ABSTRACT
Background:
Hypothyroidism due to Hashimoto's thyroiditis (HT) is the commonest autoimmune endocrine illness in which antibodies against thyroid organ result in inflammation. The disease has a complex etiology that involves genetic and environmental influences. Viral infections may be involved in triggering of the disease as their molecular mimicry enhance autoimmune responses. Human herpesvirus-6 (HHV-6) is recognized for its contribution to some autoimmune diseases.
Objective:
In the current study, the prevalence of HHV-6 active infection in patients with HT and with non-autoimmune thyroid disorders were compared with patients with euthyroidism. In addition, a correlation between presence of HHV-6 infections and HT was investigated.
Methods:
A total of 151 patients with clinically and laboratory confirmed HT, 59 patients with non-autoimmune thyroid disorders, and 32 patients with normal thyroid function were included in the study. For further confirmation of HT disease, all the precipitants were tested for anti-thyroid peroxidase (TPO), and anti-thyroglobulin (TG) antibodies. For detection of both HHV-6 types A and B, nested PCR and restriction enzyme digestion were used. HHV-6 DNA positive samples were further investigated by DNA sequencing analysis.
Results:
HHV-6A DNA was found in serum sample of 57 out of 151 patients (38%) with HT, which was significantly more often than in patients with non-autoimmune thyroid disorders (p = 0.001). However, HHV-6 DNA was not detected in serum samples of euthyroid subjects.
Conclusions:
The results support a possible role for active HHV-6A infection, demonstrated by the presence of HHV-6 DNA in sera, in the development of HT.
Keywords:
Human herpesvirus 6; Prevalence; Hashimoto disease; Autoimmune diseases
Introduction
Hashimoto's thyroiditis (HT) is a common autoimmune disease in which the immune system attacks the thyroid causing inflammation and progressive destruction of the gland. It is a common cause of hypothyroidism with a potential complication of thyroid lymphoma.11 Noureldine SI, Tufano RP. Association of Hashimoto's thyroiditis and thyroid cancer. Curr Opin Oncol. 2015;27:21-5.,22 Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Roliński J. Immune disorders in Hashimoto's thyroiditis: what do we know so far?. J Immunol Res. 2015;2015:979167.
Several factors are thought to be involved in the etiology of the disease including genetic and environmental factors.33 Shukla SK, Singh G, Ahmad S, Pant P. Infections, genetic and environmental factors in pathogenesis of autoimmune thyroid diseases. Microb Pathog. 2018;116:279-88. Viral infection may play a role in triggering autoimmunity through molecular mimicry.44 Mori K, Yoshida K. Viral infection in induction of Hashimoto's thyroiditis: a key player or just a bystander?. Curr Opin Endocrinol Diabetes Obes. 2010;17:418-24.
Recently, human herpesviruses 6 type A and type 6B were recognized as two distinct viruses with different biological features and disease associations.55 Ablashi D, Agut H, Alvarez-Lafuente R, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014;159:863-70. HHV-6B is associated with roseola infantum, a benign childhood febrile disease, which can be complicated by febrile seizures. In addition, it is the major cause of encephalitis in transplant patients.66 Abidi MZ, Hari P, Chen M, et al. Virus detection in the cerebrospinal fluid of hematopoietic stem cell transplant recipients is associated with poor patient outcomes: a CIBMTR contemporary longitudinal study. Bone Marrow Transpl. 2019;January, http://dx.doi.org/10.1038/s41409-019-0457-9.
http://dx.doi.org/10.1038/s41409-019-045...
