ABSTRACT
Aims:
To evaluate the HBeAg seroconversion rate in real clinical setting and explore its predictors in long-term nucleos(t)ide analogues (NAs) treatment for chronic hepatitis B (CHB).
Methods:
251 patients were recruited from January 2001 to September 2009 in four hospitals in Hebei province, China, for this retrospective study. Clinical and laboratory data before and after treatment with lamivudine (LAM, 100 mg daily), adefovir (ADV, 10 mg daily), telbivudine (LDT, 600 mg daily), entecavir (ETV, 0.5 mg daily), and LAM/ADV combination were compared among three groups according to treatment outcomes: synchronous HBeAg loss and HBeAg seroconversion, anti-HBe development after treatment, and no anti-HBe. Adherence was also evaluated.
Results:
In real clinical setting, cumulative HBeAg seroconversion rates were 14.3%, 32.7%, 43.0%, 46.9%, and 50.5% after 1, 2, 3, 5, and 8 years, respectively. 45 patients (17.9%) were non-adherent. Adherence (p < 0.001, Hazard Ratio (HR) = 2.203), elevated alanine aminotransferase (ALT) levels (p < 0.001, HR = 2.049), and non-vertical transmission (p = 0.006, HR = 1.656) were predictors of HBeAg seroconversion.
Conclusion:
Adherence, elevated ALT, and non-vertical transmission are predictors of HBeAg seroconversion in CHB patients treated with NAs.
Keywords:
Chronic hepatitis B; Hepatitis B and antigens; Nucleos(t)ide analogue; Adherence
Introduction
Chronic hepatitis B (CHB) remains a major public health concern, because of worldwide prevalence and the potential to cause adverse consequences such as liver cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).11 Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-2. However, CHB can be managed with effective antiviral agents. Hepatitis Be antigen (HBeAg) seroconversion is considered the treatment endpoint in HBeAg-positive patients.22 Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-83. It is correlated with clinical remission accompanied by improved necro-inflammatory activity,33 Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-52. fibrosis regression,44 Hui CK, Leung N, Shek TW, et al. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690-8. lower risk of liver cirrhosis and HCC,55 Lin SM, Yu ML, Lee CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45-52. and higher incidence of complication-free survival.66 Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-34.
Nucleos(t)ide analogues (NAs) are recommended as first-line anti-HBV agents owing to high antiviral potency, ease of use, and high tolerability in HBeAg-positive patients, especially those with high hepatitis B virus (HBV) DNA loads and liver cirrhosis. However, HBeAg seroconversion rates after treatment with five commonly prescribed oral antiviral agents are 12-23% after one year of treatment, 25-31% after two years, and up to 44% after three years according to recent clinical trials.77 Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-61. In addition, two recent studies conducted in the United States revealed that HBeAg seroconversion rate is significantly lower in real clinical setting, especially in entecavir (ETV)-treated patients.88 Liu A, Ha NB, Lin B, et al. Low hepatitis B envelope antigen seroconversion rate in chronic hepatitis B patients on long-term entecavir 0.5 mg daily in routine clinical practice. Eur J Gastroenterol Hepatol. 2013;25:338-43.,99 Lin B, Ha NB, Liu A, et al. Low incidence of hepatitis B e antigen seroconversion in patients treated with oral nucleos(t)ides in routine practice. J Gastroenterol Hepatol. 2013;28:855-60. HBeAg seroconversion rate in actual clinical setting was less than 10% during the first year of NAs therapy. A number of factors, such as older age, higher alanine aminotransferase (ALT) levels, lower HBV DNA load, genotype B (vs. C), and pathological factors, may predispose patients to HBeAg seroconversion either in the natural course or during individual NAs or interferon (IFN) therapies.1010 Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-95.
11 Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123-9.
