Abstract
Advanced HIV infection is frequently complicated by diarrhea, disruption of bowel structure and function, and malnutrition. Resulting malabsorption of or pharmacokinetic changes in antiretroviral agents might lead to subtherapeutic drug dosing and treatment failure in individual patients, and could require dose adjustment and/or dietary supplements during periods of diarrheal illness. We determined the plasma levels of antiretroviral medications in patients that had already been started on medication by their physicians in an urban infectious diseases hospital in northeast Brazil. We also obtained blood samples from patients hospitalized for diarrhea or AIDS-associated wasting, and we found reduced stavudine and didanosine levels in comparison with outpatients without diarrhea or wasting who had been treated at the same hospital clinic. There was a predominance of the protozoal pathogens Cryptosporidium and Isospora belli, typical opportunistic pathogens of AIDS-infected humans, in the stool samples of inpatients with diarrhea. We conclude that severe diarrhea and wasting in this population is associated with both protozoal pathogens and subtherapeutic levels of antiretroviral medications.
HIV; malabsorption; ddI; D4T; pharmacokinetics
ORIGINAL PAPERS
AIDS-associated diarrhea and wasting in northeast Brazil is associated with subtherapeutic plasma levels of antiretroviral medications and with both bovine and human subtypes of Cryptosporidium parvum
Richard K. Brantley; K. Robert Williams; Terezinha M.J. Silva; Maria Sistrom; Nathan M. Thielman; Honorine Ward; Aldo A. M. Lima; Richard L. Guerrant
Division of Geographic and International Medicine,Department of Internal Medicine, University of Virginia School of Medicine, Virginia; Division of Geographic Medicine and Infectious Diseases, New England Medical Center, Tufts University School of Medicine, Massassuchetts, US; Department of Physiology and Pharmacology and Clinical Research Unit of the Walter Cantidio University Hospital, Federal University of Ceará, Ceará, Brazil
Correspondence Correspondence to Dr. Richard Guerrant Box 801379 Health Science Center, Department of Internal Medicine, University of Virginia School of Medicine Charlottesville, VA 22908, US Phone: (804) 924-9672. Fax: (804) 577-5323 E-mail: rlg9a@virginia.edu
ABSTRACT
Advanced HIV infection is frequently complicated by diarrhea, disruption of bowel structure and function, and malnutrition. Resulting malabsorption of or pharmacokinetic changes in antiretroviral agents might lead to subtherapeutic drug dosing and treatment failure in individual patients, and could require dose adjustment and/or dietary supplements during periods of diarrheal illness. We determined the plasma levels of antiretroviral medications in patients that had already been started on medication by their physicians in an urban infectious diseases hospital in northeast Brazil. We also obtained blood samples from patients hospitalized for diarrhea or AIDS-associated wasting, and we found reduced stavudine and didanosine levels in comparison with outpatients without diarrhea or wasting who had been treated at the same hospital clinic. There was a predominance of the protozoal pathogens Cryptosporidium and Isospora belli, typical opportunistic pathogens of AIDS-infected humans, in the stool samples of inpatients with diarrhea. We conclude that severe diarrhea and wasting in this population is associated with both protozoal pathogens and subtherapeutic levels of antiretroviral medications.
Key Words: HIV, malabsorption, ddI, D4T, opportunistic infection, pharmacokinetics.
Both acute and chronic diarrheal illnesses, together with malnutrition and wasting ("slim disease"), are frequent complications of advanced HIV infection in the developing world [1]. HIV is recognized to alter both the structure and the function of human small intestines. This may occur due to direct viral enteropathy, or secondarily as a result of opportunistic infection. Histologically, villous blunting and inflammation are commonly seen in patients with AIDS or parasitic infection, especially those infected with Cryptosporidium [2]. These structural changes are accompanied by important functional changes in absorption. For example, the lactulose/mannitol urinary excretion ratio, a commonly used measure of transcellular and paracellular permeabilities, is significantly altered in HIV-infected individuals without diarrhea in comparison with non-infected controls [3,4]. In one study [4], a subset of patients with Cryptosporidium oocysts identified in the stool had a 7-fold increase in this ratio, reflecting profound derangement of intestinal function. Patients with cryptosporidial diarrhea also have an almost 50% reduction in jejunal-cecal transit times [5]. We found subtherapeutic peak plasma levels of antiretroviral medications in patients with AIDS-associated diarrhea and wasting in Northeast Brazil. We also found both human and bovine subtypes of the pathogen Cryptosporidium parvum in these patients, as well as two patients with undetermined species of Cryptosporidium.