While HHV-6B is common and acquired by over 70% of children by age three,77 Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-76. HHV-6A is acquired later in life and the prevalence is unknown. In preliminary reports, HHV-6A has been associated with Alzheimer's disease88 Readhead B, Haure-Mirande J-V, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99, 64–82.e7. and unexplained infertility.99 Marci R, Gentili V, Bortolotti D, et al. Presence of HHV-6A in endometrial epithelial cells from women with primary unexplained infertility. PLoS One. 2016;11:e0158304. HHV-6A/B have also been proposed as triggers for a number of autoimmune disorders, including multiple sclerosis (MS),1010 Voumvourakis KI, Kitsos DK, Tsiodras S, Petrikkos G, Stamboulis E. Human herpesvirus 6 infection as a trigger of multiple sclerosis. Mayo Clin Proc. 2010;85:1023-30.,1111 Behzad-Behbahani A, Mikaeili MH, Entezam M, et al. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. J Microbiol Immunol Infect. 2011;44:247-51. autoimmune connective tissue diseases,1212 Broccolo F, Drago F, Paolino S, et al. Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J Clin Virol. 2009;46:43-6. and HT.1313 Caselli E, Zatelli MC, Rizzo R, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8:e1002951. Seropositivity for HHV-6A/B is over 90% in many studies.1414 Ward KN, Thiruchelvam AD, Couto-Parada X. Unexpected occasional persistence of high levels of HHV-6 DNA in sera: detection of variants A and B. J Med Virol. 2005;76:563-70.
HHV-6A and HHV-6B infect T cells, monocytes-macrophages, epithelial cells, and central nervous system cells.1515 Eliassen E, Di Luca D, Rizzo R, Barao I. The interplay between natural killer cells and human herpesvirus-6. Viruses. 2017;9:367. It has been shown that CD46, a complement regulatory protein, is the only cellular receptor for HHV-6A which is a surface protein presented in all cell types,55 Ablashi D, Agut H, Alvarez-Lafuente R, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014;159:863-70.,1616 Jasirwan C, Furusawa Y, Tang H, Maeki T, Mori Y. Human herpesvirus-6A gQ1 and gQ2 are critical for human CD46 usage. Microbiol Immunol. 2014;58:22-30.,1717 Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817-27, http://www.ncbi.nlm.nih.gov/pubmed/10619434. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/10619...
whereas HHV-6B uses CD134, a member of the TNF-receptor, that is expressed mainly on activated T lymphocytes.1818 Tang H, Serada S, Kawabata A, et al. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc Natl Acad Sci. 2013;110:9096-9. The HHV-6A uses envelop gH/gL/gQ1/gQ2 glycoprotein complex to attach cellular CD46 receptor, while the HHV-6B rarely binds to CD46 with the same glycoproteins as HHV-6A.1616 Jasirwan C, Furusawa Y, Tang H, Maeki T, Mori Y. Human herpesvirus-6A gQ1 and gQ2 are critical for human CD46 usage. Microbiol Immunol. 2014;58:22-30.
A recent study suggests a potential link between HHV-6 infection of thyroid cells and the progress or activating of HT disease.1313 Caselli E, Zatelli MC, Rizzo R, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8:e1002951.,1919 Caselli E, D'Accolti M, Soffritti I, et al. HHV-6A in vitro infection of thyrocytes and T cells alters the expression of miRNA associated to autoimmune thyroiditis. Virol J. 2017;14:3. In addition, available data on in vitro infection of thyrocytes with HHV-6A indicate modulation of miRNAs which are involved in the disease development. These alterations were not seen in thyrocytes infected with HHV-6B.1919 Caselli E, D'Accolti M, Soffritti I, et al. HHV-6A in vitro infection of thyrocytes and T cells alters the expression of miRNA associated to autoimmune thyroiditis. Virol J. 2017;14:3.
The presence of HHV-6B and HHV-6A DNA in the plasma sample both indicates active infection, though HHV-6B is more likely to go into latency in peripheral blood mononuclear cells (PBMCs). Although HHV-6B DNA is detected in PBMCs of both patients and healthy individuals, analysis of serum samples indicated that none of the healthy blood donors were positive for HHV-6 DNA.2020 Alvarez-Lafuente R, Martín-Estefanía C, de Las Heras V, et al. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch Neurol. 2002;59:929-33, http://www.ncbi.nlm.nih.gov/pubmed/12056928. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/12056...
