Abstract
Human herpesvirus type 6-(HHV-6) has been associated with morbidity after liver transplantation. OBJECTIVE: The aim of this study was to determine the HHV-6 seroprevalence among donor-recipient pairs, analyze the incidence of early active infection, its clinical manifestation, interaction with CMV, and the related morbidity in the first year after kidney transplantation. METHODS: 46 donor-recipient pairs had IgG evaluated by ELISA before transplantation: HHV-6(Pambio - USA) and CMV-(Roche - USA). A frozen whole blood sample collected weekly (from the 1st to the 6th week) was retrospectively tested for HHV-6 viral load (VL) determination by real time quantitative PCR (qPCR, Nanogen - Italy). Patients were preemptively surveyed for CMV by pp65 antigenemia (Ag, APAAP, immunohistochemistry, Biotest - Germany) from the 4th to the 12th week after transplantation. Active infection was defined as qPCR-HHV6+ (viral-load/mL-VL) and Ag+ (+cells/100.000 granulocytes), for HHV-6 and CMV, respectively. DCMV was defined as simultaneous positive antigenemia and suggestive signs/symptoms. Concerning +qPCR-HHV6, associated factors, clinical manifestation, interaction with CMV and morbidity were searched. RESULTS: Pre-transplant HHV-6 seroprevalence was significantly higher among kidney recipients compared to their donors (82.6x54.8%; p = 0.005 [3.9 (1.4-10.4)]). Active infection by this virus occurred in 26.1% (12/46), with no association with previous IgG (p = 0.412). Median VL was 125 copies/mL (53-11.264), and the median Ag was 21 +cells (2-740). There was no association between HHV-6 and CMV activation after transplantation (p = 0.441), neither concerning DCMV (p = 0.596). Median highest Ag+ and days of ganciclovir treatment were similar between qPCR-HHV6 + or - (p = 0.206 and p = 0.124, respectively). qPCR-HHV6+ was associated with higher incidence of bacterial (p = 0.009) and fungal (p = 0.001) infections, and higher number (p = 0.001) of hospital admission and longer duration of hospitalization over the first 6 and 12 months post-transplantation (p = 0.033 and p = 0.001). CONCLUSION: Latent HHV-6 infection is more common among recipients than donors before transplantation. Early active infection by this pathogen after transplantation does not increase DCMV incidence or severity during the first 3 months of follow-up. However, early HHV-6 replication is associated with other infections and hospitalizations in the first year.
Cytomegalovirus infections; Herpesvirus 6; DNA virus infections; Clinical diagnosis; Polymerase chain reaction
ORIGINAL ARTICLE
Early HHV-6 replication is associated with morbidity non-related to CMV infection after kidney transplantation
Regina Barbosa Schroeder* * Corresponding author at: Laboratório de Imunologia de Transplantes, Hospital Dom Vicente Scherer, Av. Independência 75, Porto Alegre, RS, 94035-075, Brazil E-mail address: rbs_schroeder@hotmail.com (Regina Barbosa Schroeder) ; Tatiana Ferreira Michelon; Gabriela Garbin; Valter Garcia; Janaina Gomes da Silveira; Luciano Santos; Jorge Neumann; Elizete Keitel
Complexo Hospitalar Santa Casa de Misericórdia de Porto Alegre, Universidade Federal de Ciências Médicas de Porto Alegre, Porto Alegre, RS, Brazil
ABSTRACT
Human herpesvirus type 6-(HHV-6) has been associated with morbidity after liver transplantation.
OBJECTIVE: The aim of this study was to determine the HHV-6 seroprevalence among donor-recipient pairs, analyze the incidence of early active infection, its clinical manifestation, interaction with CMV, and the related morbidity in the first year after kidney transplantation.
METHODS: 46 donor-recipient pairs had IgG evaluated by ELISA before transplantation: HHV-6(Pambio - USA) and CMV-(Roche - USA). A frozen whole blood sample collected weekly (from the 1st to the 6th week) was retrospectively tested for HHV-6 viral load (VL) determination by real time quantitative PCR (qPCR, Nanogen - Italy). Patients were preemptively surveyed for CMV by pp65 antigenemia (Ag, APAAP, immunohistochemistry, Biotest - Germany) from the 4th to the 12th week after transplantation. Active infection was defined as qPCR-HHV6+ (viral-load/mL-VL) and Ag+ (+cells/100.000 granulocytes), for HHV-6 and CMV, respectively. DCMV was defined as simultaneous positive antigenemia and suggestive signs/symptoms. Concerning +qPCR-HHV6, associated factors, clinical manifestation, interaction with CMV and morbidity were searched.
