. Molecular characterization of the cytotoxicmechanism of multiwall carbon nanotubes and nanoonions on human skin fibroblast. Nano Lett 2005; 5(12):2448-2464.
- 46. Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, AR M, Gandelsmann VZ, Maynard A, Baron P. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A 2003; 24(66):1909-1926.
- 47. Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, Moore VC, Doyle CD, West JL, Billups WE, Ausman KD, Colvin VL. Functionalization density dependence of single- walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett 2006; 161(2):135.
- 48. Hu H, Ni Y, Montana V, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett 2004; 4(3):507-511.
- 49. Hu H, Ni Y, Mandal SK, Montana V, Zhao B, Haddon RC, Parpura V. Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth. J Phys Chem B 2005; 109:4285-4289.
- 50. Liopo A, Stewart M, Hudson J, Tour J, TC P. Biocompatibility of native and functionalized single-walled carbon nanotubes for neuronal interface.J Nanosci Nanotechnol2006; 6(5):1365-1374.
- 51. Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett 2005; 155(1):73-85.
- 52. Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, Bergamaschi A, Mustelin T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett 2006; 160(2):121-126.
- 53. Kam NWS, Jessop TC, Wender PA, Dai H. Nanotube molecular transporters: internalization of carbon nanotubeprotein conjugates into mammalian cells. J Am Chem Soc 2004; 126(22):6850-6851.
- 54. Kam NWS, Liu Z, Dai H. Functionalization of carbonnanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Am Chem Soc 2005; 127:12492-12493.
- 55. Kam NWS, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 2005; 102:11600-11605.
- 56. Bianco A. Carbon nanotubes for the delivery of therapeutic molecules. Expert Opin Drug Deliv 2004; 1(1):57-65.
- 57. Bianco A, Hoebeke J, Godefroy S, Chaloin O, Pantarotto D, Briand JP, Muller S, Prato M, Partidos CD. Cationic carbon nanotubes bind to CpG oligodeoxynucleotides and enhance their immunostimulatory properties. J Am Chem Soc2005; 127:58-59.
- 58. Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand J-P, Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed 2004; 43:5242-5246.
- 59. Price RL, Haberstroh KM, Webster TJ. Improved osteoblast viability in the presence of smaller nanometre dimensioned carbon fibers. Nanotechnology 2004; 15:892-900.
- 60. Nisbet C, LaGoy P. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons. (PAI-Is). Regul Toxicol Pharmacol 1992; 16:290-300.
Publication Dates
-
Publication in this collection
11 Feb 2008 -
Date of issue
Apr 2008
History
-
Received
25 Aug 2006 -
Accepted
10 May 2007