Abstract
BACKGROUND: Acute cor pulmonale is a clinical syndrome with signs of right-sided heart failure resulting from sudden increase of pulmonary vascular resistance. CASE PRESENTATION: A five-year-old male, infected by human immunodeficiency virus (HIV), was admitted at the division of infectious diseases of this hospital with cough, tachydyspnea, fever, and breathing difficulty. Computed tomography scan showed ground-glass opacities, cystic lesions, and bronchiectasis. The patient had nasal flaring, intercostal and subcostal retractions, and keeled chest. Abdomen was depressible; liver was 3 cm from the right-costal border, while spleen was 6 cm from the left-costal border. Echocardiogram examinations showed signs of acute cor pulmonale characterized by pulmonary hypertension and increased right-heart chamber dimensions. DIAGNOSTICS OUTCOME: Acquired immunodeficiency syndrome (AIDS)-B3, lymphocytic interstitial pneumonia (LIP), and acute cor pulmonale. Regressions of pulmonary hypertension and of right-heart chamber were observed after 30 days of highly active antiretroviral therapy (HAART) and chloroquine therapy. CONCLUSION: AIDS should be considered in children with recurrent pneumonia that is mostly associated with LIP rather than cystic fibrosis.
acquired immunodeficiency syndrome; adult; children; respiratory tract infections
CASE REPORT
Acute cor pulmonale due to lymphocytic interstitial pneumonia in a child with AIDS
Sandra Fagundes Moreira-SilvaI,* * Corresponding author at: Rua Natalina Daher Carneiro, 55/302, Jardim da Penha, Vitória, ES, 29060-490, Brazil. E-mail address: sandrafagundesmoreira@gmail.com (Sandra Fagundes Moreira-Silva) ; Linda Marly C. MorenoI; Mariana DazziI; Consuelo Maria Caiafa FreireI; Angelica Espinosa MirandaII
IHospital Estadual Infantil Nossa Senhora da Glória, Vitória, Espírito Santo, Brazil
IIUniversidade Federal do Espírito Santo, Vitória, Espírito Santo, Brazil
ABSTRACT
BACKGROUND: Acute cor pulmonale is a clinical syndrome with signs of right-sided heart failure resulting from sudden increase of pulmonary vascular resistance.
CASE PRESENTATION: A five-year-old male, infected by human immunodeficiency virus (HIV), was admitted at the division of infectious diseases of this hospital with cough, tachydyspnea, fever, and breathing difficulty. Computed tomography scan showed ground-glass opacities, cystic lesions, and bronchiectasis. The patient had nasal flaring, intercostal and subcostal retractions, and keeled chest. Abdomen was depressible; liver was 3 cm from the right-costal border, while spleen was 6 cm from the left-costal border. Echocardiogram examinations showed signs of acute cor pulmonale characterized by pulmonary hypertension and increased right-heart chamber dimensions.
DIAGNOSTICS OUTCOME: Acquired immunodeficiency syndrome (AIDS)-B3, lymphocytic interstitial pneumonia (LIP), and acute cor pulmonale. Regressions of pulmonary hypertension and of right-heart chamber were observed after 30 days of highly active antiretroviral therapy (HAART) and chloroquine therapy.
CONCLUSION: AIDS should be considered in children with recurrent pneumonia that is mostly associated with LIP rather than cystic fibrosis.
