Abstract
OBJECTIVE: Compare the anti-T. gondii IgG titer between HIV-1 infected and non HIV-1 infected pregnant women and report three cases of congenital toxoplasmosis resulting from reactivation of infection during pregnancy of HIV-1 infected women. METHODS: This study was conducted among 2,270 pregnant women with chronic Toxoplasma gondii infection (absence of IgM and presence of IgG), including 82 HIV-1 infected and 2,188 non-infected women. RESULTS: The average anti-T. gondii IgG titer was 127 for the 2,188 non-HIV-1 infected women, and 227 for the 82 HIV-1-infected women (p = 0,007). These results suggested that higher anti-T. gondii IgG titers in HIV-1-infected pregnant women may not be indicative of an elevated risk for fetal infection. In this study three cases of congenital toxoplasmosis that resulted from infection reactivation during pregnancy of HIV-1-infected women were manifested by fetal death, symptomatic infection, and infant without symptoms, respectively. In two of these women, a ten-fold increase in IgG levels above used cutoff was observed (2,320 UI/mL and 3,613 UI/mL, respectively). In the third pregnant women anti-T. gondii IgG titers during pregnancy did not rise despite the occurrence of congenital toxoplasmosis (204; 198; 172 UI/mL). CONCLUSIONS: Congenital toxoplasmosis resulting reactivation of infection during pregnancy in the studied group leads us to believe that it is a public health problem, especially in our population, in which seroprevalence of T. gondii infections is high. These findings also suggest that special attention is necessary during pregnancy, because the serologic diagnosis may not be indicative of toxoplasmosis reactivation.
Toxoplasma; Toxoplasmosis, congenital; HIV infections
ORIGINAL ARTICLE
Toxoplasma gondii antibody profile in HIV-1-infected and uninfected pregnant women and the impact on congenital toxoplasmosis diagnosis in Rio de Janeiro, Brazil
Márcia Antunes FernandesI; Giovanni Inácio BatistaII; Juliano da Costa Silveira CarlosII; Ivete Martins GomesIII; Kátia Martins Lopes de AzevedoIII; Sérgio SetúbalIII; Solange Artimos de OliveiraIII; Luis Guilhermo Coca VelardeIV; Claudete Aparecida Araújo CardosoI* * Corresponding author at: Rua Marquês de Paraná, 303, Centro, Niterói, RJ, 24033-900, Brazil E-mail address: claudete@huap.uff.br (Claudete Aparecida Araújo Cardoso)
IMaternal and Child Department, Medical School, Hospital Universitário Antônio Pedro, Universidade Federal Fluminense, Niterói, RJ, Brazil
IIMedical School, Universidade Federal Fluminense, Niterói, RJ, Brazil
IIIInfectious and Parasitic Diseases, Hospital Universitário Antônio Pedro, Universidade Federal Fluminense, Niterói, RJ, Brazil
IVInstitute of Mathematics and Statistics, Universidade Federal Fluminense, Niterói, RJ, Brazil
ABSTRACT
OBJECTIVE: Compare the anti-T. gondii IgG titer between HIV-1 infected and non HIV-1 infected pregnant women and report three cases of congenital toxoplasmosis resulting from reactivation of infection during pregnancy of HIV-1 infected women.
METHODS: This study was conducted among 2,270 pregnant women with chronic Toxoplasma gondii infection (absence of IgM and presence of IgG), including 82 HIV-1 infected and 2,188 non-infected women.
RESULTS: The average anti-T. gondii IgG titer was 127 for the 2,188 non-HIV-1 infected women, and 227 for the 82 HIV-1-infected women (p = 0,007). These results suggested that higher anti-T. gondii IgG titers in HIV-1-infected pregnant women may not be indicative of an elevated risk for fetal infection. In this study three cases of congenital toxoplasmosis that resulted from infection reactivation during pregnancy of HIV-1-infected women were manifested by fetal death, symptomatic infection, and infant without symptoms, respectively. In two of these women, a ten-fold increase in IgG levels above used cutoff was observed (2,320 UI/mL and 3,613 UI/mL, respectively). In the third pregnant women anti-T. gondii IgG titers during pregnancy did not rise despite the occurrence of congenital toxoplasmosis (204; 198; 172 UI/mL).