12 Kim HS, Kim HJ, Shin WG, et al. Predictive factors for early HBeAg seroconversion in acute exacerbation of patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2009;136:505-12.-1313 Wu JF, Wu TC, Chen CH, et al. Serum levels of interleukin-10 and interleukin-12 predict early, spontaneous hepatitis B virus e antigen seroconversion. Gastroenterology. 2010;138:e1-3. However, few studies have evaluated HBeAg seroconversion rates during long-term NAs therapy in real clinical setting, but not assessing factors that may predict HBeAg seroconversion.
Currently, adherence studies mainly focus on anti-HIV therapies, and many options have been tested for adherence assessment, including visual analogue scale and the so-called 3-day recall instrument.1414 Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255-66.,1515 Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74-9. Although such studies are less common in the HBV field, it was demonstrated that higher adherence to NAs treatment in CHB patients tended to result in decreased rate of viral breakthroughs.1616 Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok AS. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19:205-12.
Thus, the goal of the current study was to evaluate long-term outcomes of NAs treatment through HBeAg seroconversion rates, whose predictive factors were also assessed, in a multicenter set of CHB treatment-naive patients in real clinic in China.
Materials and methods
Study design and patient population
This was a retrospective case control study of consecutive treatment-naïve CHB patients treated with lamivudine (LAM, 100 mg daily), adefovir (ADV, 10 mg daily), telbivudine (LDT, 600 mg daily), ETV (0.5 mg daily), and LAM and ADV combination for more than three years from January 2001 to September 2009 at five departments of gastroenterology and hepatology in Chinese hospitals.
A total of 1591 patients were enrolled. Inclusion criteria were: ≥18 years of age, no previous HBV treatment, and detectable HBeAg and negative anti-HBe at baseline. Patients were excluded if there was any evidence of autoimmune hepatitis, metabolic liver diseases, heavy alcohol abuse, hepatitis C virus, hepatitis D virus, hepatitis E virus, human immunodeficiency virus (HIV), recent exposure to hepatotoxic drugs, and pregnancy for women. Patients with changed anti-HBV agents (switching to an alternate NAs monotherapy, IFN therapy, or adding another anti-HBV medication) were also excluded. A total of 251 patients were finally included in the study but only 177 were fully assessed with recorded HBeAg loss. Patient clinical records, including laboratory results and adherence reports, were reviewed using a self-report questionnaire.
Laboratory examinations were generally conducted at baseline and at 12-24 week intervals. Serum levels of ALT, alpha fetoprotein (AFP), HBV DNA, HBeAg, anti-HBe, HBsAg, and anti-HBs were evaluated. Serum HBV DNA load in CHB patients was detected by real time polymerase chain reaction (PCR) in our Hospital, and detection limits were 500 and 108 copies/mL, for lower and upper levels, respectively. HBeAg and HBsAg were measured quantitatively or qualitatively (described as “positive” or “negative”).
The study protocol was approved by the Ethics Committee of our Hospital, and the requirement for written informed consent was waived.
Assessment of treatment outcomes
In this study, HBeAg seroconversion was defined as loss of HBeAg with the development of anti-HBe, accompanied with complete viral suppression (undetectable levels of HBV DNA replication, <500 copies/mL) and normalization of ALT (<40 IU/L).
Adherence measurement
Three questions regarding adherence to anti-HBV NAs were included in a self-administered questionnaire at the last follow-up. The first two were adapted from the questionnaire established for HIV-infected patients by the AIDS Clinical Trial Group.1414 Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255-66. The third question corresponded to a visual analogue scale (VAS)1515 Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74-9. (Table 1).
Patients were classified as fully adherent if skipping no more than one medication dose, with a VAS score of 10. Other patients were classified in the non-adherence group. This approach tends to minimize adherence inflation using self-reporting to healthcare providers.1616 Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok AS. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19:205-12.
Statistical analysis
Descriptive statistics were reported as number and percentage (%) for categorical variables, and mean ± SD or median (range) for continuous variables. Groups of patients with different NAs and HBeAg seroconversion status were compared by χ2-test, log-rank test, Student's t-test, and one-way analysis of variance (ANOVA) for categorical and continuous variables, respectively. Kaplan-Meier analysis was used to assess cumulative incidence rates over time. Cox proportional hazard regression analysis was used to identify independent factors significantly associated with HBeAg seroconversion, with Wald χ2 values and hazard ratios (HR) calculated.
