Abstract
The objective of this study was to identify the frequency of coinfection by human papillomavirus (HPV) and Chlamydia trachomatis (CT) in cervical lesions and relate it with immunohistochemical expression of p16INK4a and Ki67, both oncogenicity markers. A cross-sectional study with 86 women from primary care units in southern Brazil was conducted. Cervical swabs were collected for HPV-DNA and CT-DNA detection, through the polymerase chain reaction technique (PCR). The immunohistochemical analysis was performed on biopsy cervical tissue material to identify the expression of p16INK4a and Ki67 cell cycle markers. About 83 % were positive for HPV-DNA and 19% had coinfection with CT-DNA. Among coinfected women, 56% expressed p16INK4a. There was a statistically significant association between the histological grade of the lesion and Ki67 expression. All high-grade lesions, 50% of low-grade lesions and 31% of negative biopsies expressed Ki67 (p = 0.004). A total of 37% of coinfected women expressed both markers. In conclusion, although more than half of the coinfected patients have expressed p16INK4a and more than one third have expressed both markers, these results suggest no association between those variables. However, other studies involving larger samples are necessary to corroborate such findings.
epidemiology; human papillomavirus 16; biomarkers; pharmacological
ORIGINAL ARTICLE
Chlamydia trachomatis and human papillomavirus coinfection: association with p16INK4a and Ki67 expression in biopsies of patients with pre-neoplastic and neoplastic lesions
Luciane Noal CalilI; Cristine Nascente IgansiII; Luise MeurerIII; Maria Isabel Albano EdelweissIV; Mary Clarisse BozzettiV
IPhD; Professor, Post-graduate Program in Medical Sciences, Faculty of Pharmacy, Post-graduate Program in Epidemiology at Universidade Federal do Rio Grande do Sul - UFRGS; Hospital de Clínicas de Porto Alegre - HCPA, Porto Alegre, Brazil
IIMSc, PhD; Brazilian Ministry of Health Epidemiologist
IIIMD; Pathologist, HCPA, Porto Alegre, Brazil
IVMD, PhD; Associated Professor, UFRGS, Porto Alegre, Brazil
VMD, MSc, PhD; Associated Professor, UFRGS, Porto Alegre, Brazil
Correspondence to Correspondence to: Luciane Noal Calil Avenida Ipiranga, 2752, 3º Andar, Laboratório 304D 90610-000, Rio Branco, Porto Alegre, RS Fax: +55 51 33085437 Phone: +55 51 33085276 luciane1011@gmail.com
ABSTRACT
The objective of this study was to identify the frequency of coinfection by human papillomavirus (HPV) and Chlamydia trachomatis (CT) in cervical lesions and relate it with immunohistochemical expression of p16INK4a and Ki67, both oncogenicity markers. A cross-sectional study with 86 women from primary care units in southern Brazil was conducted. Cervical swabs were collected for HPV-DNA and CT-DNA detection, through the polymerase chain reaction technique (PCR). The immunohistochemical analysis was performed on biopsy cervical tissue material to identify the expression of p16INK4a and Ki67 cell cycle markers. About 83 % were positive for HPV-DNA and 19% had coinfection with CT-DNA. Among coinfected women, 56% expressed p16INK4a. There was a statistically significant association between the histological grade of the lesion and Ki67 expression. All high-grade lesions, 50% of low-grade lesions and 31% of negative biopsies expressed Ki67 (p = 0.004). A total of 37% of coinfected women expressed both markers. In conclusion, although more than half of the coinfected patients have expressed p16INK4a and more than one third have expressed both markers, these results suggest no association between those variables. However, other studies involving larger samples are necessary to corroborate such findings.
Keywords: epidemiology; human papillomavirus 16; biomarkers; pharmacological.