Keywords: acquired immunodeficiency syndrome; adult; children; respiratory tract infections
Introduction
Lymphocytic interstitial pneumonia (LIP) is regarded as a preneoplastic disease that results from inflammatory pulmonary reaction to various external stimuli or systemic disease.1 LIP is characterized by a diffuse lymphocytic infiltrate with lymphoid hyperplasia around the enlarging airways. This may range from small benign lymphoid aggregates to high degree lymphoma in 5% of cases.1-3 Death results in approximately 33% to 50% of patients in nearly five years after the diagnosis.2
Acute cor pulmonale is a clinical syndrome characterized by signs of right heart failure (right ventricular hypertrophy) of sudden onset and results from the abrupt increase of pulmonary vascular resistance due to diseases that affect the function and/or the structure of the lung, of which pulmonary embolism is the most common cause.4
In Brazil, despite the availability of antiretroviral drugs in the public health system, the initiation of acquired immunodeficiency syndrome (AIDS) treatment is delayed.5 Late diagnosis of human immunodeficiency virus (HIV) infection in children results in reduced survival time and severe clinical conditions with greater possibility of sequelae and death. The time span between diagnosis and death is usually longer in children infected at older ages. This interval may vary from two to three months to several years6 depending on the severity of infections that arise from the early clinical course. The most common infections are recurrent bacterial infections.7
The pulmonary manifestations in children with AIDS represent 65% of the defined pathology of the syndrome and can be the first clinical symptoms to emerge in these patients.7 There is a paucity of data in the medical literature concerning the progression of LIP to cor pulmonale. LIP is very common in children with AIDS and in women with Sjögren's syndrome, an autoimmune disease.1-3,8 The commonly affected age-group is children over two years. The common findings are digital clubbing, enlarged parotids, lymphadenopathy, hepatosplenomegaly, and chronic radiographic changes of diffuse infiltrate type with hypoxia and hypergammaglobulinemia.9 The clinical symptoms include tachydyspnea, failure to thrive, cough, crackles on pulmonary auscultation, cyanosis, and digital hypocratism. Thoracic deformities, digital clubbing and heart involvement with sign of cor pulmonale are observed in the more advanced clinical forms.10
Here, a case report of a child with HIV and recurrent respiratory infections during the first five years of life who progressed to clinical conditions of cor pulmonale is described.
Case presentation
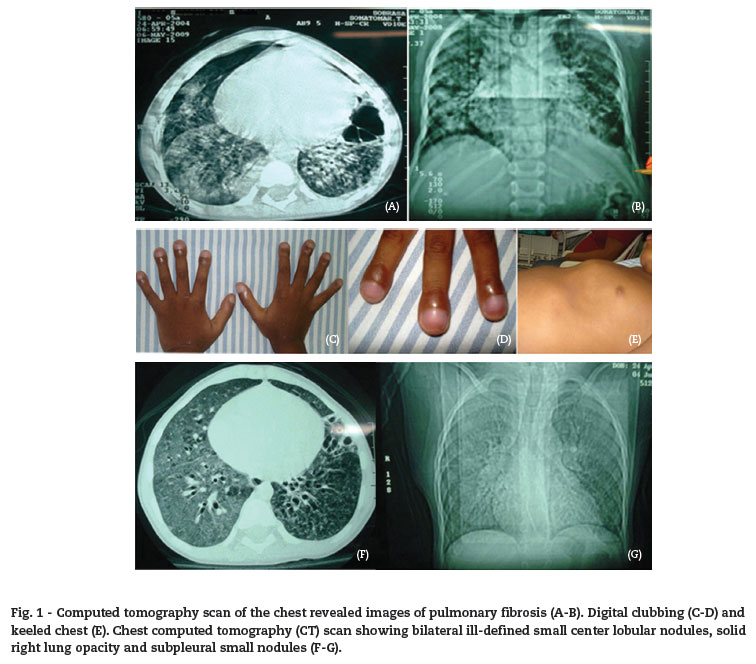
I.S.S. is a five-year-old male from the state of Bahia with the following clinical conditions: productive cough and tachydyspnea since the age of one month, and fever with worsening respiratory effort. He was hospitalized for pneumonia. He did not respond to treatment and progressed to anasarca. Computed tomography (CT) scan of the chest revealed images of pulmonary fibrosis (Fig. 1). For this reason, he was sent to the pediatric emergency room of the Hospital Infantil Nossa Senhora da Glória in Vitória, ES, Brazil, with a diagnosis of cystic fibrosis.
Upon admission, the mother reported that she had attended consultations for prenatal care irregularly in Bahia. HIV testing was not done at any moment during her pregnancy. She had no history of systemic hypertension, diabetes mellitus, or urinary tract infection during the pregnancy. Delivery was normal and at term without complication. Rapid anti-HIV test was not performed at the time of delivery. The child was breastfed for one year. He was often hospitalized for recurrent pneumonia with chronic cough and recurrent wheezing since the first year of life.