Two-tailed p < 0.05 was considered statistically significant. The SPSS software version 17 (SPSS, Chicago, IL, USA) was used for statistical analyses.
Results
Baseline characteristics of the included subjects
The patient flowchart is shown in Fig. 1. Of the 1591 patients, 739 were excluded with chronic HBV infection and HBeAg negative results. A total of 396 additional individuals were excluded for treatment duration below three years, and an additional 196 patients for changing medication/stopping treatment without a clinician's agreement and not conforming to the treatment strategy. Nine patients were lost to follow-up. Finally, 251 eligible patients remained, but only 177 were included and fully studied with recorded HBeAg loss. The number of CHB patients treated with LAM, ADV, LDT, ETV, and LAM/ADV combination were 71, 53, 7, 29, and 91, respectively. Median age was 42.3 years (range, 18-72), and 72.5% of patients were male (182/251). 56 of the 251 patients (22.3%) were diagnosed with liver cirrhosis; 155 patients (61.8%) had vertical transmission modes. Demographic and laboratory characteristics at the time of diagnosis are shown in Table 2.
Flow chart showing the selection of patients for inclusion in the study. CHB, chronic hepatitis B; HBeAg, hepatitis Be antigen.
Anti-HBV treatment responses, safety and adherence
HBeAg loss increases and seroconversion rates are not parallel
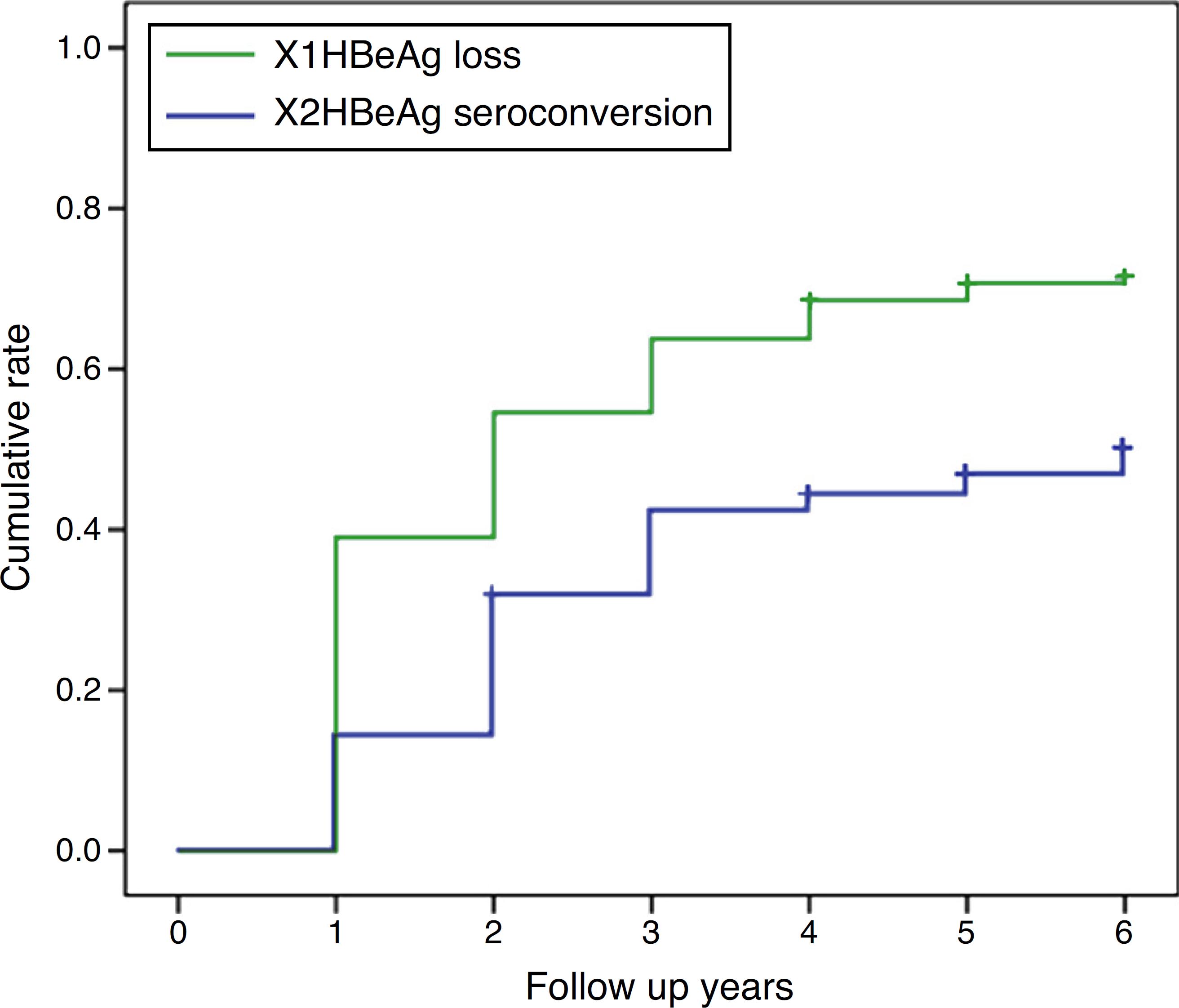