Each year, approximately 493,000 new cases of cervical cancer are diagnosed worldwide and approximately 273,000 women die from the disease, making it the second most frequent cancer in women and the second leading cause of cancer-related death worldwide among women 15 to 44 years old.1
Chlamydia trachomatis (CT) is the most common bacterial sexually transmitted infection (STI) worldwide.2 Bacterial coinfection by CT in women with a history of HPV infection has been studied as a potential factor that contributes to the development of malignacies and cervical cancer. Some authors3 have suggested that previous CT infection is associated with a high risk of developing the disease.
The p16INK4a is a cyclin-dependent kinase inhibitor (CDK) that reduces the cell cycle's speed by controlling the expression of E2F transcription factor. High-grade infection by HPV is involved in the carcinogenesis and, HPV integration in the human genome results in overexpression of E6 and E7 viral oncoproteins.4 E7 HPV protein interacts with retinoblastoma protein (pRb) and neutralizes the function of these proteins, resulting in the release of E2F transcription factor of the pRb-E2F complex. E2F accumulation induces p16INK4a activity.5
Results of a study6 showed that none of the negative cervical tissues were positive for p16INK4a, while there was a constant and significant increase in the positivity of this marker, according to histological grade, where cervical intraepithelial neoplasia (CIN) 1 had 31% positivity, 90% in CIN 2 and 100% in CIN 3 and carcinomas (p < 0.0001). Thus, p16INK4a overexpression has consistently shown high sensitivity (84%) and specificity (98%) to detect the high-risk HPV with a high positive predictive value (97%). Ki67 monoclonal antibody reacts with a non-histone nuclear protein that is expressed in the nucleus of proliferating cells through the cell cycle, except in G0 and in early G1 phase. Some authors7 have shown a relationship between Ki67, lesion severity or growth rate, and its utility in the study of vulvar and vaginal lesions caused by HPV. Therefore, the determination of Ki67 seems to be a relevant additional and complementary examination in the detection and distinction of different lesion grades of the uterine cervix.
It is known that high-risk HPV, through oncoproteins, can deplete the action of suppressor genes like the p53 and pRb, causing overexpression of CDK inhibitors. However, in coinfected patients (HPV/CT), this effect has not yet been studied. The purpose of this study was to verify p16INK4a and Ki67 immunohistochemical expression in women with positive HPV-DNA and/or CT-DNA who attended the primary health care services in southern Brazil.
This was a cross-sectional study that enrolled 86 women from primary care services, selected from participants of a cohort study8 started in February 2003. This sample included women who were tested for HPV-DNA/CT-DNA and performed uterine cervix biopsy for histopathological analysis. HPV-DNA and CT-DNA detection in uterine cervical samples were performed through the PCR technique. DNA extraction from material collected with cervical-vaginal swabs was carried out using the proteinase K protocol.9 The technique was directed to the L1 gene and the primers used were My09 and My11. PCR conditions were described by Bauer et al. (1993) and Coutlée et al. (2002). For the PCR reaction internal control, β-globin primers were used and tested in all samples.10,11 For CT-DNA, the technique was standardized by Becker D9 using the CTP1 and CTP2 primers that amplify a 201pb segment of the ORF number 4 of the cryptic Chlamydia trachomatis plasmid. This segment is situated 2940pb from the single BamHI restriction site:
CTP1 5'... TAGTAACTGCCACTTCATCA... 3'
CTP2 5'... TTCCCCTTGTAATTCGTTGC... 3'
In order to control the reactions, along with each DNA PCR of clinical samples, a positive control was tested, which consisted of a 450bp fragment corresponding to Caski and Siha cells, a 201bp fragment corresponds to cells infected by CT; and a negative control with the omission of any DNA. In all tested samples for HPV PCR and CT PCR, multiplex PCR were performed using specific primers for each microorganism and primers complementing the human β-globin gene (gH20 and PC04). β-globin primers were used to verify the viability of tested DNA. All positive samples in the HPV-DNA screening were tested for high risk (HR) HPV types (HPV16, 18, 31), using specific primers. The amplification conditions and the primers used are based on the methodology described by Cuzick et al.12 and are HPV-16 (5'...GC GAT CCT GTC TGC TTT TAT ACT AA...3' [sense]; 5'...AAG GCC AAC TAA ATG TCA C...3' [antisense]) and the temperature was 54ºC; HPV-18 (5'...TGC AGC ACG AAT GGC ACT GGC CTG...3' [sense]; 5'...CAC GGC GAC CCT ACA AGC TAC CTG...3' [antisense]), temperature was 70ºC. For HPV-31 (31A 5'...TACCTGTGTTTCTGTTAAC...3' [sense]; S5'...AGAAAGACCTCGGAAATTG...' [antisense])and the temperature was 52ºC.