On his physical examination, the patient was tachydyspneic, hypohydrated (2+/4+), pale (2+/4+), acyanotic, and presenting facial edema. On auscultation, he had regular cardiac pacing, 2T, normal heart sounds without murmurs, heart rate of 128 bpm, and blood pressure of 96/68 mmHg. He had pronounced digital clubbing and keeled chest (Fig. 1). He had respiratory physiological vesicular murmur with crackles and diffuse bilateral rales. His respiratory rate was 49 bpm with a peripheral oxygen saturation of 91% on room air. He had nasal flaring and retraction of intercostals and subcostals. Abdomen was depressible; liver was three cm from the right costal border while the spleen was six cm from the left costal border. He was admitted to the pulmonology ward due to acute respiratory failure.
During hospitalization, his cardiac examination revealed an echocardiogram of mild pulmonary hypertension. His pulmonary arterial pressure was 38 mmHg. There was a mild to moderate increase of his right heart chambers. The left ventricle had a mild contractile deficit. Of note, these observations might have been a result of the paradoxical movement caused by the enlargement of the septum of the right ventricle. There were no congenital heart defects. In light of the above observations, the diagnostic hypothesis was cor pulmonale. He was put on digoxin, captopril, and furosemide. The hypothesis that he had cystic fibrosis was also maintained.
As his clinical conditions were not improving, the opinion of an infectologist was sought. AIDS and LIP were proposed. ELISA test of anti-HIV1 and 2 was positive for both mother and child and was confirmed by Western-blot. He was then transferred to the infectology ward where HIV viral load determination and CD4+ and CD8+ T-cell counting were performed. His HIV viral load was 35,268 copies/mL (Log 4.54). The CD4+ and CD8+ T-cell counts were 715 cells/mL (9.79%) and 4,829 cells/mL (66.16%), respectively, with a ratio of CD4+/CD8+: 0.15.
Serological tests for opportunistic infections were performed. A new CT scan of the chest was also performed, confirming the diagnosis of LIP. There was considerable change of the parenchymal opacities with bilateral ground-glass opacities. The air cysts were scattered. Small solid pulmonary nodules and bronchiectasis were present, especially to the left. Bilateral ill-defined small center lobular nodules, solid right lung opacity, and subpleural small nodules were also observed (Fig. 1).
Following the results of the CT scan of the chest with a final diagnosis of LIP and acute cor pulmonale and AIDS due to mather-to-child transmission, the patient was treated with chloroquine, antiretroviral zidovudine (AZT), lamivudine (3TC), and efavirenz (EFV). His AIDS status was classified as clinicoimmunological B3, i.e., severe immunosuppression. He was also put on sulfamethoxazole/trimethoprim (SMZ/TMP) prophylaxis for P. jirovecii.
Cystic fibrosis was ruled out after the molecular detection of cystic fibrosis mutation (CFTR delta F508) was absent and after negative sweat chloride tests repeated twice. The first sweat chloride test results were: 0.064 g weight of sweat; 30.50 mEq/L (normal 00 to 40 mEq/L). The results of the second test were: 0.2392 g; 10.30 mEq/L. Tuberculosis was also ruled out as the sputum acid fast bacilli (AFB) test and culture were negative and the PPD was 00 mm. Bronchoscopy showed normal larynx, trachea, bronchioles with mucopurulent secretion, and free lobar orifices. Microbiology test on bronchial lavage was performed, and it was negative for AFB. Leucocytes, fungi, and aerobic organisms were present in the bronchial lavage. Amphotericin B was started for the treatment of fungal pneumonia.