RESULTS: Pre-transplant HHV-6 seroprevalence was significantly higher among kidney recipients compared to their donors (82.6x54.8%; p = 0.005 [3.9 (1.4-10.4)]). Active infection by this virus occurred in 26.1% (12/46), with no association with previous IgG (p = 0.412). Median VL was 125 copies/mL (53-11.264), and the median Ag was 21 +cells (2-740). There was no association between HHV-6 and CMV activation after transplantation (p = 0.441), neither concerning DCMV (p = 0.596). Median highest Ag+ and days of ganciclovir treatment were similar between qPCR-HHV6 + or - (p = 0.206 and p = 0.124, respectively). qPCR-HHV6+ was associated with higher incidence of bacterial (p = 0.009) and fungal (p = 0.001) infections, and higher number (p = 0.001) of hospital admission and longer duration of hospitalization over the first 6 and 12 months post-transplantation (p = 0.033 and p = 0.001).
CONCLUSION: Latent HHV-6 infection is more common among recipients than donors before transplantation. Early active infection by this pathogen after transplantation does not increase DCMV incidence or severity during the first 3 months of follow-up. However, early HHV-6 replication is associated with other infections and hospitalizations in the first year.
Keywords: Cytomegalovirus infections; Herpesvirus 6, human; DNA virus infections; Clinical diagnosis; Polymerase chain reaction
Introduction
Viral infections are one of the major causes of morbidity and mortality after organ and tissue transplants. Besides the etiological agent, the risk of a viral infection depends on the pathogen epidemiology and host's immunity. Because transplants imply the use of immunosuppressant drugs to avoid graft rejection, the diagnosis of a viral infection relies on its kinetics and clinical suspicion, frequently before signs/ symptoms. One of the most studied families of virus in transplantation is the Herpesviridae, which encompasses eight different viruses. The majority of them are highly prevalent in the general population and shows an immunomodulatory effect.1-5
The deleterious role of the cytomegalovirus (CMV) after transplantation is well recognized, and its active replication is systematically checked. Initially, this active infection was associated with high mortality and morbidity.1-10 These were the reasons that brought about the current practice of early diagnosis and treatment in risk populations. Nowadays, the morbidity and cost related to the specific antiviral treatment are still major concerns. In addition, late recurrences of CMV, slow decrease of viral replication rate, or even drug resistance have concerned clinicians.11-15
CMV replication has been surveyed after kidney, kidney-pancreas, lung, liver, heart, and hematopoietic stem cell transplantations in order to avoid end-organ disease.3,13,15-21 In our hospital, a preemptive strategy for CMV was introduced in 1993 using antigenemia (Ag) from the 4th to 12th week post-solid organ transplantation and whenever there is clinical suspicion. Based on this, the cumulative incidence of probable CMV disease (pCMVD) in the first 3 months, among kidney recipients, has ranged from 27-38%, and severe cases have not been frequent.3,13
Nevertheless, there can be sporadic patients subjected to more than 21 days of intravenous ganciclovir; cases with low cellularity on Ag showing signs/symptoms; and, sometimes, unusual clinical manifestations for patients being preemptively surveyed (as severe bone marrow suppression or central nervous systems involvement). These observations raised the hypothesis that another viral agent could be implicated, such as HHV-6, which also has a known immunomodulatory potential.1,5,7,18-23
HHV-6, as other herpesviruses, can remain latent in the host's cells and reactivate as soon as the immunosuppression starts. Usual sites for latency after primary infection include salivary glands, lymph nodes, mononuclear cells, and liver and renal parenchyma.6 Clinically, HHV-6 causes a mononucleosis-like syndrome, lymphadenopathy, hepatitis, bone marrow suppression, interstitial pneumonitis, and severe focal encephalitis, well-reported in liver transplant recipients.4,7,8
Understanding that the epidemiology and the clinical role of the latent and early-active HHV-6 infection after kidney transplantation are not clear, we designed this study. The purpose was to determine HHV-6 seroprevalence in donor-recipient pairs, the incidence of early viral replication after kidney transplant, its clinical repercussion, interaction with CMV, and association with morbidity during the first year after transplantation.
Patients and methods
This was a cohort study that included all the adult kidney transplants performed between April and September/2002 in a tertiary hospital, which is a national reference for transplants (n = 46).
There, donor's and recipient's serology, collected before transplantation, were analyzed for latent infection determination. HHV-6 active infection was described as viral load (VL) measured by real time quantitative polymerase chain reaction (qPCR-HHV6) in peripheral blood collected between the 1st and 6th weeks and frozen at -80ºC.
Patients were surveyed preemptively, as routine, with serial CMV-Ag from the 4th to the 12th week post-transplantation. Intravenous ganciclovir was administered prophylactically during 14 days in CMV-IgG negative recipients (n = 3). CMV active infection was defined as CMV-Ag+, and pCMVD was defined as more than ten +cells on Ag independent of the signs/symptoms or increasing number of +cells combined with signs/symptoms, according to our previous study.13 Treatment was also performed using intravenous ganciclovir for 14 days or more, until Ag became negative.