HBeAg loss and seroconversion were observed in 70.5% (177/251) and 49.0% (123/251) of patients, respectively. The total rates of HBeAg loss for patients with treatment duration beyond three, five, and eight years were 65.4% (70/107), 73.8% (76/103), and 75.6% (31/41), respectively; however, total HBeAg seroconversion rates were only 50.5% (54/107), 47.6% (49/103), and 48.8% (20/41), respectively (Fig. 2). The cumulative rates of HBeAg loss and seroconversion are shown in Fig. 3. In real clinical setting, cumulative HBeAg loss rates were 39%, 54.6%, 63.7%, 70.7%, and 74.4% after one, two, three, five, and eight years of treatment, respectively. Meanwhile, cumulative HBeAg seroconversion rates were only 14.3%, 32.7%, 43%, 46.9%, and 50.5% in one, two, three, five, and eight years, respectively.
Comparison of total rates of HBeAg loss and seroconversion with different treatment durations. Total rates of hepatitis Be antigen (HBeAg) loss (grey bars) and HBeAg seroconversion (black bars) in patients treated for 3-5 years, 5-8 years, and over 8 years.
Cumulative rates of HBeAg loss and seroconversion over different treatment durations. Cumulative rates of hepatitis Be antigen (HBeAg) loss (X1 line) and HBeAg seroconversion (X2 line) in patients treated for 6 years.
Safety results
LAM, ADV, and ETV were well tolerated, and no patients discontinued treatment due to adverse events. No patients died during the study. Creatine kinase elevation (mild) occurred during the first year in four patients on LDT monotherapy.
Adherence to HBV medication is suboptimal
A total of 206 patients (82.1%) fully adhered to HBV medication. At the last follow-up, 45 patients reported to have missed HBV medication for ≥1 day in the last 30 days. The most common reasons for missing medication were forgetfulness (n = 27) and financial difficulties (n = 7).