Materials and Methods
Study Location. The São Jose Hospital is a state infectious disease hospital in urban Fortaleza, Brazil. It is the only provider of dedicated care to the HIV-infected population, through its inpatient and outpatient services. Its catchment area includes both the capital city of 2.5 million and the remainder of the State of Ceará, a relatively arid region of northeast Brazil. Cryptosporidium is hyperendemic as a diarrheal pathogen of both immunocompetent children [6,7] and in patients with AIDS in this region, particularly during the rainy season, which is when this study was conducted (Feb-Mar, 2000).
Subjects. Volunteers were recruited from the hospital's inpatient AIDS ward and from the hospital's outpatient ambulatory clinic, which serve a large population of HIV-infected individuals. Written informed consent was obtained and the project was approved by the Human Investigation Committees of the Federal University of Ceara, the Sao Jose Hospital, and the University of Virginia. Entry criteria included the ability to give informed consent to participate and a history of a positive serological test for HIV together with at least one AIDS-defining illness. Diarrhea/wasting subjects were inpatients who reported diarrhea [defined as three or more stools with decreased consistency] during at least eight of the ten days prior to enrollment, or intermittent diarrhea for two weeks over the two months prior to entry together with a weight loss of greater than 10% below baseline during those two months. Approximately three patients per week with diarrhea and wasting were admitted to the hospital during this period. About two thirds of these patients met entry criteria and were invited to participate; all did so. Controls were outpatients with a history of a serological HIV test and an AIDS-defining illness, but who had experienced no diarrhea or weight loss in the preceding two months. All subjects had been receiving the antiretroviral regimens indicated by their physicians during at least four dosing intervals before the samples were obtained. Treatment regimens and patient demographics are outlined in Table 1. However, each medication was not sampled in every patient due to differences in the timing of peak plasma levels. As assays were not available for all medications, it was decided to concentrate resources on the nucleoside analogs. Exclusion criteria were (1) women who were pregnant, nursing, or not practicing effective contraception, (2) inability to give informed consent, and (3) evidence of severe hepatic disease or renal failure. Chart review and patient interviews were used to compile baseline descriptive characteristics.
Laboratory. Diarrheal pathogens from inpatients with diarrhea and wasting were identified whenever possible by microscopy of stool specimens and by stool culture at the hospital laboratory and/or at the Clinical Research Unit. Stool samples were promptly frozen at 80oC for genetic studies. Medications were provided by the Ministry of Health to the hospital's pharmacy, which supplied both inpatients and outpatients. Therapy prescribed by the patient's treating physician was directly observed and timed (never before the fourth regularly scheduled dose in the hospital), and then phlebotomy performed at the time of predicted peak plasma concentration, based on prior pharmacokinetic studies for each drug provided by a U.S. commercial laboratory (Specialty Laboratories, Santa Monica CA) in order to determine antiretroviral drug levels. Both d4T (stavudine) and AZT (zidovudine) were sampled 35-45 minutes after directly observed therapy. ddI levels (didanosine) were obtained 45 to 65 minutes after administration. 3TC (lamivudine) was sampled 55-95 minutes after oral therapy. Levels of antiretroviral medications were determined by validated assays using liquid chromatography with tandem mass spectrometry at Specialty Laboratories following collection in Brazil, immediate centrifugation, and storage of plasma at - 70oC until the assay.
Cryptosporidium Genotyping. Stool samples, were frozen at 80°C and shipped to Boston on dry ice. DNA was isolated by a previously described method [8]. PCR-RFLP analysis at the small-subunit rRNA locus was used to determine the species of Cryptosporidium according to the method described by Xiao et al. [9]. A nested PCR reaction, using previously described primers and conditions, was performed. The final PCR product was digested with Ssp I at 37°C for 1 hour. For genotypic analysis of C. parvum isolates, PCR-RFLP of the thrombospondin-related adhesive protein of Cryptosporidium (TRAP-C1) and Cryptosporidium outer wall protein (COWP) was performed using previously described primers and conditions [10,11]. PCR products were then digested with Rsa I at 37°C for 1 hour.