Donor and recipient demographic data (age and gender) and transplant characteristics (donor source, isolated kidney/ simultaneous pancreas-kidney, cold ischemia time, initial immunosuppression, induction therapy and delayed graft function) were analyzed. Delayed graft function was defined as the necessity for dialysis in the first week post-transplantation.
Clinical and laboratory parameters studied that could be associated with HHV-6 included: total leukocytes and lymphocytes (1st-6th week), liver enzymes (aspartate- and piruvate-amminotransferase, 1st-12th week). Serum creatinine levels were evaluated as a graft function marker (1st-12th weeks, monthly until the 6th month, and annually until 4th year). Morbidity in the first year was evaluated by: biopsy-proven acute graft rejection, development of other infections (non-HHV-6 and non-CMV), hospital admission (number and duration), and graft loss and death. Information was taken from medical records.
The variables above described were compared, qualitative and quantitatively, as indicated, between patients who developed HHV-6 active infection (+qPCR-HHV6) and those who remained negative. In order to avoid a bias due to CMV infection, all comparisons were performed between positive and negative patients, as follows: a) HHV-6 active infection (qPCR-HHV6+), b) CMV active infection (CMV-Ag+) and c) active infection by both viruses (qPCR-HHV6 + CMV-Ag+) after transplantation, each one analyzed during its higher risk period.
For the study of HHV-6 latent and early replication effects upon CMV infection, the following associations were analyzed: a) incidence of CMV active infection, b) incidence of pCMVD and c) pCMVD severity (highest Ag+ and days of ganciclovir treatment).
This study was approved by the institutional Ethics Committee, and all patients included signed an informed consent.
HHV-6 and CMV serology
Immunoenzimatic test (ELISA) for specific IgG was performed in the sera of all donor-recipient pairs collected before the transplant, either for HHV-6 (Pambio - USA) and CMV (Roche - USA). Inconclusive results were repeated, and those that remained undetermined were excluded from the analysis (inconclusive HHV6-IgG: n = 4 donors).
CMV pp65 antigenemia (CMV-Ag)
Antigenemia was performed in blood samples collected with EDTA by immunohistochemistry method after granulocyte isolation. A monoclonal antibody (C10/C11) directed against pp65 CMV matrix protein was applied (APAAP, Biotest - Germany). This test gives a quantitative result, indicating the number of positive cells that represent those with viral replication (+cells/105 granulocytes).
HHV-6 quantitative real time PCR (qPCR-HHV6)
HHV-6 VL was determined by qPCR using DNA extracted (Invisorb, Spin Blood mini kit, Invitek - Germany) from whole blood collected with EDTA. A commercial kit for HHV-6 (HHV6Q-PCR Alert AmpliMIX, Nanogen - Italy) was used in an ABI Applied Biosystems 7300 device. This test is a multiplex reaction, including an internal control (human β-globin gene) simultaneously amplified with the HHV-6 ORF13R region as target. This region is common for both variants of HHV-6 (A and B). The standard-curve has four known quantitative points of VL (102,103,104, 105), allowing a precise quantification of the initial sample VL. The limit for detection is 40 copies/reaction. The provided software for analysis shows the following results: a) negative, b) < 2000 copies/mL and c) absolute number of copies when > 2000 copies/mL. For the purposes of this study, the following formula was applied to determine absolute number of copies in positive samples showing VL < 2000 copies/mL: cN (initial copy number in the sample) = Fe (unit for VL description in the sample; 1mL) x eE (extraction method equivalent efficiency; 1/1,0=1) x Fa (extracted and amplified DNA volume ratio; 200µL/5µL = 40) x number of copies (VL obtained in the reaction).
Statistical analysis
Variables were described as percentage, mean and standard deviation (SD), or median. Chi-square with Yates correction or Fisher's exact and Student's t test or Mann-Whitney was applied, as indicated, using the Statistical Package for Social Sciences (SPSS 14.0) software. p < 0.05 was considered significant, being described the respective relative risk (RR) and 95% confidence interval (95% CI).
Results
Pre-transplant HHV-6 and/or CMV latent infection
Prevalence of HHV-6 latent infection was significantly higher among recipients than donors (recipients: 82.6% [38/46] x donors: 54.8% [23/42], respectively; p = 0.005; RR = 3.9 [1.4-10.4]). CMV-IgG seroprevalence was similar between the groups at the time of transplantation (recipients: 93.5% [43/46] x donors: 84.8% [39/46]; p = 0.315).