Considering that a possible relationship exists between infection with human herpesvirus 6 (HHV-6) and autoimmune diseases, the purpose of this study was to determine the prevalence of HHV-6A/B DNA in serum of patients with HT, non-autoimmune thyroid disorders, and patients with normal thyroid gland function (euthyroid) to identify if HHV-6A/B active infection may be a possible risk factor for triggering HT disease.
Material and methods
Patients and clinical samples
Thyroiditis were classified based on the American Thyroid Association definition. Based on clinical, laboratory, and ultrasonography findings, 151 patients with a definite diagnosis of HT, 59 patients with non-autoimmune thyroid disorders, and 32 subjects with normal thyroid gland function were included in the study. To confirm the results, thyroid function tests were repeated prior to taking any drug that could possibly affect thyroid function. None of them had concomitant diseases.
The patients were initially diagnosed with thyroiditis in routine clinical practice by general practitioners and referred to the endocrinology clinic of the University Hospital for further investigation. The study was approved by the University Ethics Committee. Informed consent was collected from all participants before sample collection.
Blood samples were collected in serum gel separator tubes and were then centrifuged to separate the serum and stored in a sterile tube at −20ºC until use.
Thyroid function measurements and antibody assays
Serum TSH (normal range, 0.3-5.3 µIU/mL), free T3 (normal range, 1.9-5.7 pmol/L), free T4 (normal range, 10-22 pmol/L), thyroid peroxidase antibodies (anti-TPO normal range, <35 IU/mL), and thyroglobulin antibodies (Tg Ab < 115 IU/mL) were measured in all subjects by using an immuno-chemiluminescence technique (Cobas E411 system). Anti-HHV-6 antibody in the serum samples was determined by enzyme immunoassay according to the manufacturer's instruction (Biotin, Dublin, Ireland) as described before.1111 Behzad-Behbahani A, Mikaeili MH, Entezam M, et al. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. J Microbiol Immunol Infect. 2011;44:247-51. An index value for HHV-6 antibody in serum samples for both patient and control samples was calculated as suggested by the manufacturer (Biotin).
DNA isolation
Total DNA was isolated from the serum samples with the use of DNeasy Blood & Tissue Kit (Qiagen, Germany) according to the manufacturer's protocol. The DNA was then re-suspended in a final elution buffer volume of 50 µL and stored at −20ºC until further analysis.
Detection of HHV-6 DNA in serum samples
The presence of HHV-6 DNA in serum samples was detected by nested PCR with primers designed to specifically targeting U67 gene in a region, a conserved between HHV-6A and HHV-6B. For the amplification of HHV-6 DNA, the outer primers (PF1: 5'GCTAGAACGTATTTGCTG3'; PR1: 5'ACAACTGTCTGACTGGCA3') which amplify a fragment of 252 base pairs (bp) and the inner primers (PF2: 5'CTCAAGATCAACAAGTTG3'; PR2: 5'TCACGCACATCGGTATAT3') which amplify a fragment of 154 bp were used. Primer-BLAST was performed to check the specificity of the primers (https://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Nested PCR
For the amplification of target genes, PCR was run in two separate steps. To amplify a 154 bp DNA fragment of the major capsid protein (MCP) of HHV-6A and HHV-6B genotype genes, the first round of PCR amplification was carried out with 2 µL of extracted DNA from serum samples, 1× PCR master mix, and 0.5 µM of each outer primers in a final volume of 20 µL. To construct the plasmid control for HHV-6A and HHV-6B, the PCR product fragments of HHV-6A and HHV-6B isolated from clinical samples and confirmed by DNA sequencing, U67 fragments were amplified as follows: initial denaturation at 95ºC for 5 min, 35 cycles of 95ºC for 30 s, 50ºC for 30 s, and 72ºC for 30 s, followed by a final extension at 72ºC for 5 min.
The second round of PCR was performed as mentioned for the first round, except that one microliter of the first PCR product was added to the second PCR reaction mixture. The PCR products were analyzed on 2% agarose gel electrophoresis and stained with GelRed TM (Biotium, UK), and visualized under UV light.
