Abstract
There are various strategies to improve the effectiveness of antibiotics in hospitals. In general, the implementation of guidelines for appropriate antibiotic therapy and the participation of infectious disease (ID) physicians deserve considerable attention. This study was a prospective ecological time-series study that evaluates the effectiveness of the ID physician's opinion to rationalize and control the use of antibiotics in medical-surgical intensive care units (ICU), and the impact of their intervention on treatment expenditures. There was significant change in the pattern of use of antimicrobials, this pattern approximating that of a medical-surgical ICU that participates in the ICARE (Intensive Care Antimicrobial Resistance Epidemiology) Project. For example, there was a significant increase in the consumption of antimicrobials of the ampicillin group (Relative Risk [RR]=3.39; 95% CI: 2.34-4.91) and antipseudomonal penicillins (RR=2.89; 95% CI: 1.70-4.92). On the other hand, there was a significant reduction in the consumption of 3rd/4th generation cephalosporins (RR=0.66; 95% CI: 0.57-0.77) and carbapenems (RR=0.43; 95% CI: 0.33-0.56). On average, for every patient-day antibiotic expense was reduced 37.2% during calendar year 2001, when compared with 2000. The ID specialists' opinion and the adoption of guidelines for empirical antibiotic therapy of hospital-acquired pneumonia contributed to a reduction in the use of antimicrobials in medical-surgical ICU. However, further studies that have more control over confounding variables are needed to help determine the relevance of these discoveries.
Antibiotics; cost control; infection control; drug resistance; microbial
ORIGINAL PAPERS
Effectiveness of the actions of antimicrobial's control in the intensive care unit
Edilson Floriano dos SantosI, II; Antonio Emanuel SilvaIII; Henrique M. Sampaio PinhatiII; Marcelo de O. MaiaII
ICatholic University of Brasília
IISanta Luzia Hospital
IIIUniversity of Brasília, Brasília/DF, Brazil
Correspondence Correspondence to Dr. Edilson Floriano dos Santos, MD, MSc Serviço de Controle de Infecção Hospitalar/Hospital Santa Luzia SHLS 716 conjunto E Zip code: 70390-903 Brasília, DF E-mail: ccih@hsl.com.br
ABSTRACT
There are various strategies to improve the effectiveness of antibiotics in hospitals. In general, the implementation of guidelines for appropriate antibiotic therapy and the participation of infectious disease (ID) physicians deserve considerable attention. This study was a prospective ecological time-series study that evaluates the effectiveness of the ID physician's opinion to rationalize and control the use of antibiotics in medical-surgical intensive care units (ICU), and the impact of their intervention on treatment expenditures. There was significant change in the pattern of use of antimicrobials, this pattern approximating that of a medical-surgical ICU that participates in the ICARE (Intensive Care Antimicrobial Resistance Epidemiology) Project. For example, there was a significant increase in the consumption of antimicrobials of the ampicillin group (Relative Risk [RR]=3.39; 95% CI: 2.34-4.91) and antipseudomonal penicillins (RR=2.89; 95% CI: 1.70-4.92). On the other hand, there was a significant reduction in the consumption of 3rd/4th generation cephalosporins (RR=0.66; 95% CI: 0.57-0.77) and carbapenems (RR=0.43; 95% CI: 0.33-0.56). On average, for every patient-day antibiotic expense was reduced 37.2% during calendar year 2001, when compared with 2000. The ID specialists' opinion and the adoption of guidelines for empirical antibiotic therapy of hospital-acquired pneumonia contributed to a reduction in the use of antimicrobials in medical-surgical ICU. However, further studies that have more control over confounding variables are needed to help determine the relevance of these discoveries.
Key Words: Antibiotics, cost control, infection control, drug resistance, microbial.