Post-transplant HHV-6 and/or CMV active infection
HHV-6 active infection occurred in 26.1% (12/46) of the recipients during the first six weeks of transplantation. Primary infection was seen in 37.5% (3/8) of the HHV-6-IgG negative, and reactivation and/or reinfection in 23.7% (9/38) of the IgG+ patients (p = 0.412). Median VL was 125 copies/mL (53-11.264), mostly showing < 2000 copies/mL (83.3% [10/12]). HHV-6 latent infection did not significantly change the incidence of: a) HHV-6 active infection (IgG+: 23,7% [9/38] x IgG-: 37,5% [3/8]; p = 0,412); b) CMV active infection (HHV-6 IgG+: 76.0% [29/38] x IgG-: 75.0% [6/8]; p = 0.999) and c) pCMVD (HHV-6 IgG+: 42.0% [16/38] x IgG-: 50.0% [4/8]; p = 0.713).
There was also no association between early active replication of HHV-6 and later CMV-Ag+ (qPCR+: 66.7% [8/12] x qPCR-: 79.0% [27/34]; p = 0.441) or pCMVD (qPCR+: 50.0% [6/12] x qPCR-: 41.2% [14/34]; p = 0.596). Elevated CMV-Ag+ was also not associated with HHV-6 active infection, neither among patients who developed pCMVD (qPCR+: 93[6-600] x qPCR-: 53[15-740]+cells; p = 0.857) nor among those who did not develop (qPCR+: 7[3-11] x qPCR-: 5[2-12]+cells; p = 0.387). The mean duration of ganciclovir treatment for pCMVD was similar between patients who previously had + or - qPCR-HHV6 respectively (18.2+3.8 x 15.5+3.0 days; p = 0.124).
CMV viremia occurred in 76.1% (35/46) of the recipients. The CMV-IgG negative transplanted patients needed additional ganciclovir treatment, despite the intravenous prophylaxis with the same drug administered during the first 14 days post-transplantation. Among CMV-IgG+ patients, 74.4% (32/43) developed Ag+ (IgG-:100.0% x IgG+:74.4%; p = 0.999). pCMVD occurred in 43.5% (20/46), being 100.0% among those who had primary infection and 39.5% among those who reactivated and/or had reinfection after transplantation (IgG-:3/3 x IgG+: 17/43; p = 0.075). Median highest Ag+ was 21 +cells (2-740), being 65 +cells (6-740) and 5 +cells (2-12) among patients with or without pCMVD, respectively (p = 0.001).
Active infection by both viruses occurred in 17.4% (8/46) of the recipients during the first 3 months, but 15.2% (n = 7) became always negative for both, and 8.7% (n = 4) had only qPCR-HHV6+. Additional 58.7% (n = 27) of the sample had only CMV-Ag+.
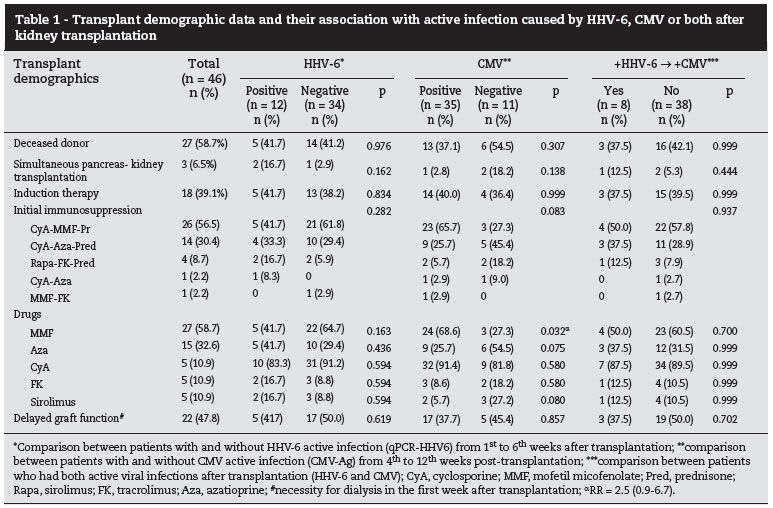
Associated factors with active viral replication after transplantation
Table 1 presents the general transplant characteristics and their association with active infection by HHV-6, CMV, or both, after kidney transplant. Mean age was similar between positive or negative qPCR-HHV6 after transplantation (qPCR+: 32.7 ± 13.9 x qPCR-: 39.1 ± 13.7 years; p = 0.178), and also between double positive HHV-6+CMV along the first weeks than the others (+:38.4 ± 13.6 x -:37.2 ± 14.2; p = 0.836). CMV-Ag positivity occurred more frequently among older patients at the time of transplantation (Ag+:40.4 ± 13.7 x Ag-:28.0 ± 10.0; p = 0.008). Gender (male 23/46, 50.0%) and cold ischemia time (21.5 ± 6.9 hours [12.2-36.0]) were not different between patients + or - for HHV-6, CMV or both infections (data not shown). Donor source, kidney or simultaneous pancreas-kidney transplant, induction therapy, and delayed graft function were not associated with HHV-6, CMV or both viral replications during the follow-up.