To test for possible plasmid contamination, PCR reaction was performed using T7 universal primer of pTZ57R/T vector 5'TAATACGACTCACTATAGGG3' and PF1 primer of HHV-6 5'GCTAGAACGTATTTGCTG3' that amplify a 320 bp fragment including a portion of HHV-6 and T7 promoter sequences. In addition, extensive precautions were used to prevent PCR contamination. To ensure efficient DNA extraction and PCR amplification, a 177-bp fragment of the human β-actin gene was amplified together with the samples using 5'ATCGTGCGTGACATTAAGGAG3' and 5'GAAGGAAGGCTGGAAGAGTG3' primers.
The sensitivity of nested PCR assays
To evaluate the sensitivity of HHV-6 PCR detection, a set of serum sample negative for HHV-6A and HHV-6B DNA were spiked with known 10-fold serial dilutions of HHV-6 A and HHV-6B DNA in a separate reaction. After DNA extraction, two stages of consecutive amplification were used for each virus separately. The product of the first-round amplification (252 bp) was used as targets for the second round of nested PCR (154 bp) amplification. The last positive dilution of the first-round PCR product (252 bp) and dilutions which in the first round PCR failed to yield a PCR product were used as targets for the determination of the second round of nested PCR sensitivity test (154 bp). The amplified products were then analyzed by gel electrophoresis and GelRedTM (Biotium, USA) post-staining.
Sequence analysis
To investigate the specificity of PCR, the amplified products were subjected to nucleotide sequencing analysis. To verify the accuracy of sequence determination, each nested PCR product was gel purified and cloned into pTZ57R/T plasmid vector. Sequence analysis was performed (150-200 ng/µL) in both forward and reverse directions using specific primers by ABI 3730XL DNA analyzer (Bioneer, Inc.).
Restriction fragment analysis for HHV-6A/B determination
A 154 bp fragment of the U67 gene was amplified. This fragment includes an XhoI restriction site in the HHV-6A genome absent in type B. XhoI digestion was performed on the PCR product, producing two fragments of 105 and 49 bp for HHV-6A. The procedure was performed according to the manufacturer's instruction (Thermo scientific).
Statistical analysis
Statistical analysis was performed using SPSS software version 18 (SPSS Inc., Chicago, IL, USA). Pearson Chi-Square and Likelihood Ratio were used to determine any relation between the variables. Two-tailed p-values < 0.05 were considered as statistically significant.
Results
General characteristics
Overall, 242 patients were enrolled in the study. The demographic characteristics of patients and the results of thyroid function tests are shown in Table 1.
For the whole study group, there was a significant difference between age and TSH levels of participants (p < 0.05). In addition, a significant correlation between age and the levels of FT3, FT4, anti-TPO, and anti-Tg (p < 0.01) was found.
Anti-IgG in patients with Hashimoto thyroiditis and in control groups
Anti-HHV-6 IgG antibody was detected in 69.5% of patients with HT, in 77.8%, patients with non-autoimmune thyroid disorders, and in 40.6% of euthyroid subjects. Based on anti-HHV-6 antibody detection, there was a significant difference between euthyroid subjects and patients suffering from thyroid dysfunctions (p < 0.001).
HHV-6 DNA in serum samples
Serum samples collected from 151 HT patients and 59 patients with non-autoimmune thyroid disorders as well as 32 euthyroid patients were analyzed for the presence of HHV-6 DNA by nested PCR using primers specific for a highly conserved sequence of U67 gene corresponding to the major capsid protein gene of HHV-6A/B. When evaluated by gel electrophoresis and GelRed staining, the lower limits of detection using nested PCR assay for HHV-6A/B DNA were determined to be five copies/reaction of HHV-6A or HHV-6B DNA.
To ensure impartiality, and avoid errors arising from bias, all the samples were analyzed in double-blind tests. HHV-6 DNA was detected in the serum sample of 57 out of 151 (38%) patients with HT, in contrast to five out of 59 (8%) patients with non-autoimmune thyroid disorders and 0/32 (0%) in euthyroid subjects. The prevalence of HHV-6 DNA in HT patients compared to that of the two other control groups was significantly different (p = 0.001). Distribution of HHV-6 DNA in serum samples of the patients is shown in Table 1.