Independent predictors of HBeAg seroconversion in patients with NAs induced HBeAg loss
HBeAg loss was observed in 70.5% (177/251) of the patients. In 61 patients, HBeAg loss was accompanied with anti-HBe production; 62 achieved HBeAg seroconversion after a median time on therapy and follow-up of 48 weeks; the remaining 54 patients maintained HBeAg and anti-HBe negative after a median therapy and follow-up of 245 weeks at the end of the study. Univariate analysis revealed that vertical/non-vertical transmission (p = 0.009), adherence (p = 0.001), baseline ALT (p = 0.025), and baseline log HBV DNA (p = 0.001) were significantly different among groups as shown in Table 3. These factors were entered to a Cox proportional hazards model, and adherence (Wald χ2 = 12.228, p < 0.001, HR = 2.203), high ALT level (Wald χ2 = 14.750, p < 0.001, HR = 2.049), and non-vertical transmission (Wald χ2 = 7.570, p = 0.006, HR = 1.656) were independent predictive factors of HBeAg seroconversion in patients with NAs induced HBeAg loss (Table 4). The effects of the three identified factors on HBeAg seroconversion were further assessed by the Kaplan-Meier method (Fig. 3).
Discussion
The aim of this study was to evaluate the HBeAg seroconversion rate in patients with CHB treated in real clinical setting, and to determine factors that predict seroconversion with long term NAs treatment. The results showed that cumulative HBeAg seroconversion rates during NAs therapy were 14.3%, 32.7%, 43.0%, 46.9%, and 50.5% at one, two, three, five, and eight years, respectively. Adherence to HBV medication, high ALT level, and non-vertical transmission were predictive factors of HBeAg seroconversion.
In registered clinical trials as low as 20% of CHB patients treated with NAs achieve HBeAg seroconversion after one year of treatment, despite small increases in LDT-treated patients.1010 Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-95.,1717 Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-88.
18 Hou J, Yin YK, Xu D, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47:447-54.-1919 Chan HL, Heathcote EJ, Marcellin P, et al. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med. 2007;147:745-54. However, registered clinical trials strictly select patients on the basis of inclusion criteria such as age, gender, and baseline ALT levels, all of which are predictive factors of anti-HBV responses. Clinical trials also require close adherence to strict protocol guidelines, which may improve the measured medication adherence levels and consequently the HBeAg seroconversion rates. Thus, the HBeAg seroconversion rate might be even lower in real clinical setting. Indeed, in a recent study conducted in real clinical setting in the United States, the HBeAg seroconversion rate at month 12 was as low as 8.2%.99 Lin B, Ha NB, Liu A, et al. Low incidence of hepatitis B e antigen seroconversion in patients treated with oral nucleos(t)ides in routine practice. J Gastroenterol Hepatol. 2013;28:855-60. Another study reported an even lower rate of 4.8% at 12 months of ETV treatment.88 Liu A, Ha NB, Lin B, et al. Low hepatitis B envelope antigen seroconversion rate in chronic hepatitis B patients on long-term entecavir 0.5 mg daily in routine clinical practice. Eur J Gastroenterol Hepatol. 2013;25:338-43. In this study the HBeAg seroconversion rate at one year was 14.3%. Potential reasons for the difference between HBeAg seroconversion rates in the current study and the previous trials may include differences in baseline characteristics, adherence of the patient population, and the genotypic distribution of HBV. In addition, this study retrospectively recruited patients on the basis of NAs treatment duration. Another explanation for the seemingly higher HBeAg seroconversion rate in the current study may be selection bias because we included only patients still being treated, and some patients might have been excluded from follow-up due to suboptimal anti-HBV responses or drug resistant mutations during the first three years.
In clinical trials, however, after long-term follow-up, the HBeAg seroconversion rate increases with therapy duration to more than 30% and 40% at three and five years, respectively.77 Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-61.,2020 Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-22.
21 Marcellin P, Chang TT, Lim SG, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750-8.
22 Zoutendijk R, Reijnders JG, Brown A, et al. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naive patients with a partial virological response. Hepatology. 2011;54:443-51.