Several opportunistic diseases were also investigated. Serology tests were negative for VDRL, HCV, HBsAg, anti-HBs, toxoplasmosis (both IgG and IgM), CMV (both IgG and IgM), hepatitis A (both IgG and IgM), histoplasmosis, and paracoccidioidomycosis. Substantive examination of the eyes was normal. Abdominal ultrasonography showed homogeneous hepatosplenomegaly with no signs of nodules. Stool fat was 1.8 g/24 hours (> 2.0 g). Parasitology tests were negative for helminthes and protozoa. Cryptosporidium and Isopora beli were absent in the stools.
After 30 days of antiretroviral therapy, cardiological and LIP specific treatment, significant improvement with regression of cardiac abnormalities described above was observed. At the last echocardiogram examination, there were regressions of the previously observed abnormalities. There was only mild tricuspid regurgitation. Digoxin and furosemide were discontinued. His clinical condition was significantly improved. CT scan of the chest showed improved tomographic image. Only bronchiectasis was still present. He was kept on antiretroviral regimen, SMZ/TMP, and chloroquine. He showed good adherence. After hospital discharge, he was followed at the service center for pediatric AIDS of the children's hospital in Vitória. His clinical condition and laboratory tests continued to improve.
Discussion
HIV infection in children has a broad clinical spectrum that may include severe immune deficiency, hepatosplenomegaly, parotitis, growth retardation, persistent fever or chronic diarrhea.11,12 In the first year of life, lymphadenopathy, splenomegaly, and hepatomegaly are observed in more than 50% of HIV-infected children. Other clinical signs that are very often observed, including growth retardation, fever, diarrhea, and secondary infections that define AIDS, may also appear late in the first year of life.13,14
Children with AIDS are at greater risk of developing pneumonia and more severe diseases than immunocompetent children.15-17 Chronic non-infectious lung diseases such as LIP, immune reconstitution inflammatory syndrome, malignant disease, and pneumonitis have also often been reported in older children with AIDS. The most common chronic changes observed in chest radiography are reticular bronchovascular lesions and bronchiectasis.17,18
There are few reports about LIP in children with AIDS. The clinical history of recurrent pneumonia leading to changes such as digital clubbing, chest deformity, chronic respiratory failure, and cardiac involvement with signs of cor pulmonale need to be investigated for LIP.13 The definitive diagnosis of LIP in children with AIDS is made by histopathological examination of the affected lung tissue. However, presumptive diagnosis, through analysis of chest X-ray showing bilateral reticulonodular interstitial infiltrate for at least two months with no definitive etiologic agent and lack of response to antibiotic treatment, or through CT scan of the chest showing images of bilateral reticulonodular pattern with ground-glass opacities and the presence of bronchiectasis, are also accepted.15,16
The clinical manifestations of LIP may arise insidiously. Initially, the child may have intermittent dry cough, which is often mistaken for viral infection or allergic disease.18 Later, other clinical manifestations such as dyspnea, exercise intolerance, and cyanosis are observed. The laboratory evaluation of the patient includes blood cell counts, hemoculture, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and anti-HIV test, complemented by chest radiography or CT scan of the chest.7 The standard radiographic interstitial characteristic is a lymphocytic infiltrate in the walls and alveolar septa. The presence of LIP in children with AIDS has been correlated with poor prognosis, especially concerning respiration. The pathophysiological mechanisms involved in LIP are not clear. Also, it is not known if other viruses such as Epstein Barr virus associated with AIDS may be involved in its development.7,18,19
Pitcher & Zar wrote a review of articles published between 1982 and 2007 about cases of LIP to compare diagnosis used to those with radiological criteria for presumptive diagnosis of LIP in children as defined by the Centers for Disease Control and Prevention, USA. The inclusion criteria were children under 13 years, HIV infection, and radiological and pathological data such as lung biopsy and chest X-ray. There were 25 reports analyzing 128 cases of LIP in children with AIDS aged 7 to 108 months. All had lung biopsy. Chest X-ray was described as bilaterally diffuse and symmetrical interstitial infiltrate in 90% of the published cases. A reticulonodular or parenchymal nodular pattern was described in 75% of the cases. Diffuse pulmonary changes, asymmetric in 8% and focal in less than 2% of cases, were reported.18
The differential diagnosis of LIP with other chronic lung diseases in HIV-infected children should be considered, as it is difficult to distinguish LIP from miliary tuberculosis in areas of high prevalence of tuberculosis.19 Pediatrians must routinely include the investigation of HIV infection in children presenting with recurrent respiratory infections.