CONCLUSIONS: Congenital toxoplasmosis resulting reactivation of infection during pregnancy in the studied group leads us to believe that it is a public health problem, especially in our population, in which seroprevalence of T. gondii infections is high. These findings also suggest that special attention is necessary during pregnancy, because the serologic diagnosis may not be indicative of toxoplasmosis reactivation.
Keywords: Toxoplasma; Toxoplasmosis, congenital; HIV infections
Introduction
Toxoplasmosis is an infection that is typically manifested as a nonspecific or asymptomatic presentation in immunocompetent individuals. The infection can remain latent after the acute phase and is characterized by the presence of the parasite within tissue cysts.1 In cases where the immune system is not able to control parasite replication, clinical disease may ensue and cause complications, especially visual and neurological.2 The most susceptible individuals to the more serious forms of the disease are fetuses and newborns of pregnant women who become infected during pregnancy, and immunosuppressed patients.3
The fetal transmission of toxoplasmosis occurs predominantly when the mother is infected during pregnancy, a situation where the diagnosis is usually made through serological tests. However, congenital toxoplasmosis may also be associated with reactivation of a chronic maternal infection, especially in HIV-1-infected and immunosuppressed women,4-11 or with reinfection by a different strain of Toxoplasma gondii.12,13
The diagnosis of reactivated T. gondii infection during pregnancy has been the subject of much controversy. Some studies have tried to correlate higher anti-T. gondii IgG serum titers with reactivation of the infection irrespective of the levels of CD4+ T-lymphocytes.5,14 However, recent research in southern Brazil has shown higher anti-T. gondii IgG levels in HIV-1-infected than in non-HIV-1-infected pregnant women. These results suggest that the increase in antibody titers (IgG) could be the result of polyclonal activation of B-lymphocytes, which are not parasite-specific.15 Therefore, the interpretation of such IgG titers is not necessarily indicative of a reactivated infection, as proposed by other authors.16-18
The objectives of this study were: 1) to compare anti-T.gondii IgG class antibody levels among HIV-1-infected and uninfected pregnant women previously exposed to the parasite; 2) to correlate anti-T. gondii IgG class antibody levels with the levels of CD4+ T-lymphocytes and HIV plasma viral load in HIV-1-infected pregnant women.
Patients and methods
This was a retrospective study conducted at the Hospital Universitário Antônio Pedro at Universidade Federal Fluminense (HUAP-UFF), Niterói, Rio de Janeiro, Brazil, from June 2002 to June 2009.
The entire pregnant population of this study received medical assistance at the public health system.
The inclusion criteria for this study were: 1) availability of serological tests for toxoplasmosis during the prenatal period or upon admission of the patient in labor to HUAP-UFF from June 2002 to June 2009; 2) presence of a serological profile compatible with chronic toxoplasmosis infection, defined as the presence of serum anti-T. gondii IgG class antibodies and the absence of anti-T. gondii IgM antibodies.
We compared anti-T. gondii IgG levels among HIV-1-infected and uninfected pregnant women.
The serological results for toxoplasmosis in non-HIV-1-infected pregnant women were taken from the patient record archives of the immunology sector of the HUAP-UFF Laboratory. For the HIV-1-infected pregnant women pertinent information (age, delivery date, confirmation of HIV diagnosis, anti-T.gondii IgG titers, CD4+ T-lymphocyte count and HIV plasma viral load) were taken from medical records at the Infectious and Parasitic Disease Service.
Among the HIV-1-infected pregnant women chronically infected with T. gondii, those who had their serology, CD4+ count and HIV-1 viral load determination collected at the same day were analyzed separately.
Children born to HIV-1-infected mothers received medical assistance at the Pediatric AIDS Clinic of the HUAP-UFF. As a routine service, serology for toxoplasmosis was performed during pregnancy or during the child's first consultation. Asymptomatic children who had anti-T. gondii IgG titer lower than the mother's were examined every two months until it turned negative. Symptomatic children and/or children with positive anti-T. gondii IgM and/ or toxoplasma IgG titer equal to or higher than the mother's, underwent transfontanellar ultrasound and a fundoscopic examination to confirm the diagnosis of congenital toxoplasmosis.