23 Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106:1264-71.-2424 Gane EJ, Wang Y, Liaw YF, et al. Efficacy and safety of prolonged 3-year telbivudine treatment in patients with chronic hepatitis B. Liver Int. 2011;31:676-84. The current study found a similar HBeAg seroconversion rate of 43% and 46.9% at three and five years, respectively, corroborating previous studies in real clinical setting.88 Liu A, Ha NB, Lin B, et al. Low hepatitis B envelope antigen seroconversion rate in chronic hepatitis B patients on long-term entecavir 0.5 mg daily in routine clinical practice. Eur J Gastroenterol Hepatol. 2013;25:338-43.,99 Lin B, Ha NB, Liu A, et al. Low incidence of hepatitis B e antigen seroconversion in patients treated with oral nucleos(t)ides in routine practice. J Gastroenterol Hepatol. 2013;28:855-60. Thus, medical adherence might only affect anti-HBV responses during the initial year. Several studies have also reported that persistence and adherence rates to anti-HBV agents and other medicines for chronic diseases dramatically drop after the first six months of therapy.2525 Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455-61.
26 Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462-7.
27 Donnelly LA, Doney AS, Morris AD, Palmer CN, Donnan PT. Long-term adherence to statin treatment in diabetes. Diabet Med. 2008;25:850-5.-2828 Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54:12-8. Although an even longer duration of eight years on NAs treatment was associated with increased HBeAg seroconversion rate of more than 50%, the NAs induced HBeAg seroconversion is not durable.2929 Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139:491-8. Thus, in real-life clinical settings, HBeAg-positive patients should be counselled about the possibility of long treatment duration being required to achieve and maintain HBeAg seroconversion.
Increases in HBeAg loss and seroconversion rates were not parallel because the rate of HBeAg loss continued to increase with time while HBeAg seroconversion almost levelled off after three years. This may be partially related to insufficient anti-HBV related immune responses in some patients with NAs induced HBeAg loss.
Prediction of the response to anti-HBV treatment has become increasingly important in therapy individualization for CHB patients. Baseline predictive factors can assist physicians to select the best timing for treatment initiation as well as antiviral agents. Baseline ALT and HBV DNA levels are important factors for HBeAg seroconversion. Indeed, patients with higher baseline ALT levels and lower HBV DNA replication have a higher probability of HBeAg seroconversion, both in the natural course3030 Bae SK, Yatsuhashi H, Hashimoto S, et al. Prediction of early HBeAg seroconversion by decreased titers of HBeAg in the serum combined with increased grades of lobular inflammation in the liver. Med Sci Monit. 2012;18:CR698-705.,3131 Yuen MF, Yuan HJ, Hui CK, et al. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416-9. and during NAs or interferon therapy.77 Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-61.,1010 Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-95.,3232 Wang CC, Tseng KC, Peng CY, et al. Viral load and alanine aminotransferase correlate with serologic response in chronic hepatitis B patients treated with entecavir. J Gastroenterol Hepatol. 2013;28:46-50.,3333 Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-95. This may be related to more severe inflammatory processes, and subsequent more rapid viral loads and HBeAg titre decrease in patients with higher baseline ALT levels.
Adherence was found to be an important predictor of HBeAg loss/seroconversion in the current study. Its predictive value of virological suppression or breakthrough in patients receiving anti-HBV NAs treatment was also demonstrated in clinical practice.1616 Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok AS. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19:205-12.,3434 Sogni P, Carrieri MP, Fontaine H, et al. The role of adherence in virological suppression in patients receiving anti-HBV analogues. Antivir Ther. 2012;17:395-400. However, this is the first study of this kind in the Chinese population in real clinical setting. Thus, adherence might be seriously taken into consideration while treating CHB patients with NAs, especially during the first year. Indeed, more time should be spent counselling patients regarding the importance of adherence to HBV treatment and the risks of medication discontinuation.