Conflict of interest
All authors declare to have no conflict of interest.
Received 26 August 2011
Accepted 13 February 2012
- 1. Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid Interstitial Pneumonia: A Narrative Review. Chest. 2002;122:2150-64.
- 2. Pulmonary Case of the Week 11 04 2005. Virginia Commonwealth University Cardiothoracic Pulmonary Imaging Correlation Conference Case of the Week - November 4, 2005.
- 3. Koulaouzidis A, Karagiannidis A, Prados S, Pattenshetty D, Deramon A, Tan WC. Lymphocytic interstitial pneumonitis (LIP)-The liver and the lung. Ann Hepat. 2006;5(3):170-171
- 4. Ota JS, Pereira CAC. Cor Pulmonale. Medicina, Ribeirão Preto. 1998;31:241-6. Available from: http://www.fmrp.usp.br/revista/1998/vol31n2/cor_pulmonale.pdf
- 5. Ministério da Saúde (Brazil). Coordenação Nacional de DST/AIDS. Boletim Epidemiológico Ano XV; nş 4. Outubro de 2001 a março de 2002.
- 6. Pizzo PA, Wilfert CM . The Pediatric AIDS Siena Workshop II. Markers and determinants of disease progression in children with HIV infection. J AIDS and Hum Retrovirol. 1995;8:30-44.
- 7. Brockmann PV, Viviani TS, Peña AD. Compromiso pulmonar en la infección por virus de inmunodeficiencia humana en niños. Rev Chil Infect. 2007;24(4):301-5. Available from: http://www.scielo.cl/pdf/rci/v24n4/art07.pdf
- 8. Galli L, De Martino M, Tovo PA, et al. Onset of clinical signs in children with HIV-1 perinatal infection. AIDS. 1995;9:455-61.
- 9. European Collaborative Study. Natural history of vertically acquired human immunodeficiency virus-1 infection. Pediatrics. 1994;94:815-9.
- 10. Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246-7.
- 11. American Academy of Pediatrics. Human immunnodeficiency virus infection. In: Peter G, editor. Red Book: Report of the Committee on Infectious Diseases. 27th ed. Elk Grove Village IL: American Academy of Pediatrics; 2006. p. 378-401.
- 12. Barbosa MP, Silva JF. Doenças pulmonares císticas adquiridas. Brasília Med. 2007;44(4):277-87.
- 13. Yparraguirre ITR, Sant'Anna CC, Lopes VGS, Madi K. Acometimento pulmonar em crianças com a síndrome da imunodeficiência humana (AIDS): Estudo clínico e de necropsia de 14 casos. Rev Assoc Med Bras. 2001;47(2):129-36.
- 14. Paiva MA, Amaral SM. Chronic intertitial lung disease in children. J. Pediatr (Rio J.). 2007;83(3):233-40.
-
15Ministério da Saúde (Brazil). Secretaria de Vigilância em Saúde, Programa Nacional de DST e AIDS. Critérios de definição de casos de AIDS em adultos e crianças. n. 60. Brasília: Ministério da Saúde; 2004.
- 16. Gray DM, Zar HJ. Community-acquired pneumonia in HIV-infected children: a global perspective. Curr Opin Pulm Med. 2010,16:208-216.
- 17. Theron S, Andronikou S, George R, et al. Non-infective pulmonary disease in HIV-positive children. Pediatr Radiol. 2009;39:545-554.
- 18. Pitcher RD, Zar HJ. Radiographic features of pediatric pneumocystis pneumonia - a historical perspective. Clin Radiol. 2008;63:666-672.
- 19. Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol. 2008;43:1-10.
Publication Dates
-
Publication in this collection
20 June 2012 -
Date of issue
June 2012
History
-
Received
26 Aug 2011 -
Accepted
13 Feb 2012