Antibiotic therapy is the most complex part of clinical medicine. This is due to the great number of antimicrobial agents available, the varied bacterial flora capable of causing infection in human beings, particularly in those admitted to hospitals, and the impossibility of foreseeing the sensibility of these microorganisms to antibiotics. Also, aspects of the doctor-patient-society relationship, such as the doctor's good intentions in always looking for the best way to treat the patient, the fear of litigation, time constraints, pressure from the pharmaceutical industry and deficiencies in medical formation on infectious diseases, can interfere in the quality of prescriptions by these doctors, resulting in inadequate use of antimicrobials [1,2].
There are several strategies to improve the use of antibiotics in hospitals. Some of them are purely restrictive and others are educational. In general, educational methods are less likely to generate conflict among doctors [3], and they are used in antimicrobial therapy rationalization programs.
Among educational actions, the implementation of guidelines for appropriate antibiotic therapy and the participation of infectious disease (ID) specialist physicians deserve attention. Several studies have shown the value of the ID physician's participation, interacting with the assisting doctor, and promoting appropriate antibiotic therapeutic regimes, with benefits for the patient and the institution [4-7]. These benefits are most evident in patients admitted to intensive care units (ICU).
Our objective was to evaluate the effectiveness of the ID physician's opinion to rationalize and control the use of antibiotics in a medical-surgical ICU, particularly how it affected the consumption of 3rd/4th generation cephalosporins and carbapenems, and the impact on treatment expenditures.
Materials and Methods
A prospective ecological study was carried out, analyzing time-series in the ICU of a private, general hospital, with about 10,000 admissions per year, located in the capital of Brazil (Brasília). It is a 14 bed medical-surgical ICU that logs about 3,500 patient-days per year.
All the patients admitted in this medical-surgical ICU, from January to December 2000 and from January to December 2001, with suspicion or diagnosis of infection, were considered.
In January, 2001, some general measures for the control of hospital infections were introduced, such as to elevating the head of the bed to an angle of 30° to 45° for patients at high risk for aspiration pneumonia; touching the patient and their utensils only when necessary; washing hands before and after procedures and appropriate use of gloves, masks and overcoats. These actions were complemented with measures for greater rationalization of the use of antimicrobials: revision of the hospital antibiotics form; elaboration of guidelines for empirical antibiotic therapy of hospital-acquired pneumonia (the most frequent type of infection in the ICU); and using the ID physician's opinion for defining the antibiotic therapy to be used on patients with known or suspected infection.
In January, 2001, at least one doctor of the team of ID specialists visited the medical-surgical ICU in the morning, daily, from Monday to Friday, and he evaluated, together with the doctors of the ICU, all the patients with suspicion of infection. For patients who began or changed antimicrobial therapy during weekends, holidays or at other hours, the decision regarding antibiotic therapy was made by the ICU doctor, and later evaluated by the ID physician.
Customary data, such as the number of patient-days, were collected daily, at night, by the ICU nurses. The data on the consumption of antibiotics (and other materials and medicines), beginning with the medical prescription were registered by a hospital employee, entered into the computer system and directed to the hospital pharmacy.
The following indicators were used to calculate the consumption of antibiotics: 1) defined daily doses (DDD) this is the average daily dosage in grams of a specific antimicrobial agent given to an average adult patient (Table 1 shows the use of selected oral and parenteral antimicrobial agents in DDD); 2) number of defined daily doses; this is calculated by dividing the total grams of the antimicrobial agent used in a hospital area by the number of grams in an average daily dose given to an adult patient (the DDD); 3) consumption density division of the number of defined daily doses of the antimicrobial agent by the total number of patient-days; 4) attributed fraction this means the part of the consumption density of the antimicrobial agent that came to be used due to the new actions; 5) prevented fraction this means the part of the consumption density of the antimicrobial agent that was no longer being used due to the new actions [8].
The parameter to evaluate antimicrobial consumption adaptation in this medical-surgical ICU came from American hospitals that participate in the ICARE (Intensive Care Antimicrobial Resistance Epidemiology) project [9,10].
The statistical analysis of this continuous variable was made with the t test. The categorical variables, appraised through the consumption density, during the periods considered in the time series, were compared using Chi-square tests. The level of significance was 0.05 and the confidence interval (CI) was 95%. We used the computer program Epi Info 6.04d [11].