Serological diagnosis of toxoplasmosis in the pregnant women and children were performed using IgG and IgM class antibody determination. The laboratory technique employed for these tests was the enzyme linked fluorescent assay (ELFA) using the VIDAS System (bioMérieux - Lyon, France), with the following reference values for positive results: IgM > 0.65 and IgG > 8 UI/mL. Pregnant women were classified into three groups according to their serological profiles: 1) Susceptible: absence of anti-T. gondii IgG and IgM antibodies; 2) Acute infection: presence of anti-T. gondii IgM and IgG antibodies; 3) Chronic infection: presence of anti-T. gondii IgG antibodies and absence of anti-T. gondii IgM antibodies.
The database for the analysis was prepared using the Statistical Package for Social Sciences (SPSS) version 18.0 for Windows©. We used the Chi-square test (χ2) to compare the prevalence of chronic T. gondii infection among HIV-1 infected and uninfected pregnant women, as well as for comparing age groups and analyzing the influence of age on the prevalence of immunity in the two groups. We used the Spearman correlation coefficient to assess associations between the numerical variables. For the comparison between continuous variables in the two groups were used Student's t-test. A p-value less than 0.05 was considered statistically significant.
This study was approved by the Research Ethics Committee at the HUAP-UFF, under the number 0268.0.258.000-09.
Results
From June 2002 to June 2009, we evaluated 2,270 pregnant women, among whom 2,188 were not infected with HIV-1 and 82 had a confirmed diagnosis of HIV-1 infection. Fig. 1 describes the study population and the respective serological profile.
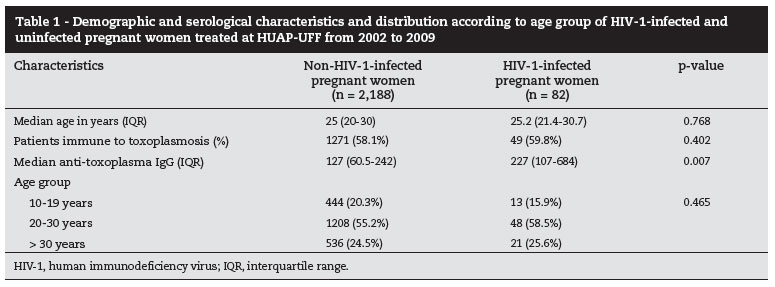
Table 1 shows the demographic and serological characteristics of the two groups of pregnant women studied, as well as their distribution according to age group.
Among the 49 HIV-1-infected patients with a chronic T. gondii infection, 38 had their serologies, CD4+ T-lymphocyte counts and HIV-1 plasma viral load collected on the same day. Within this group, the median anti-T. gondii IgG titer was 247 IU/mL (IQR: 116.8-807.8), the median CD4+ T-lymphocyte count was 330 cells/mm3 (IQR: 228-538) and the median plasma viral load of HIV-1 was 3,066 copies/mL (IQR: 80-13,500). Among these 38 pregnant women, anti-T. gondii IgG titers were neither significantly different among those with CD4+ T-lymphocyte counts below or above 100 cells/mm3 nor above or below 300 IU/mL (p = 0.0934). The IgG values considered were not absolute but a classification from a cut-off point (below or above 300 IU/mL). There was no significant difference of anti-T. gondii IgG titers of HIV-1-infected pregnant women between those with undetectable (below 80 copies/mL) and detectable HIV-1 viral load (p = 0.477).
Of the 49 fetuses born to HIV-1-infected mothers with chronic T. gondii infection, three children were diagnosed with congenital toxoplasmosis. Previously published data4 suggest that such unfavorable outcome resulted from reactivation of maternal T. gondii infection during pregnancy. The first pregnant woman had low titers of anti-T. gondii IgG antibody and lacked IgM antibody. At the 21st week of pregnancy, the IgG titers were 2,320 IU/mL (ELFA/VIDAS) with high IgG antibody avidity. At the 28th week, fetal death was confirmed. A histological study of the placenta and embryo confirmed the diagnosis of congenital toxoplasmosis.
In the second case, IgM antibodies were not detected and increasing IgG titers during pregnancy (364 IU/mL in the 20th week of pregnancy and 3,613 IU/mL in the 36th week) with high-avidity IgG antibodies were observed. The child was born with anti-T. gondii IgM antibodies and severe clinical manifestations of congenital toxoplasmosis and was treated during the first year of life.