The paraffin blocks of biopsy material have been stained by the hematoxylin-eosin technique for histological classification, as follows: LSIL, HSIL, where LSIL category included the results with CIN1, and HSIL the findings with CIN 2 and 3 diagnoses. Absence of malignant alterations was reported when no abnormalities were observed. Later, the cases were reviewed by trained pathologists (MIE and LM) from Hospital de Clínicas de Porto Alegre (HCPA), in Porto Alegre, southern Brazil.
The paraffin blocks were cut, deparaffinized in xylene and then rehydrated. Antigen retrieval was made in citrate buffer pH 6.0 and, later taken to microwave for 20 minutes. The sections were placed at room temperature and washed three times with phosphate buffer. After the endogenous peroxidase blockage, the sections were rehydrated and the slides were submitted to immunohistochemistry process.
The antibodies used were p16INK4a, "Neomarkers, CA, USA", clone 16P07 at a dilution of 1:75 and antiKi67, "DAKO, Glostrup, Denmark", clone MIB-1, at a dilution of 1:30. The p16INK4a pattern expression for squamous intraepithelial lesions (SIL) was graduated as suggested in Bulten J. et al.:13 negative (-) if none of the cells were colored; positive (+, weak) if the percentage of expressing cells ranged between 1-25%; (++, moderate) if the percentage ranged between 26-75%; and (+++, strong) if over 75% of the cells were colored, both in the cytoplasm and nucleus. The cells' expression pattern was divided into focal if the expression was concentrated in some areas, and diffuse if the expression was distributed throughout the slide.
Ki67 was considered positive if the cell's nucleus was brown-colored. In all samples, 100 to 200 cells were counted and the results were expressed in percentage. All samples were evaluated by two pathologists (MIE e LM) and, when necessary, they reached a consensus.
The study outcomes were genital infection by HPV and/or Chlamydia trachomatis and histopathological diagnosis of uterine cervical lesions. The relation of p16INK4a and Ki67 expression in cervical lesions of women infected by HPV and Chlamydia trachomatis p16INK4a and Ki67 expressions to such outcomes were assessed. The independent variables studied included demographic characteristics, sexual behavior, reproductive data and previous morbidities. Data was stored in a database by double-entry, where 20% of the questionnaires were selected at random and compared to the original. Possible errors and discrepancies were corrected.
Chi-square test or Fisher's exact test when indicated were used to compare categorical variables. Continuous variables were analyzed using the student's t test and ANOVA. The findings with p-value < 0.05 were considered statistically significant. Sensitivity, specificity and the positive and negative predictive values for both markers, using the histological results as gold-standard, were calculated with the correspondent 95% confidence intervals.
A total of 86 patients were analyzed, among which 83% (71/86) were positive for HPV-DNA and 19% (16/86) for CT-DNA. All women with positive CT-DNA were also positive for HPV-DNA (p = 0.06). About 40% (34/86) were positive for HR-HPV, among which 37% (6/34) were positive for CT-DNA. The most frequent HR-HPV was HPV-16 (50%), followed by HPV-31 (38%) and HPV-18 (12%).
Table 1 describes the characteristics of women who presented coinfection (HPV-DNA and CT-DNA). There was noted a statistically significant association between coinfection and education level (p = 0.04).
No statistically significant association was observed between lesion histology and coinfection (p = 0.75).