Results
The numbers of patient-days followed in the medical-surgical ICU, during 2000 and 2001 were 3,636 and 3,842, respectively.
The patient populations in the ICU from January to December 2000 and from January to December 2001 had similar sex, age, hospital infection and mortality distributions (Table 2).
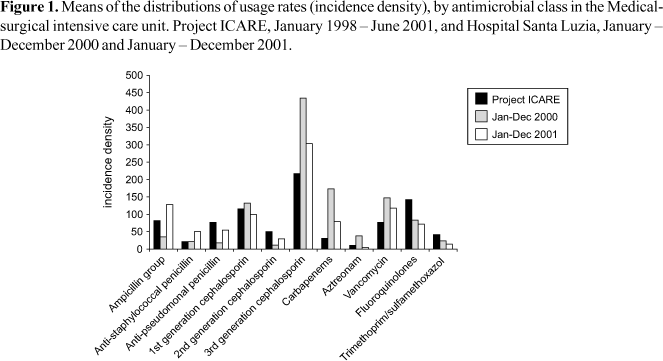
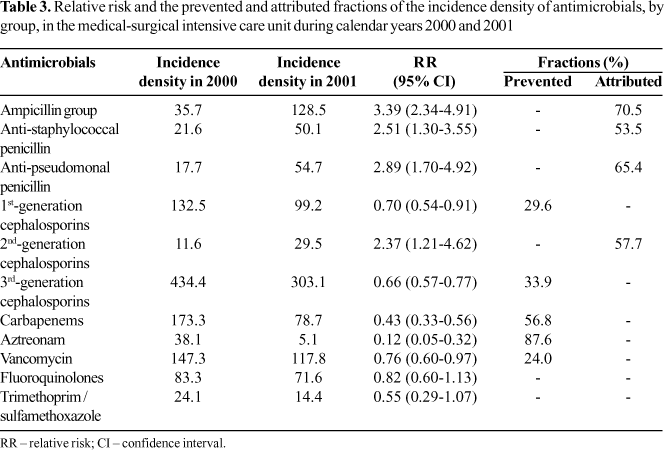
There was a significant change in the pattern of antimicrobial use, approximating that of the medical-surgical ICU that participates in the ICARE project (Figure 1). For instance, there was a significant increase in the consumption of antimicrobials of the ampicillin group (Relative Risk [RR]=3.39; 95%CI: 2.34-4.91), antistaphylococcal penicillins (RR=2.51; 95% IC: 1.30-3.55), antipseudomonal penicillins (RR=2.89; 95% CI: 1.70 4.92) and 2nd-generation cephalosporins (RR=2.37; 95% CI: 1.21-4.62). This increase was 53.5% to 70.5%. On the other hand, there was significant reduction in the consumption of 1st generation cephalosporins (RR=0.70; 95%CI: 0.54-0.91), 3rd/4th generation cephalosporins (RR=0.66; 95% CI: 0.57-0.77), carbapenems (RR=0.43; 95% CI: 0.33-0.56), aztreonam (RR=0.12; 95% CI: 0.05-0.32) and vancomycin (RR=0.76; 95% CI: 0.60-0.97). The amount of reduction was 24.0% to 87.6%. There was no significant change in the consumption of the fluoroquinolones (RR=0.82; 95% CI: 0.60-1.13) and trimethoprim/sulfamethoxazole (RR=0.55; 95% CI: 0.29-1.07) (Table 3).
Together with the change in the pattern of antimicrobial consumption in the ICU, after the visits of the ID physician began and the other new measures were adopted; there was also a reduction in the funds spent on these antibiotics. From January to December 2000, the amount spent on these antimicrobials in the medical-surgical ICU was R$ 261,600 (about US$ 87,200 1 dollar = R$ 3). During the 2001, calendar year the value was R$ 173,720 (about US$ 57,918). On average, the reduction in the amount spent on antibiotics per patient-day was reduced 37.2% in the calendar year 2001, compared to 2000.