In the third patient, at the 33rd week of pregnancy anti-T. gondii antibody test was inconclusive for the IgM class and positive for the IgG class (204 IU/mL). At the 36th week of pregnancy, uveitis caused by toxoplasmosis was diagnosed and specific treatment begun. On this occasion, T. gondii serology showed high avidity anti-T. gondii IgG antibody titers at 198 IU/mL in the absence of IgM (ELFA). The child was delivered at the 38th week and IgM and IgG (titer: 172 IU/mL - ELFA) anti-T. gondii antibodies were detected. During follow-up visits, the child was diagnosed with HIV-1 infection. Anti-retroviral treatment and toxoplasmosis treatments were initiated.
Discussion
This study demonstrates a similar prevalence of T. gondii infection in HIV-1-infected pregnant women when compared to uninfected women. The results are consistent with other studies that have demonstrated similar serological prevalence between the two groups, as well as in the general population.11,19-22
Persistence of high seroprevalence could reflect greater circulation of the parasite in our environment, leaving the pregnant women, already exposed, susceptible to reinfection, either by new strains or reactivation of the chronic infection.
There was no difference in the seroprevalence of toxoplasmosis among HIV-1-infected and uninfected pregnant women adjusted for age. Thus, the susceptibility to a primary infection was equal for both groups, which may be explained by the same regional origin of the groups of pregnant women.
In our study anti-T. gondii IgG titers were higher in the group of HIV-1-infected than uninfected pregnant women. This finding is similar to other reports where elevated anti-T.gondii IgG serological titers in HIV-1-infected patients were seen.15,23 However, other studies have not found any difference in anti-T. gondii IgG antibody titers.21,24,25 Several factors may explain these discrepancies, such as difference in age and/or study population. Among HIV-1-infected patients, hypergammaglobulinemia is common and seems to be caused by the activation of polyclonal B-cells. This may explain the higher anti-T. gondii IgG titers among HIV-1-infected patients without taking into account the duration of the infection or its reactivation state.16,18
This study found three HIV-1-infected pregnant women who were chronically infected with T. gondii and who transmitted toxoplasmosis to their fetuses during pregnancy. When we analyzed their respective serological profiles, these patients did not have anti-T. gondii IgM class antibodies during pregnancy, but only IgG antibodies. One of the pregnant women had a significant increase in anti-T. gondii IgG antibody titers during the course of her pregnancy. This finding may be related to reactivation of a latent infection and subsequent vertical transmission of the infection. Therefore, although the anti-T. gondii IgG antibody titers were typically elevated in HIV-1-infected pregnant women, monitoring these titers during pregnancy may be useful for the early detection of reactivation. However, the serological profiles in these pregnant women were different, and there were no large variations in the anti-T. gondii IgG class antibody titers. In one pregnant woman, reactivation of ocular lesions was caused by toxoplasmosis. This finding is consistent with reports of vertical transmission of the infection as a result of ocular toxoplasmosis reactivation.26,27 The reactivation of ocular lesions caused by toxoplasmosis in the absence of IgM antibodies and the presence of anti-T. gondii IgG antibodies are suggestive of infection reactivation as opposed to reinfection with another strain.28 Three cases of congenital toxoplasmosis were identified. In the first case, fetal death occurred in the 28th week of pregnancy. Fetal autopsy showed hepatosplenomegaly, anasarca, pericarditis and myocarditis. In the second case, the infant showed chorioretinal scars, intracranial calcification on brain CT scan and neurodevelopment delay; and in the last case the newborn was HIV-infected but congenital toxoplasmosis was asymptomatic. These cases have already been published.4
Some authors have documented that HIV-1-infected pregnant women who acquire toxoplasmosis during pregnancy (in contrast to immunocompetent women) do not have anti-T. gondii IgM antibodies and have IgG titers that remain low.15 In HIV-1 infections, hypergammaglobulinemia has been observed in conjunction with the selective loss of specific antibody production.18,29 Reduction of the immune response to specific antigens is associated with a reduction in the number of memory B-cells.18 Thus, due to their reduced humoral response against recently acquired antigens, in immunocompromised patients, the absence of IgM antibodies and low levels of anti-T. gondii IgG antibodies may be indicative of an acute infection, unlike in immunocompetent patients.16,18,29
In this study, there was no relationship between either the levels of CD4+ T-lymphocyte count or the magnitude of HIV-1 viral load and anti-T. gondii IgG titers. The interpretation of these results is limited due to the small sample of HIV-1-infected pregnant women. Therefore, our results should not be generalized.