The strengths of the current study are the relatively large sample size, availability of data for the four different NAs used in routine clinical practice, and a broad representation of the database. However, several limitations of the study should be mentioned. First, selection bias was inevitable in this retrospective analysis. For instance, although HBeAg seroconversion depends on HBV genotype, we did not determine HBV genotypes, which could not be assessed as an influencing factor in this study. In addition, treatment duration may result in an overestimation of anti-HBV responses. Second, patients who switched to or added other anti-HBV medications due to suboptimal responses or drug resistant mutations were excluded. This would definitely impact HBeAg seroconversion rates, although the effect is expected to be relatively small. Third, the number of LDT-treated patients in the current data set was small, which limited our ability to make recommendations regarding the indicated agent. Fourth, adherence was evaluated only once, at last follow-up, and related data during the first three years were missing. However, adherence to antiviral therapy might be lower than expected, leaving patients at risk of delayed HBeAg seroconversion, increased virological breakthrough, and disease progression.
In real clinical setting, cumulative HBeAg seroconversion rates during NAs therapy are lower in the first year but similar after long term treatment to values reported in clinical trials. Adherence to HBV medication, higher ALT level, and non-vertical transmission are predictive factors of HBeAg seroconversion in patients treated with NAs.
References
-
1Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-2.
-
2Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-83.
-
3Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-52.
-
4Hui CK, Leung N, Shek TW, et al. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690-8.
-
5Lin SM, Yu ML, Lee CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45-52.
-
6Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-34.
-
7Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-61.
-
8Liu A, Ha NB, Lin B, et al. Low hepatitis B envelope antigen seroconversion rate in chronic hepatitis B patients on long-term entecavir 0.5 mg daily in routine clinical practice. Eur J Gastroenterol Hepatol. 2013;25:338-43.
-
9Lin B, Ha NB, Liu A, et al. Low incidence of hepatitis B e antigen seroconversion in patients treated with oral nucleos(t)ides in routine practice. J Gastroenterol Hepatol. 2013;28:855-60.
-
10Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-95.
-
11Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123-9.
-
12Kim HS, Kim HJ, Shin WG, et al. Predictive factors for early HBeAg seroconversion in acute exacerbation of patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2009;136:505-12.
-
13Wu JF, Wu TC, Chen CH, et al. Serum levels of interleukin-10 and interleukin-12 predict early, spontaneous hepatitis B virus e antigen seroconversion. Gastroenterology. 2010;138:e1-3.
-
14Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255-66.
-
15Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74-9.
-
16Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok AS. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19:205-12.
-
17Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-88.
-
18Hou J, Yin YK, Xu D, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47:447-54.
-
19Chan HL, Heathcote EJ, Marcellin P, et al. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med. 2007;147:745-54.
-
20Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-22.
-
21Marcellin P, Chang TT, Lim SG, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750-8.
-
22Zoutendijk R, Reijnders JG, Brown A, et al. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naive patients with a partial virological response. Hepatology. 2011;54:443-51.
-
23Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106:1264-71.
-
24Gane EJ, Wang Y, Liaw YF, et al. Efficacy and safety of prolonged 3-year telbivudine treatment in patients with chronic hepatitis B. Liver Int. 2011;31:676-84.
-
25Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455-61.
-
26Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462-7.
-
27Donnelly LA, Doney AS, Morris AD, Palmer CN, Donnan PT. Long-term adherence to statin treatment in diabetes. Diabet Med. 2008;25:850-5.
-
28Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54:12-8.
-
29Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139:491-8.
-
30Bae SK, Yatsuhashi H, Hashimoto S, et al. Prediction of early HBeAg seroconversion by decreased titers of HBeAg in the serum combined with increased grades of lobular inflammation in the liver. Med Sci Monit. 2012;18:CR698-705.
-
31Yuen MF, Yuan HJ, Hui CK, et al. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416-9.
-
32Wang CC, Tseng KC, Peng CY, et al. Viral load and alanine aminotransferase correlate with serologic response in chronic hepatitis B patients treated with entecavir. J Gastroenterol Hepatol. 2013;28:46-50.
-
33Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-95.
-
34Sogni P, Carrieri MP, Fontaine H, et al. The role of adherence in virological suppression in patients receiving anti-HBV analogues. Antivir Ther. 2012;17:395-400.
Publication Dates
-
Publication in this collection
May-Jun 2017
History
-
Received
16 Nov 2015 -
Accepted
10 Mar 2017