The distribution of different combinations of drugs for initial immunosuppression was not different between patients with or without active infection by HHV-6, CMV, or both. However, mofetil mycofenolate (MMF) appeared to be more frequently associated with the CMV replication (p = 0.032; RR = 2.5[0.9-6.7]).
Clinical outcomes related to the post-transplant active infections by HHV-6, CMV or both
Laboratory parameters analyzed in this study were not associated with HHV-6 infection, neither qPCR-HHV6+ nor HHV-6+CMV. Patients who had CMV-Ag+ showed higher serum creatinine at the following moments compared to those who remained negative for this virus: on the 6th week post-transplantation (1.9 ± 0.8 versus 1.3 ± 0.4 mg/dL; p = 0.038), on the 7th (1.7 ± 0.6 x 1.3 ± 0.4 mg/dL; p = 0.037), on the 10th (1.5 ± 0.5 x 1.2 ± 0.3 mg/dL; p = 0.034) and on the 12th (1.6 ± 0.5 x 1.2 ± 0.3 mg/dL; p = 0.018).
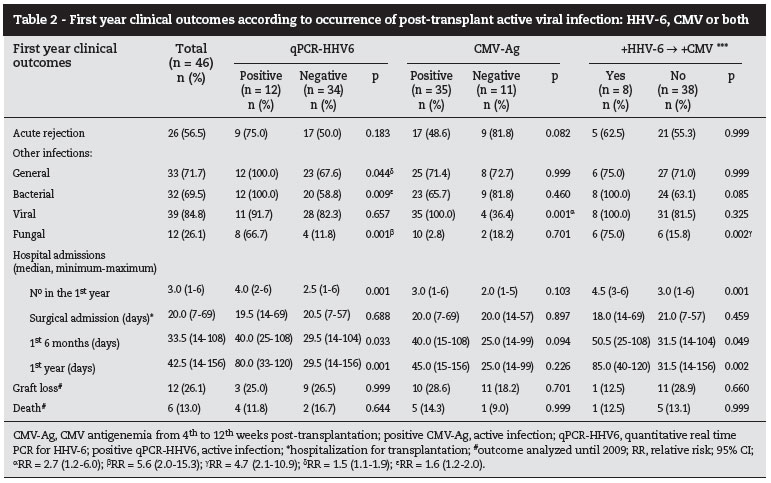
Table 2 describes first year post-kidney transplant clinical outcomes, presenting cumulative incidences and their distributions according to the early viral infection: HHV-6, CMV or both, respectively.
qPCR-HHV6+ was associated with higher incidence of other infections in the first year after transplantation (qPCR+: 100% x qPCR-: 67.6%; p = 0.044; RR = 1.5[1.1-1.9]), mainly caused by: bacteria (qPCR+: 100% x qPCR-: 58.8%; p = 0.009; RR = 1.6[1.2-2.0]) and fungi (qPCR+: 66.7% x qPCR-: 11.8%; p = 0.001; RR = 5.6[2.0-15.3]). Even though there was no case of deep mycosis, the only two cases that achieved HHV-6 VL > 2000 copies/mL presented fungal diseases and severe bacterial infections.
CMV viremia was associated with higher incidence of other viral infections (non-HHV-6 and non-CMV) (p = 0.000, RR = 2.7[1.2-6.0]). These other viral infections were not dependent on the MMF initial immunosuppression (MMF: 92.6% [25/27] x no MMF: 57.9% [14/19]; p = 0.107). In this series, viral infections (non-HHV6 and non-CMV) were diagnosed based on clinical and laboratory findings, including: human papillomavirus, simplex herpesvirus (types 1 and 2), varicella-zoster virus (both clinical presentations), upper respiratory viral infections, Verruca vulgaris, Molluscum contagiosum, and meningitis. Bacterial clinical syndromes observed along the follow-up included: piodermitis, wound infection, arteriovenous fistulae infection, cystitis, prostatitis, graft's acute pyelonephritis, perirenal abscess, sinusitis, bronchopneumonia, spontaneous bacterial peritonitis, and sepsis. Fungal infections were, respectively: genital candidiasis, Tinea cruris, dermatophytosis, and onicomycosis.
The single case of viral meningitis had no etiological definition and occurred in a diabetic recipient of simultaneous pancreas-kidney transplant. This patient had received daclizumab induction therapy and combined immunosuppression with sirolimus, tacrolimus and MMF. She developed pCMVD and several bacterial infections, some of them very severe, but never showed early qPCR-HHV6+ in the follow-up screening.
Duration of hospitalization after transplant surgery was similar for patients who did or did not develop HHV-6 and/or CMV replication thereafter. On the other hand, patients who had qPCRHHV6+ during the first 6 weeks after transplantation had more hospitalizations with longer duration at 6th and 12th months post-transplantation. These outcomes were not associated with CMV viremia. These results can be seen in Table 2.