The rate of vertical transmission of toxoplasmosis in HIV-1-infected pregnant women previously exposed to T. gondii has not yet been defined. Some authors have reported that congenital toxoplasmosis due to the reactivation of a maternal infection is less frequent than usually expected.9,30 In this study, we observed three cases of vertical transmission of toxoplasmosis due to reactivation of maternal infection in HIV-1-infected pregnant women. In our literature review, three additional cases of fetal infection due to reactivation of maternal toxoplasmosis in HIV-1-infected women were found in the last three years.7,31,32
Our study has some limitations. The results were based on cross-sectional data from HIV-positive patients with chronic T. gondii infection who may not represent the hypothetical original cohort of patients, among whom losses were not random. In addition, the current data are based on HIV-1-infected patients selected from a public hospital, who may not have been truly representative of the HIV-infected population of the municipalities of study from which they came. On the other hand, there is no reason to suppose that Toxoplasma infected individuals would be more likely to be seen at our hospital. These selection factors limit generalizability of our estimates of Toxoplasma reactivation infection rates in HIV-1- infected pregnant women.
Further multicenter studies should be conducted to address the poorly understood issues regarding
T. gondii infections in HIV-1-infected pregnant women. Such studies would allow for early implementation of effective preventive and therapeutic measures aimed at reducing the morbidity and mortality of this disease in the pediatric population.
Acknowledgements
We would like to thank Dr. Rosa Vieira and her team, from the Immunology Laboratory, HUAP-UFF, for carrying out the serological tests for toxoplasmosis. This study received financial support from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Conflict of interest
All authors declare to have no conflict of interest.
Received 30 August 2011
Accepted 4 October 2011
- 1. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;12:1965-1976.
- 2. Fuentes I, Rubio JM, Ramirez C, Alvar J. Genotypic characterization of toxoplasma gondii strains associated with toxoplasmosis in Spain: direct analysis from clinical samples. J Clin Microbiol. 2001;39:1566-1570.
- 3. Remington JS, Thulliez P, Montoya JG. Minireview. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol. 2004;42:941-945.
- 4. Azevedo KML, Setúbal S, Lopes VGS, et al. Congenital toxoplasmosis transmitted by human immunodeficiency-virus infected women. Braz Infect Dis J. 2010;14:186-189.
- 5. Belanger F, Derouin F, Grangeot-Keros L, Meyer L, HEMOCO and SEROCO Study Groups. Incidence and risk factors of toxoplasmosis in a cohort of human immunodeficiency virus-infected patients: 1988-1995. Clin Infect Dis. 1999;28:571-581.
- 6. Desmonts G, Couvreur J, Thulliez PH. Congenital toxoplasmosis: five cases with mother-to-child transmission of pre-pregnancy infection. Presse Med. 1990;19:1445-1449.
- 7. Fernandes RCSC, Vasconcelos VP, Araújo LC, et al. Vertical transmission of HIV and toxoplasmosis by reactivation in a chronically infected woman. Braz J Infect Dis. 2009;13:70-71.
- 8. Marty P, Bongain A, Rahal A, et al. Prenatal diagnosis of severe fetal toxoplasmosis as a result of Toxoplasmic reactivation is an HIV-1 seropositive woman. Prenat Diag. 2004;14:414-415.
- 9. Minkoff H, Remington JS, Holman S, et al.Vertical transmission of Toxoplasma by human immunodeficiency virus-infected women. Am J Obstet Gynecol. 1997;176:555-559.
- 10. Montoya JG, Kovacs JA, Remington JS. Toxoplasma gondii In: Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases, 6th edition, Elsevier, Churchill, Livingstone, 2005, p 3170-3198.
- 11. Nogueira SA, Guedes AL, Machado ES, et al. Toxoplasmic encephalitis in an HIV infected pregnant woman: successful outcome for both mother and child. Braz J Infect Dis. 2002;6:201-205.
- 12. Fortier B, Aissi E, Ajana F, et al. Spontaneous abortion and reinfection by Toxoplasma gondii Lancet. 1991;338:444.