Results
Twelve patients were enrolled during the study period, and descriptive data was obtained through patient interview and chart review. Patients with diarrhea and wasting had a more recent diagnosis of AIDS, a weight loss of approximately 25%, a lower median BMI, lower CD4 counts, and increased viral load (Table 2) in comparison with the outpatients concurrently recruited from the hospital's clinic, who indicated no diarrhea or weight loss. Stool studies demonstrated a protozoal etiology for diarrhea in seven of the 11 inpatients, with Cryptosporidium spp. found in five, making it the most frequently identified agent. Isospora belli was found in two patients, and Strongyloides was present as a coinfection in one patient.
Genetic analysis of stool specimens from the patients in which Cryptosporidium was visually identified in either the hospital or Clinical Research Unit laboratories revealed three cases of C. parvum and one of non-C. parvum. Of the C. parvum isolates, one was of the human (type 1) genotype and two were of the bovine (type 2) genotype. Five additional specimens positive for cryptosporidia by microscopy were obtained from similar inpatients admitted with diarrhea and wasting between November 1997 and August 1998. Genetic analysis of these specimens demonstrated three cases of C. parvum (human genotype), and one contained a non-C. parvum (undetermined species) isolate. One specimen was PCR negative.
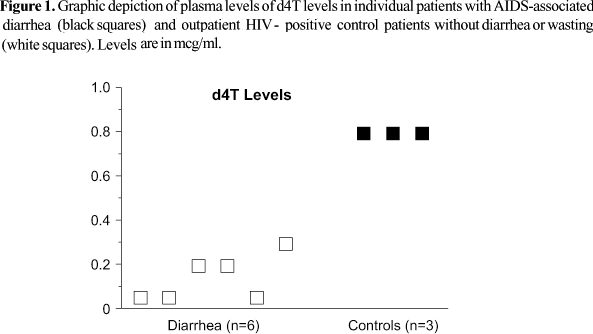
Plasma d4T levels differed strikingly between the inpatients with diarrheal illness/wasting in comparison with outpatients receiving treatment at the same hospital (Figure 1). d4T levels among patients with diarrhea/wasting were subtherapeutic (less than 0.3 mcg/ml) in five of the six patients tested (Figure 1). By comparison, outpatients had a higher median peak d4T level (p<0.02; Mann-Whitney U test), with three of three patients near the high end of the therapeutic range. Among the inpatients taking ddI, all four had profoundly low levels with three of the four falling below the detection limit of the assay (<0.1 ug/ml), whereas the one outpatient taking this drug had a higher, albeit still subtherapeutic, level of 0.5 mcg/ml (therapeutic range 1.0-1.3). The patients taking zidovudine (AZT) and lamivudine (3TC) had highly variable levels, but without a discernable trend.
Discussion
At an urban infectious disease hospital in NE Brazil, diarrhea and wasting are associated with low median CD4 counts, reduced body-mass index, and increased viral load. This syndrome was also associated, more often than not in our small series, with the presence of protozoal enteric pathogens. In the patients with AIDS, wasting, and diarrhea, protozoal pathogens were frequently found, including both human and bovine subtypes of C. parvum, as well as non-C. parvum isolates (of an undetermined species). Specimens obtained from the same patient population in a 1997-1998 study also showed the importance of both C. parvum and non-C. parvum isolates in this setting.
Cryptosporidiosis is an emerging, highly infectious threat to both immunocompetent and immunocompromised patients [12]. Recently, the application of molecular genetics tools to a prior taxonomy based on morphology and host species has led to the demonstration of both new species and an understanding that non-human strains can cause significant human disease [13]. Two recent reports have indicated that non-human cryptosporidioses are significant pathogens in humans. A study of children in a poor urban community in Peru demonstrated that 21% of 85 episodes of cryptosporidial infection in children in this community were due to non-human species. Cryptosporidium parvum (bovine genotype), C. parvum (dog genotype), C. meleagris, and C. felis were identified [14]. None of the children infected with these species were HIV positive. Similarly, HIV-infected individuals participating in a longitudinal study of enteric parasitosis and chronic diarrhea in the United States were found to be infected with non-human types of C. parvum (bovine and dog genotypes) and also with C. felis [15,16]. As both immunocompetent and immunocompromised individuals can be infected by a variety of cryptosporidial agents, non-human cryptosporidiosis may soon be recognized as clinically important. The two isolates described here were non-C. parvum species, and their identity is currently being determined by sequence analysis at the SSU rRNA locus.