Besides qPCR-HHV6+, viral (p = 0.001) and fungal infections (p = 0.002) were associated with more hospitalizations in the first year, whereas the use of initial immunosuppression including prednisone showed an inverse association (with prednisone: 2.9+1.4 x without: 5.5+0.7; p = 0.023). However, different infectious agents were associated with duration of hospitalization at 6 or 12 months, respectively: bacterial: p = 0.058 and p = 0.022; viral: p = 0.001 and p = 0.001; fungal: p = 0.004 and p = 0.011. pCMVD was an independent risk factor for bacterial and viral infection in univariate analysis bacterial: RR = 1.9[1.2-2.8]; p = 0.001 and viral: RR = 1.3[1.0-1.7]; p = 0.014).
Graft loss and death were not associated with the studied viral infections (HHV-6, CMV or both). In this series, patients returned to dialysis (n = 6; 13.0%) only after the fourth year of transplantation, all due to chronic allograft nephropathy. Among the other six (13.0%) patients who died during the study, three (50.0%) died during the first year: one had sepsis due to spontaneous bacterial peritonitis, the second had a cardiac arrest and the third died by external cause (trauma), in the presence of stable graft function.
Discussion
In the last decade, HHV-6 has become increasingly important, mainly as an emergent or co-pathogen in complex diseases. It is possible that transplants with higher immunological risk that have been performed using numerous new immunosuppressant agents could have contributed to the emergency of a new pathogen potentially dangerous to the graft or host.1,5,7,18,23 HHV-6 can be one of them, and can compromise the clinical outcome of the transplant. This virus has been studied especially among liver and hematopoietic stem cell recipients. Its role in kidney transplant is not yet clear.
Our healthy population represented by organ donors showed a marginally lower incidence of latent infection by this virus when compared to North American reports (55% x 59-100%).4,5,9,15,18 It was significantly lower than the rate observed among kidney transplant recipients immediately before engraftment, which could be explained by the relative immunosuppression conferred by chronic renal disease and hemodialysis.1,5 Nevertheless, the prevalence of HHV-6 latent infection before kidney transplantation in our study was similar to that described for kidney and liver recipients in other centers.22 It should be pointed out that HHV-6 serology before transplantation was not as helpful as CMV-IgG determination for predicting subsequent viral active infection, as suggested by others, needing confirmation with bigger studies.1,3,5,7,18
The incidence of HHV-6 active infection in the present study was also relatively lower (26%) than that observed in other solid organ transplant centers (31-50%).5,7,8,10,14 Nevertheless, the technical methodology applied and social and economic factors were not considered in these analyses. The time of diagnosis could be another difference. Strategically, we investigated HHV-6 active replication between the 1st and the 6th week post-transplantation because this is the period with greater risk. According to some authors, this is the period when the majority of primary infections are seen, usually developing into severe diseases with involvement of end-organs. This is also the risk period for secondary disease by HHV-6 virus, appearing generally from the 2nd to the 6th week post-transplantation, which was exactly what was observed in this study.1,2,11,17,18
No clinical or transplant-related factor appears to be clearly associated with early HHV-6 replication. However, as it was expected, prophylaxis or treatment with anti-CD3 has already been described as an independent risk factor.12,23
The clinical importance of HHV-6 among liver recipients is well documented, mainly because the major signs/ symptoms related to this infection are also the traditional presentation of graft dysfunction, imposing specific differential diagnosis. Besides hepatitis and liver graft loss, HHV-6 can cause meningoencephalitis due to its peculiar neuroinvasive potential and predisposition for opportunistic infections.1,9,17,20,22,24 Although acute onset of neurological symptoms is not common early after kidney transplant, HHV-6 might be an important differential diagnosis whenever a meningoencephalitis occurs after transplantation.1,4,5,11,18
Many authors have explored the indirect effects of HHV-6, mainly simultaneously with CMV infection, with contradictory results. Some of them described greater viral disease severity, while others did not find it.14-16,24,25 Recently, Humar et al. showed that co-infection with HHV-6 can compromise CMV disease outcomes treated with ganciclovir or valganciclovir, being associated with frequent recurrence of replication.23
In order to clarify if early HHV-6 infection could affect CMV active replication between the 4th and the 12th week post-kidney transplant, the present study showed that it did not increase the incidence of CMV infection nor its severity. Besides the sample size, the apparent low clinical impact of early HHV-6 replication among the studied population could result from the strict preemptive strategy on CMV monitoring and treatment. In this study, patients were followed under preemptive strategy for CMV according to the previously defined Ag cut-off of 10 positive cells for starting ganciclovir treatment. As many other viruses, CMV and HHV-6 can have similar clinical manifestations at the beginning, making it hard to tease out the role of each virus in a mild syndrome, especially under immunossuppresion. The stratification of the analysis and the combination of a sensitive and specific laboratory tool were applied to identify any independent effect of both viruses.13 It is probable that diagnosing or treating CMV infection only in symptomatic patients could better reveal a negative interaction of these viruses.3 This is supported by reports showing some degree of HHV-6 response to ganciclovir, even though no specific antiHHV-6 treatment is available until now.24,25
In the present study, every HHV-6 VL was considered significant, and it was associated with higher incidence of bacterial and fungal infections, besides more hospitalizations with longer duration during the first 6 and 12 months post-transplantation. It was not clear if these infections were cause or consequence of the longer hospitalizations. Clarifying this association is extremely valuable because, at least in liver transplantation, fungal infections have been the major cause of morbidity and mortality related to the HHV-6 infection.1,17,22 Even though there was no case of severe fungal infection among the studied kidney recipients justifying greater necessity for hospitalization, toxicity of antifungal drugs and/ or their pharmacological interaction with immunosuppressive drugs might be considered. Some drugs were also associated with other infections. The paradoxical effect of MMF was interesting, apparently increasing the risk of CMV and decreasing the risk of fungal infection. The independence of all these factors can only be checked by a multivariate analysis in studies with larger sample size.