In the immunohistochemical analysis, 56% of the coinfected patients expressed p16INK4a, (p = 0.38), which ranged from moderate to strong in approximately 37% and the expression pattern was diffuse in 50%. In HPV-DNA positive biopsies, 78% expressed p16INK4a, ranging from moderate to strong in 54% and with a diffuse pattern in 56%. Regarding histology, 83% of the high-grade lesions expressed p16INK4a. Among low-grade lesions and negative biopsies, 72% and 60% expressed this protein, respectively (p = 0.43).
About 50% (8/16) of coinfected women expressed Ki67 antibody. In HPV infected women, this antibody was present in 38% (p = 0.73).
There was a statistically significant association between the grade of lesion histology and Ki67 expression, which was expressed in 100% of high-grade lesions, 50% of low-grade lesions and 31% of negative biopsies (p = 0.004).
Approximately 37% (p = 0.31) of coinfected women and 29% (p = 0.16) of those without coinfection expressed both markers.
In relation to lesion histology among coinfected patients, the sensitivity of p16INK4a was 25% (95%CI: 0.7-49.3%), the specificity, 72.9% (95%CI: 61.7- 84%), PPV, 17.4% (95%CI: 0-35%) and NPV of 81% (95%CI: 70.5-91.40%). Regarding Ki67, the sensitivity was 50% (95%CI: 23-78.0%), specificity, 66% (95%CI: 54-79%), PPV de 25% (95CI%: 8.4-42%), and NPV 85% (95%CI: 75-96%).
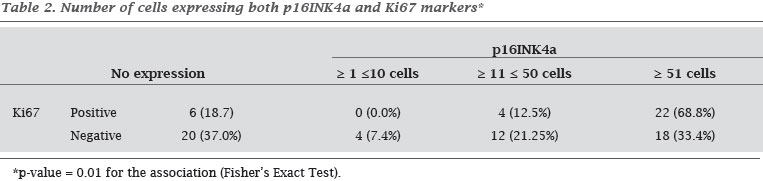
Table 2 describes the distribution of the number of cells (%) that expressed both p16INK4a and Ki67 markers.
Studies have shown that the HPV is an important factor for the development of cervical precursor lesions and cancer; however, cofactors such as age, use of oral contraceptives, other hormones, parity, smoking and previous exposure to other sexually transmitted agents, such as Clamydia trachomatis and herpes simplex virus 2, have been mentioned.1,14
CT is necessarily an intracellular agent that infects epithelial cells of the genital tract and the ocular tissue. It is one of the most common infection worldwide with 89 million new cases/year.15 Asymptomatic infection is common, but nonetheless it is considered as a cause of morbidity in sexually active women, causing severe sequelae such as pelvic inflammatory disease, ectopic pregnancy and tubal infertility. It is also associated with hypertrophy and induction of metaplasia, suggesting that it may have a role in cervical cancerogenesis.15
In addition, there are evidences that HPV-infected women present a higher risk to contract another infection, like CT infection.3 In the present study, the frequency of HPV infection was of 83% and CT infection 19%. These rates are higher than previous reports,16 possibly because this was a high risk sample, provided that every women had undergone cervical biopsy. A previous report16 found an 8% prevalence of CT infection and significantly associated with high risk HPV (OR = 2.5, p < 0.005), especially in women over 41 years old.
Education was significantly associated with coinfection in the present study. It maybe suggested that education may interfere in the level of knowledge about risk factors, exposure and information about means of prevention. A higher risk of infection was also observed in the HPV-infected patients.15 In relation to the viral types tested, the present study shows that HPV-16 was the most frequent high-risk viral type, which is in agreement with other authors.15
A study17 that enrolled 5,328 women, aged between 15-44 years, in eight countries presented a CT infection prevalence of 0.2% (95%CI: 0.0-0.7%) in Spain and 5.6% (95%CI 3.4-7.8%) in Nigeria, being higher in the age range between 15-24 years (4.5, 95%CI: 3.4-8.0%) than the age range of 25-44 years (2.6, 95%CI: 2.1-3.1). The same investigators17 believe that such prevalence data are low and do not express the reality for high-risk women.