HHV-6 DNA sequencing and typing
The primers were not able to differentiate between the two types on the basis of the size of the PCR product. For discriminating between HHV-6A and HHV-6B, restriction endonuclease XhoI and nucleotide sequences were used. In all samples positive for HHV-6 DNA detected in patients either with HT or non-autoimmune thyroiditis diseases, where accurate typing result was achieved, the virus was HHV-6 type A.
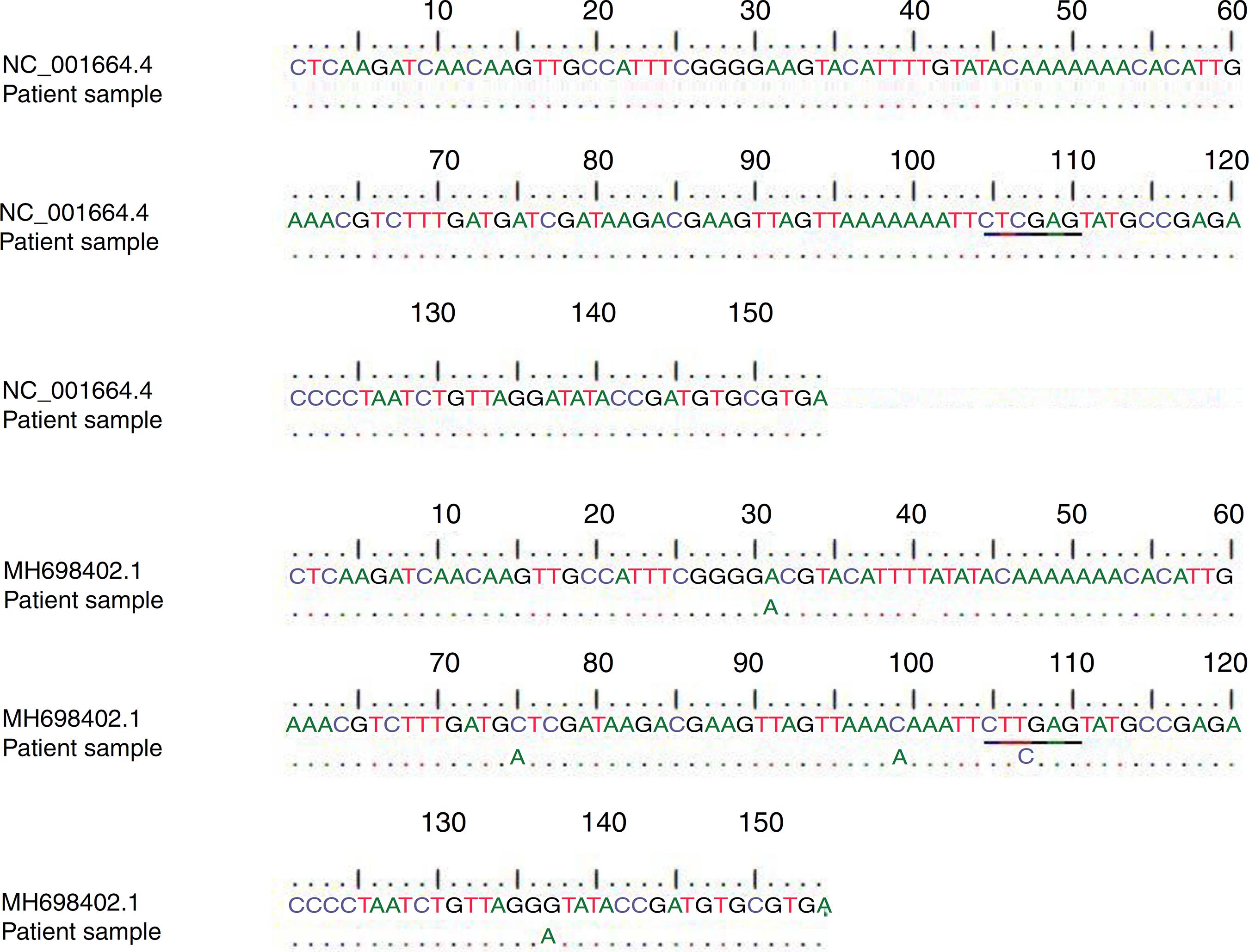
In addition, the obtained sequences were subjected to a BLAST search against the GenBank database for the HHV-6A (accession no. NC_001664.4) and HHV-6B (GenBank accession no. MH698402.1) (Fig. 1). A type was assigned to each sample based on the homology of our sequence data with published sequences in GenBank as shown in Fig. 1.