The low immunological risk of the selected patient population (adults receiving a first graft) and the small sample size were limitations of the present study. However, the use of two different methodologies to evaluate HHV-6 and CMV, respectively was perhaps the major limitation. Both Ag and qPCR are well recognized for this purposes, but measure different aspects of the same condition - the viral replication.1,23,26-28 The way to attenuate this bias would be to apply a qualitative analysis, but it could still compromise the analysis of the association between two viral infections.
The clinical meaning of low viral activity has been difficult to interpret, mainly when the aim is to avoid signs/symptoms. Concerning the Ag, we previously determined that 10 positive cells is a risk for pCMV disease, suggesting that treatment would indicated.13 This cut-off of cells when analyzed by qPCR corresponds to > 7000 copies/mL of whole blood, quite similar to that which has been practiced by other centers worldwide. It is important to note that sometimes, at least for CMV, VL up to 3000 copies/mL can not be detected by Ag test.9 Then, it would be reasonable to consider that the low HHV-6 VLs found in this study (indicating low viral replication rate) could explain the benign presentation of the registered events. However, as of this moment there is no study defining clinically the HHV-6 VL level that could differentiate latency from active viral replication.29 Corroborating the presented results, an important study recently published by Humar et al. describes 31% of HHV-6 active infection among 253 patients under CMV disease treatment. They found a median highest VL of 281 copies/mL of whole blood, being seldom as high as 100.000 copies/mL.12,23
Finally, this study showed that early HHV-6 replication after kidney transplant appears to be weakly significant, even though it was associated with more bacterial and fungal infections, more hospital admissions and longer duration of hospitalization. These evidence suggest that HHV-6 is not an innocuous virus, and probably is a marker of excessive immunosuppression.4,15,16,24 Nevertheless, there is no reason for systematic follow-up of this virus, at least in patients under preemptive monitoring for CMV. Until now, there is no evidence for HHV-6 worsening CMV outcomes in the highest risk period, even though its interference with treatment outcome of pDCMV with ganciclovir should be confirmed in larger studies. HHV-6 was not associated with graft dysfunction or graft loss/death in the first year after kidney transplant.
Conclusion
Latent HHV-6 infection is highly prevalent (> 80%), being more common among kidney transplant candidates than healthy donors. The incidence of early active infection after transplantation is 26%. Despite not affecting CMV prognosis, it is associated with more bacterial and fungal infections and longer duration of hospitalization in the first 6 and 12 months.
Acknowledgements
This study received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflict of interest
All authors declare to have no conflict of interest.
Received 29 July 2011
Accepted 17 August 2011
- 1. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18(1):217-45.
- 2. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N. Engl J. Med. 2005;342:768.
- 3. Schroeder R, Michelon T, Fagundes I, et al. Cytomegalovirus disease latent and active infection rates during the first trimester after kidney transplantation. Transplant Proc. 2004;36:896-898.
- 4. Singh N, Peterson D. Encephalitis caused by human herpesvirus-6 in transplant recipients. Transplantation. 2000;69:2474-2479.
- 5. Brennan DC. Highlights From The Sixth Annual Ast Winter Symposium - Transplantation meets infection: microbes, rejection, atherosclerosis, and malignancy. Medscape Transplantation. 2002;3(1):1-14
- 6. Tianssheng C, Hudnall D. Anatomical mapping of human herpesvirus reservoirs of infection. Mod Pathol. 2006;19:726-737.