De Paula et al.18 investigated the presence of CT-DNA and HPV-DNA in 250 cervical-vaginal samples of normal patients or patients with cytological alterations. Among them, 70% were negative, 40% were HPV-DNA positive and 5.2% had a positive diagnosis of CT infection. Among samples with abnormal cytology, HPV-DNA was detected in 73.3% and CT-DNA, in 9.33%. The higher coinfection rate, 15.4%, was found in samples diagnosed as atypical squamous cells/glandular cells of undetermined significance. An association between HPV infection and cytological alterations was verified; however, for CT infection or coinfection, such association was not observed. Taking that into account and relating it to the results found in the present study, it can be suggested that the association was not verified due to the low number of CT cases. However, it can be noticed that only 4/16 (25%) of the cases had coinfection and normal histology.
In a study19 with 114 cases, where 72 were incident and 42 were prevalent at the study entrance, HPV-16 seropositivity was associated with cervical cancer (OR = 6.33, 95% CI: 3.45-11.62). In general, the CT infection was not associated with the cervical cancer; however, there was an association when incidental cases were evaluated (OR = 2.94, 95% CI: 1.16-8.47).19
The p16INK4a expression has been mentioned5,6 as a promising marker for HR-HPV-induced lesions. However, studies did not evaluate if its expression is increased in HPV and CT coinfection. In HPV positive patients, 78.2% expressed the protein, where the intensity was moderate to strong in 54.5% of the cases and the pattern was diffuse in 56.4%, as expected. The p16INK4a appeared as overexpressed by the interaction of high-risk HPV E7 oncoprotein and the pRb, resulting in the release of the E2F transcription factor. This interaction is analogous to the CDK-mediated phosphorylation, resulting in the p16INK4a inhibitor accumulation. However, among patients with coinfection in the present study, the p16INK4a expression occurred in 56.3%.
Ki67 is a proliferation antigen that has been related to carcinogenesis in different tumor types. High-risk HPV infections and cell cycle alterations with higher Ki67 expression have been mentioned.9,10 CT infection has been suggested as a risk factor for HPV and, consequently for carcinogenesis.3,14 The objective of the present study was to verify if coinfection increases proliferation, through Ki67 determination in relation to HPV infection, provided that studies verifying this association were not found in literature. In this study, Ki67 was expressed in 50% of the coinfected cases and in 34% of the positive cases of HPV-DNA.
Through this immunohistochemical analysis, we had intended to investigate if some of these markers or the combination of both could suggest a larger association in the patients with coinfection. No significant difference was found in the p16INK4a and Ki67 expression in patients with coinfection, in relation to HPV-DNA positivity alone. Just like the results observed by Fehér and Szalmáz,20 our findings suggest that the presence of CT infection does not seem to be associated with cervical carcinogenesis. Thus, although more than a half of the coinfected patients expressed p16INK4a and more than one third expressed both markers studied, the results suggest no association between these variables. However, other studies involving larger samples are necessary to corroborate such findings in a more conclusive manner.
Submitted on 8/10/2010
Approved on: 12/10/2010
Financial Support: FIPE/ HCPA and CNPq
We declare no conflict of interest.
- 1. Velicer C, Zhu X, Vuocolo S et al. Prevalence and incidence of HPV genital infection in women. Sex Transm Dis 2009; 36(11):696-703.
- 2. Bilardi FJ, Fairley CK, Hopkins CA et al. Experiences and Outcomes of partner notification among men and women recently diagnosed with Chlamydia and their views on innovative resources aimed at improving notification rates. Sex Transm Dis 2010; 37(4):253-258.
- 3. Wallin KL, Wiklund F, Luostarinen T et al. A population-based prospective study of Chlamydia trachomatis infection and cervical cancer carcinoma. Int J Cancer 2002; 101(4):371-374.
- 4. Doeberitz VK. New markers for cervical dysplasia to visualise the genomic chaos created by oncogenic human papillomavirus infections. Eur J Cancer 2002; 38:2229-2242.