- 13. Gavinet MF, Robert F, Firtion G, et al. Congenital toxoplasmosis due to maternal reinfeccion during pregnancy. J Clin Microbiol. 1997;35:1276-1277.
- 14. Israelski DM, Chmiel JS, Poggense L, et al. Prevalence of toxoplasma infection in a cohort of homosexual men at risk of AIDS and toxoplasmic encephalitis. J Acquir Immune DeficSyndr. 1993;6:414-418.
- 15. Lago EG, Conrado GS, Piccoli CS, et al. Toxoplasma gondii antibody profile in HIV-infected pregnant women and the risk of congenital toxoplasmosis. Eur J Clin Microbiol Infect Dis. 2009;28:345-351.
- 16. Nagase H, Agematsu K, Kitano K, et al. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmcytosis. Clin Imunol. 2001;100:250-259.
- 17. Hunziker L, Recher M, Macpherson AJ, et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4:343-349.
- 18. De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity inHIV-1 infection. Blood. 2004;103:2180-2186.
- 19. Coutinho SG, de Souza WJS, Camillo-Coura L, et al. Levantamento dos resultados das reações de imunofluorescência indireta para toxoplasmose em 6.079 pacientes de ambulatório em gestantes no Rio de Janeiro realizadas durante os anos de 1971 a 1977. Inst Med Trop São Paulo. 1981;23:48-56.
- 20. Meirelles Filho J. Toxoplasmose e gravidez inquérito sorológico em gestantes e seus recém-nascidos na maternidade escola da Universidade Federal do Rio de Janeiro. J Bras Ginec. 1985;95:393-401.
- 21. Doehring-E, Reiter-Owona I, Bauer O, et al. Toxoplasma gondii antibodies in pregnant women and their newborns in Darses Salaam, Tanzânia. Am J Trop Med Hyg. 1995;52:546-548.
- 22. Falusi O, French AL, Seaberg EC, et al. Prevalence and predictors of Toxoplasma seropositivity in women with and at risk for human immunodeficiency virus infection. Clin Infec Dis. 2002;35:1414-1417.
- 23. Morvan JM, Mambely R, Selekon B, et al. La toxoplasmose à l'Institut Pasteur de Bangui, Rèpublique Centrafricaine (1996-1998): donneés sérologiques. Bull Soc Pathol Exot. 1999;92:157-160.
- 24. Sýkora J, Zástera M, Stañková M. Toxoplasmic antibodies in sera of HIV- infected persons. Folia Parasitol (Praha). 1992;39:177-180.
- 25. Sukthana Y. Difference of Toxoplasma gondii antibodies between Thai and Austrian pregnant women. Southern Asian J Trop Med Public Health. 1999;30:38-41.
- 26. Silveira C, Ferreira R, Muccioli C, et al. Toxoplasmosis transmitted to a newborn from the mother infected 20 tears Earlier. Am J Ophthalmol. 2003;136:370-371.
- 27. Andrade GMQ, Vasconcelos-Santos DV, Carellos EVM, et al. Congenital toxoplasmosis from a chronically infected woman with reactivation of retinochoroiditis during pregnancy - an undersestimated event? J Pediatr (Rio J). 2010;86:85-86.
- 28. Avelino MM, Campos DJ, Parada JCB, et al. Pregnancy as a risk factor for acute toxoplasmosis soroconversion. Eur J Obstet Gynecol Reprod Biol. 2003;108:19-24.
- 29. Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617-622.
- 30. European Collaborative Study and Research Network on Congenital toxoplasmosis. Low incidence of congenital toxoplasmosis in children born to women infected with human immunodeficiency virus. Eur J Obstet Gynecol Reprod Bio. 1996;68:93-96.
- 31. Cruz MLS, Cardoso CA, Saavedra MC, et al. Congenital toxoplasmosis infection in an infant born to an HIV-1 infected mother. Braz Infect Dis J. 2007;11:610-611.
- 32. Vita WP, Cardoso CA, Oliveira LP, et al. Congenital toxoplasmosis infection in an infant HIV-exposed (abstract number: A-181-0009-00861). In: Abstract Book: 6th World Congress of World Society for Pediatric Infectious Diseases, 2009.
Publication Dates
-
Publication in this collection
25 Apr 2012 -
Date of issue
Apr 2012
History
-
Received
30 Aug 2011 -
Accepted
04 Oct 2011