Discussion
The antibiotic therapeutics oriented by ID physicians and by guidelines for empirical antibiotic therapy of hospital-acquired pneumonia approximated the rate of antimicrobial consumption of a medical-surgical ICU that participated in the ICARE project [9,10]. Also, the amount spent on antibiotics was reduced, on average, 37.2% during the study period.
Educational programs traditionally compose the arsenal of strategies implemented to rationalize the use of antimicrobials in hospitals [3]. Among these strategies, the promotion of interactions between the ID specialist as an advisor for the ICU doctor to define antibiotic therapy has the smallest potential for conflict [2,12]. The authors of these studies found an association between the ID physician's opinion and the most adequate antibiotic therapy [4-7,13]. We also found an improved adaptation of antibiotic therapy, observing that the consumption of these medicines in our hospital approached the level found in a medical-surgical ICU, used as a standard of excellence [9,10]. Byl et al. [6], for example, studying 428 episodes of bacteremia, suggested that the ID specialist's opinion indeed prevents unnecessary use of broad-spectrum antimicrobials. The change in the profile of antimicrobial use in our hospital, starting from the intervention of the ID physician and the implementation of new guidelines for empirical antibiotic therapy of hospital-acquired pneumonia, so that broad-spectrum 3rd/4th generation cephalosporins, carbapenems were substituted by narrow spectrum antibiotics, also indicates that the new measures contributed to a decrease in the unnecessary use of broad-spectrum antibiotics.
It was not our intention to determine if there was an association between the participation of the ID physician and the frequency of isolation of multi-resistant bacteria. However, Quale et al. [14] and Rahal et al. [15], for example, observed a significant reduction in the prevalence of S. aureus and K. pneumoniae when they restricted the use of vancomycin and 3rd generation cephalosporins, substituting them with beta-lactams/beta-lactamase group antimicrobials (ampicillin/sulbactam and piperacillin/tazobactam). Frank et al. [13] also found a significant reduction in the infection/colonization rates by S. aureus and Stenotrophomonas during the period when their program was totally implemented. We did not find a significant reduction of trimethoprim/sulfamethoxazole use in the ICU. However, there was a reduction in the frequency of isolation of bacteria, such as Burkholderia cepacia and Stenotrophomonas maltophilia, which are sensitive to this antimicrobial medicine and resistant to the aminoglycosides, and can become resistant to the 3rd/4th generation cephalosporins [2]. Actions to control the consumption of antibiotics and basic measures to control hospital infection, implemented by the hospital infection control program probably contributed to the reduction in the detection and spread of these bacteria and consequently reduced the consumption of antibiotics.
The frequently published results concerning antibiotic use programs report a contention of expenses. Frank et al. [13], for example, reduced the cost of antimicrobials by 23%. Other studies have found similar results [16,17]. We obtained a reduction of 37.2%.
There are basically two ways to evaluate the adaptation of antibiotic therapies in hospitals: audits of antibiotics and benchmarking.
In an audit the adaptation of medical prescription of antibiotics can be checked in two ways: starting from its comparison with the technical routines of antimicrobial therapeutics developed by specialists; and/or through a comparison of the isolated bacterial sensibility to the prescribed antimicrobials [18]. The disadvantage of this methodology is the cost of its application. Each prescription of antibiotics should be evaluated individually and compared with the technical routines or with the bacterial sensibility profile to the antibiotics.
Benchmarking is a process destined to identify the best means to reach a certain result among institutions that resemble each other [16]. With a competitive vision ("competitive benchmarking"), we can defined it as the comparison of our work processes in relation to the best competitor, with the objective of revealing an effectiveness level that eventually can be overcome [19]. This process to identify a standard for antimicrobial use in the ICU has been used in the ICARE project. The objective of this project was to influence the doctors' behavior by providing this kind of comparabale information [9,10]. The results published by the ICARE project were used as a standard in our study (benchmarking), which made it possible to work with aggregation data, the density of consumption of antibiotics, avoiding the individual evaluation of each medical prescription of an antibiotic.