We found substantial relative differences in plasma levels of the antiretroviral medications d4T and ddI in patients with severe diarrhea and wasting. Reduced levels of these medications might occur as a result of increased drug metabolism or excretion, altered distribution within the body's compartments, or highly delayed Tmax (time to maximum plasma concentration). However, we feel that a more plausible explanation is impaired absorption due to loss of mucosal surface area and inflammation. The possibility that the bowel's structural and functional changes due to AIDS and cryptosporidial infection could provoke altered drug levels in the plasma is not entirely unexpected. For example, profound malabsorption of cyclosporine occurred in a study of allogenic bone marrow transplant patients with diarrhea of multiple causes (infectious, graft-versus host disease, and chemo-radiation; ref. 17). The malabsorption was so severe that it led the authors to propose intravenous administration of the drug during periods of gut dysfunction. Reduced plasma levels of antimycobacterial agents have been documented in patients coinfected with HIV [18-20]. Most of these authors suggest malabsorption as the underlying cause, presumably secondary to enteral disruption. Contradictory findings have been reported in tests of the absorption of zidovudine in patients with diarrhea, wasting, and impaired fat absorption. One study reported a reduced peak concentration (Cmax) and Tmax, but no change in AUC (area under the curve, ref. 21). Other authors have found no effect on any measure of AZT level when the patient has diarrhea [22]. Paradoxically, enhanced bioavailability of clindamycin is seen in persons with AIDS, when compared with healthy volunteers [23]. This may result primarily from reduced first-pass metabolism in the gut wall as a similar finding has been reported in patients with celiac disease [24]. Additionally, oral ganciclovir produces a two-fold increase in Cmax and a three-fold increase in AUC in AIDS patients with chronic diarrhea and wasting when compared with those without diarrhea and weight loss [25].
Many people in developing countries who are severely ill with HIV infection and diarrheal illness need treatment. Our preliminary findings of low plasma d4T and ddI levels together with multiple cryptosporidial subtypes in Northeast Brazil suggest potential problems with adequate treatment of those most in need. Although our sample size is limited, this work emphasizes the need for further study of the delivery of antiretroviral medications in resource-poor regions of the world. The practicalities of assuring plasma and intracellular levels of antiretroviral medications adequate for virological suppression must be addressed in patient populations where severe diarrhea and wasting ("slim disease") often define AIDS. If indeed malabsorption exists, then viral resistance might result from periodic subtherapeutic treatment and thereby complicate treatment. Transient decreases in drug absorption might be addressed through planned increased oral doses, development of parenteral agents, or the use of dietary supplements [26,27] to speed the repair of the damaged epithelium and to improve absorptive function.
Acknowledgements
We acknowledge Leah Barrett for technical assistance, the assistance of Dr. Carlos Roberto, and the AIDS patients at the Sao Jose Hospital and Ambulatory Clinic who graciously gave their time.
Received on 12 January 2002
Revised 04 December 2002
Sources of support: Benjamin Keane Fellowship of the ASTMH (RKB); NIH/NIAID/TMRC grant #AI 30439 (RLG, AAML); NIH/NIAID/ICIDR supplement #AI 26512 (HW,AAML,RLG); and the University of Virginia School of Medicine Dean's Office (KRW)
- 1. Lew E.A., Poles M.A., Dieterich D.T. Diarrheal illnesses associated with HIV infection. Gastroent Clin N Amer 1997;26:259-90.
- 2. Goodgame R.W., Kimball K., Ou C.N. Intestinal function and injury in AIDS- related cryptosporosis. Gastroenterol 1995;108:1075-82.
- 3. Tepper R.E., Simon D., Brandt L.J., et al. Intestinal permeability in patients infected with human immunodeficiency virus. Scand J. Gastroent. 1994;28:573-80.
- 4. Lima A.A.M., Silva T.M.J., Gifoni A.M.R., et al. Mucosal injury and disruption of intestinal barrier function in HIV-infected individuals with and without diarrhea and cryptosporidiosis in Northeast Brazil. Am. J. Gastroenterol 1997:92:1861-5.
- 5. Sharpstone D., Neild P., Crane R., et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhea. Gut 1999;45:70-6.
- 6. Wuhib T., Silva T.M., Newman R.D., et al. Cryptosporidial and microsporidial infections in HIV-infected patients in northeastern Brazil J Infect Dis 1994;170:494-7.
- 7. Newman R.D., Zu S.-X., Wuhib T., et al. Household epidemiology of Cryptosporidium parvum imfection. Ann Int Med 1994;120:500-5.