- 7. Lusso P. Human herpesvirus 6 (HHV-6). Antiviral Research. 1996;31:1-21.
- 8. Schmidt AC, Wilborn F, Weiss K, et al. A prospective study of human herpesvirus type 6 detected by polymerase chain reaction after liver transplantation. Transplantation. 1995;61:662.
- 9. Benito N, Moreno A, Pumarola M, et al. Virus del herpes humano tipo 6 y tipo 7 em receptores de transplante. Enferm Infecc Microbiol Clin 2003; 21(8):424-32.
- 10. Rogers J, Rohal S, Carrigan D, et al. Human herpesvirus-6 in liver transplant recipients. Transplantation. 2000;6:2566-73.
- 11. Ratnamohan V, Chapman J, Howse H. Cytomegalovirus and human herpesvirus 6 both cause viral disease after renal transplantation. Transplantation. 1998;66:877-82.
- 12. Desjardin JA, Cho E, Supran S, et al. Association of human herpesvirus 6 reactivation with severe cytomegalovirusassociated disease in orthotopic liver transplant recipients. Clin Infect Dis. 2001;33(8):1358-62.
- 13. Schroeder R, Michelon T, Fagundes I, et al. Antigenemia for cytomegalovirus in renal transplantation: choosing a cutoff for the diagnosis criteria in cytomegalovirus disease. Transplant Proc. 2005;37:2781-2783.
- 14. Lautenschlager I, Linnavuori K, Höckerstedt K. Human herpesvirus-6 antigenemia after liver transplantation. Transplantation. 2000;69:2561-66.
- 15. Reddy S, Manna P. Quantitative detection and differentiation of human herpesvirus 6 subtypes in bone marrow transplant patients by using a single real-time polymerase chain reaction assay. Biol Blood Marrow Transplant. 2005;11(7):530-41.
- 16. Visser AM, Van Doornum GJ, Coernelissen JJ, et al. Severe amnesia due to HHV-6 Encephalitis after allogenic stem cell transplantation. Eur Neurol. 2005;54(4):233-4.
- 17. Boutolleau D, Duros C, Bonnafous P, et al. Identification of human herpesvirus 6 variants A and B by primer-specific real-time PCR may help to revisit their respective role in pathology. J Clin Virol. 2006;35(3):257-63.
- 18. Komaroff Al, Jacobson S. Highlights from 5th international conference on HHV-6 and -7. Herpes. 2006;13(3):81-2.
- 19. Deborska-Materkowska D, Lewandowski Z, Sadowska A, et al. Fever, human herpesvirus 6 (HHV-6) seroconversion, and acute rejection episodes as a function of the initial seroprevalence for HHV-6 in renal transplant recipients. Transplant Proc. 2006;38:139-143.
- 20. Lautenschlager I, Harma M, Hockerstedt K, et al. Human herpesvirus-6 infection is associated with adhesion molecule induction and lymphocyte infiltration in liver allografts. J Hepatol. 2002;37(5):648-54.
- 21. Mendez JC, Dockrell DH, Espy MJ, et al. Human beta-herpesvirus interactions in solid organ transplant recipients. J Infect Dis. 2001;183(2):179-184.
- 22. Ohashi M, Sugata K, Ihira M, et al. Human herpesvirus 6 infection in adult living related liver transplant recipients. Liver Transpl. 2008;14:100-109.
- 23. Humar A, Asberg A, Kumar D, et al. An assessment of herpesvirus co-infection in patients with CMV disease: correlation with clinical and virologic outcomes. Am J Transplant. 2009;9:374-381.
- 24. Desjardin JA, Gibbons L, Cho E, et al. Human herpesvirus 6 reactivation is associated with cytomegalovirus and syndromes in kidney transplant recipients at risk for primary cytomegalovirus infection. J Infect Dis. 1998,178:1783.
- 25. Pacsa AS, Essa S, Voevodin A, et al. Correlation between CMV genotypes, multiple infections with herpesviruses (HHV-6,7) and development of CMV disease in kidney recipients in Kiwait. FEMS Immunol and Med Microbiol. 2003;35:125-130.
- 26. Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4:337-48.
- 27. Espy MJ, Uhl JR, Sloan LM, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165-256.
- 28. Gunson RN, Collins TC, Carman WF. Practical experience of high throughput real-time PCR in the routine diagnostic virology setting. J Clin Virol. 2006;35:355-67.
- 29. Schroeder R, Michelon T, Adamy R, et al. Infection after kidney transplantation: comparison between pp65 antigenemia and real time PCR. (resumo PO135). In: Programa e resumos: IX Congresso Luso Brasileiro de Transplantação. Porto: Portugal 2010.
Publication Dates
-
Publication in this collection
25 Apr 2012 -
Date of issue
Apr 2012
History
-
Received
29 July 2011 -
Accepted
17 Aug 2011