Considering the advantages of this type of study, including simplicity, low cost, speed and usefulness to evaluate interventions [8], it is not possible to separate, in its interpretation, the effects of other interventions that are eventually adopted, as for instance, the basic measures to control hospital infection and modifications in the basic organization of the hospital. As a consequence of the difficulty of clarifying the relationship degree among the variables in the ecological studies, there can be an overestimation of the value of the actions on the control of use of antimicrobials.
In conclusion, the ID specialists' opinion and the adoption of guidelines for empirical antibiotic therapy of hospital-acquired pneumonia apparently contributed to control the use of antimicrobials in a medical-surgical ICU. However, new studies that allow control of confounding variables are needed to confirm this hypothesis.
Received on 11 June 2003
revised 29 September 2003
- 1. Kunin C.M. Problem of antibiotic usage. Definitions, causes, and proposed solutions. Ann Intern Med 1978; 89(5 Pt 2 Suppl):802-5.
- 2. Fishman N.O. Antimicrobial management and cost containment. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and practices of infectious diseases. Florida: Churchill Livingstone, Inc., 2000:540-6.
- 3. John J.F., Jr., Fishman N.O. Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital. Clin Infect Dis 1997;24(3):471-85.
- 4. Gomez J., Conde Cavero S.J., Hernandez Cardona J.L., et al. The influence of the opinion of an infectious disease consultant on the appropriateness of antibiotic treatment in a general hospital. J Antimicrob Chemother 1996;38(2):309-14.
- 5. Fowler V.G., Jr., Sanders L.L., Sexton D.J., et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: Experience with 244 patients. Clin Infect Dis 1998;27(3):478-86.
- 6. Byl B., Clevenbergh P., Jacobs F., et al. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin Infect Dis 1999;29(1):60-6.
- 7. Fluckiger U., Zimmerli W., Sax H., et al. Clinical impact of an infectious disease service on the management of bloodstream infection. Eur J Clin Microbiol Infect Dis 2000;19(7):493-500.
- 8. Pereira M.G. Epidemiologia. Teoria e Prática. 1st ed. Rio de Janeiro: Guanabara Koogan, 1995
- 9. Fridkin S.K., Steward C.D., Edwards J.R., et al. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: Project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis 1999; 29(2):245-52.
- 10. National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992-June 2002, issued August 2002. Am J Infect Control 2002;30(8):458-75.
-
11Epi Info version 6: A word-processing, database, and statistics program for public health on IBM-compatible microcomputers. Atlanta, Georgia, U.S.A.: Centers for Disease Control and Prevention, 1995
- 12. McGowan J.E., Jr. Do intensive hospital antibiotic control programs prevent the spread of antibiotic resistance? Infect Control Hosp Epidemiol 1994;15(7):478-83.
- 13. Frank M.O., Batteiger B.E., Sorensen S.J., et al. Decrease in expenditures and selected nosocomial infections following implementation of an antimicrobial-prescribing improvement program. Clin Perform Qual Health Care 1997;5(4):180-8.
- 14. Quale J., Landman D., Saurina G., et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23(5):1020-5.
- 15. Rahal J.J., Urban C., Horn D., et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella JAMA 1998;280(14):1233-7.
- 16. Bhavnani S.M. Benchmarking in health-system pharmacy: Current research and practical applications. Am J Health Syst Pharm 2000; 57(Suppl 2):S13-S20.
- 17. Gross R., Morgan A.S., Kinky D.E., et al. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis 2001; 33(3):289-95.
- 18. Gross P.A., Barrett T.L., Dellinger E.P., et al. Quality standard for the treatment of bacteremia. The Infectious Diseases Society of America. Infect Control Hosp Epidemiol 1994;15(3):189-92.
- 19. Camp R.C., Tweet A.G. Benchmarking applied to health care. Jt Comm J Qual Improv 1994;20(5):229-38.
-
20http://www.sph.emory.edu/ICARE/Phase3Appendix.htm (accessed 09/02/2003).
Publication Dates
-
Publication in this collection
22 Jan 2004 -
Date of issue
Oct 2003
History
-
Received
11 June 2003 -
Reviewed
29 Sept 2003