- 5. Queiroz C, Silva TC, Alves VAF et al. p16INK4a and Ki67 expression as a potential prognostic marker in ceical pre-neoplastic and neoplastic lesions. Pathology Research and Practice 2006; 202:77-83.
- 6. Benevolo M, Mottolese M, Marandino F et al. Immunohistochemical expression of p16INK4a is predictive of HR-HPV infection in cervical low-grade lesions. Mod Pathol 2006; 19(3):384-391.
- 7. Sarian LO, Derchain SF, Yoshida A et al. Expression of cycloxygenase-2 (COX-2) and Ki67 as related to disease severity and HPV detection in squamous lesions of the cervix. Gynecologic Oncology 2006; 102(3):537-541.
- 8. Rosa MI, Fachel JM, Rosa DD et al. Persistence and clearance of human papillomavirus infection: a prospective cohort study. Am J Obstet Gynecol. 2008 Dec;199(6):617. e1-7. Epub 2008 Sep 16.
- 9. Becker D. Detecção de Chlamydia trachomatis em amostras cervicais por Reação em cadeia da Polimerase (Dissertação de Mestrado). Porto Alegre: Universidade Federal do Rio Grande do Sul, Programa de Pós-Graduação em Biologia Celular e Molecular. 2005. P. 90.
- 10. Bauer HM, Hildesheim A, Schiffman MH et al. Determinants of genital human papillomavirus infection in low-risk women in Portland, Oregon. Sex Transm Dis 1993; 20(5):274-8.
- 11. Coutlee F, Gravitt P, Kornegay J et al. Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol 2002; 40(3):902-7.
- 12. Cuzick J, Terry G, Ho L et al. Type-specific human papillomavirus DNA in abnormal smears as a predictor of high-grade cervical intraepithelial neoplasia.Br J Cancer 1994; 69(1):167-71.
- 13. Bulten J, van der Avoort IA, Melchers WJ et al. p14ARF and p16INK4a, two products of the same gene, are differently expressed in cervical intraepithelial neoplasia. Gynecol Oncol 2006; 101(3):487-494.
- 14. Bosh FX, de San Jose S. The epidemiology of human papilomavirus infection and cervical cancer. Dis Markers 2007; 23(4):213-17.
- 15. De Lucca G.D, Marin H, Schelover E et al. Infeccion por Chlamydia trachomatis y Papilomavirus en mujeres con alteraciones citohistologicas de cuello uterino. Medicina (Buenos Aires) 2006; 66:303-306.
- 16. Denks K, Spaeth EL, Jérs K et al. Coinfection of Chlamydia trachomatis, Uresplasma urealyticum and human papillomavirus among patients attending STD clinics in Estonia. Scand J Infect Dis 2007; 39(8):714-8.
- 17. Franceschi S, Smith JS, Van den Brule A et al. Cervical infection with Chlamydia trachomatis and Neisseria gonorrhoeae in women from ten areas in four continents. A cross-sectional study. Sex Transm Dis 2007; 34(8):563-9.
- 18. De Paula FD, Fernandes AP, Carmo BB et al. Molecular detection of Chlamydia trachomatis and HPV infections in cervical samples with normal and abmormal cytopathological findings. Diagn Cytopathol 2007; 35(4):198-202.
- 19. Naucler P, Chen NC, Persson K et al. Seroprevalence of Human Papillomaviruses and Chlamydia trachomatis and cervical cancer risk: nested case-control study. J Gen Virol 2007; 88(3):814-22.
- 20. Fehér E, Szalmás A. Prevalence of Chlamydia trachomatis and oncogenic human papillomavirus types in cytologic atypia of the uterine cervix. Acta Microbiol Immunol Hung 2006; 53(4):479-87
Correspondence to:
Publication Dates
-
Publication in this collection
06 Apr 2011 -
Date of issue
Apr 2011
History
-
Received
10 Aug 2010 -
Accepted
10 Dec 2010