- 8. McLauchlin J., Pedraza-Diaz S., Amar-Hoetzeneder C., Nichols G.L. Genetic characterization of Cryptosporidium strains from 218 patients with diarrhea diagnosed as having sporadic cryptosporidiosis. J Clin Microbiol 1999;37(10):3153-8.
- 9. Xiao L., Escalante L., Yang C., et al. Phylogenetic analysis of Cryptosporidium parasites based on the small- subunit rRNA gene locus. Appl Environ Microbiol 1999;65(4):1578-83.
- 10. Spano F., Putignani L., McLauchlin J., et al. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett 1997;150(2):209-17.
- 11. Le Blancq S.M., Tchack L., Tzipori S., Widmer G. Multilocus genotypic analysis of Cryptosporidium parvum isolates from different hosts and geographical origins. J Clin Microbiol 1998;36(11):3255-9.
- 12. Guerrant R.L. Cryptosporidiosis, an emerging, highly infectious threat. Emer Infect Dis 1997;3.
- 13. Xiao L., Morgan U.M., Fayer R., et al. Cryptosporidium systematics and implications for public health. Parasitology Today 2000;16(7):287-92.
- 14. Xiao L., Bern C., Limor J., et al. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis 2001;183:492-7.
- 15. Pieniazek N.J., Bornay-Llinares F.J., Slemenda S.B., et al. New Cryptosporidium genotypes in HIV infected persons. Emerg Infect Dis 1999;5(3):444-9.
- 16. Navin T.R., Weber R., Vugia D.J., et al. Declining CD4+ lymphocyte counts are associated with increased risk of enteric parasitosis and chronic diarrhea: results of a 3-year longitudinal study. J Acqir Immune Defic Syndr Hum Retroviral 1999;20:154-9.
- 17. Atkinson K., Biggs J.C., Britton K., et al. Oral administration of cyclosporin A for recipients of allogenic marrow transplants: implications of clinical gut dysfunction. Br J Haematology 1984;56:223-31.
- 18. Patel K.B., Belmonte R., H.M. Crowe. Drug malabsorption and resistant tuberculosis in HIV-infected patients. New Engl J Med 1995;332(5):336-7.
- 19. Gordon S., Horsburgh C.R., Peloquin C.A., et al. Low seurm levels of antimycobacterial agents in patients with disseminated Mycobacterium avium complex. J Infect Dis 1993;168:1559-62.
- 20. Peloquin C.A., Nitta A.T., Burman W.J., et al. Low tuberculosis drug concentrations in patients with AIDS. Ann. Pharmacother 1996;30:919-25.
- 21. Kapembwa M.S., Fleming S.C., Orr M., et al. Impaired absorption of zidovudine in patients with AIDS-related small intestinal disease. AIDS 1996;10:1509-14.
- 22. Mcnab K.A., Gill M.J., Sutherland L.R., et al. Zidovudine absorption and small intestinal function in HIV seropositive patients. J Antimicrob Chemotherapy 1996;37:825-9.
- 23. Gatti G., Flaherty J., Bubp J., et al. Comparative study of bioavailabilities and pharmacokinetics of clindamycin in healthy volunteers and patient with AIDS. Antimicrob Agents Chemother 1993:1137-43.
- 24. Parsons R.L., Hossack G., Paddock G. The absorption of antibiotics in adult patients with coeliac disease. J Antimicrob Chemotherapy 1975:1:39-50.
- 25. Mouly S., Aymard G., Diquet B., et al. Oral ganciclovir systemic exposure is enhanced in HIV-infected patients with diarrhea and weight loss. JAIDS 2000:24:344-51.
- 26. Silva A.C., Santos-Neto M.S., Soares A.M., et al. Efficacy of a glutamine-based oral rehydration solution on the electrolyte and water absorption in a rabbit model of secretory diarrhea induced by cholera toxin. J Ped Gastro Nutr 1998;26:513-9.
- 27. Lima A.A.M., Soares A.M., Freire J.E., Guerrant R.L. Cotransport of sodium with glutamine, alanine, and glucose in the isolated rabbit ileal mucosa. Braz J Med Biol Res 1992;25 637-40.
Publication Dates
-
Publication in this collection
02 Dec 2003 -
Date of issue
Feb 2003
History
-
Reviewed
04 Dec 2002 -
Received
12 Jan 2002