Alignment of patients' samples sequence with HHV-6A (NC_001664.4) and HHV-6B (MH698402.1) sequences in the GenBank database. Underlined letters indicate the position of the restriction site sequence.
Discussion
Among different environmental factors associated with autoimmune thyroiditis disease (AITD), viral infections are believed to trigger autoimmunity and may act alone or in concert with other environmental factors.2121 Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150:605-18, http://www.ncbi.nlm.nih.gov/pubmed/15132715. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/15132...
,2222 Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. Herpesviruses, in particular, have been involved in the development or exacerbation of disease.2222 Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5.
23 Janegova A, Janega P, Rychly B, Kuracinova K, Babal P. Rola infekcji wirusem Epstein-Barr'a w rozwoju autoimmunologicznych chorób tarczycy. Endokrynol Pol. 2015;66:132-6.-2424 Dittfeld A, Gwizdek K, Michalski M, Wojnicz R. A possible link between the Epstein-Barr virus infection and autoimmune thyroid disorders. Cent Eur J Immunol. 2016;3:297-301. Much attention has been paid to infection with HHV-6A and AITD.
The important role of HHV-6 infection in association with autoimmune HT has generally been considered in a recent study.1313 Caselli E, Zatelli MC, Rizzo R, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8:e1002951. The virus was detected in 82% fine needle aspirations from HT patients. However, HHV-6 DNA was found only in 10% of the samples from patients with benign thyroid lesions. Interestingly, all samples that tested positive for HHV-6 DNA were found to be HHV-6A type, indicating that HT possibly is associated with HHV-6A infection and not with the more common HHV-6B. The evidence associated with infected thyroid cells with HHV-6 indicates that they are susceptible to natural killer cells. Therefore, the involvement of the virus in the induction of autoimmunity in HT would be expected.
In addition, detection of HHV-6 DNA, mRNA, and antigen in tissue samples of patients with autoimmune thyroiditis indicated the association of HHV-6 infection with this autoimmune disease.1313 Caselli E, Zatelli MC, Rizzo R, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8:e1002951.,2525 Sultanova A, Cistjakovs M, Gravelsina S, et al. Association of active human herpesvirus-6 (HHV-6) infection with autoimmune thyroid gland diseases. Clin Microbiol Infect. 2017;23, 50.e1-50.e5. However, the genotype of HHV-6 had not been determined. Yet, detection of HHV-6 DNA in serum samples of patients with HT and non-autoimmune thyroiditis and in patients with euthyroid diseases had not been reported.
In our study, using a sensitive nested PCR, 38% of patients with HT were positive for HHV-6 DNA in serum samples, whereas in patients with non-autoimmune thyroid disorders only 8% was found, and in none from samples of euthyroid subjects. In terms of viral DNA present in serum samples, there was a significant difference between HT and control groups (p = 0.001). HHV-6 DNA was not detected in serum samples from euthyroid patients (normal thyroid gland function with positive HHV-6 antibody). However, HHV-6 DNA was detected in serum samples from patients with autoimmune HT and in patients with non-autoimmune thyroid disorders in which anti-HHV-6 antibody was positive. Although the mean age of patients with non-autoimmune thyroiditis was higher than the other two groups, it had no influence on laboratory and HHV-6 results.
Interestingly, the variant analysis performed by XhoI restriction enzyme and DNA sequence analysis of HHV-6 DNA positive subjects indicates that all samples harbored HHV-6A, suggesting an association between thyroid diseases, in particular, HT to this virus.
As mentioned, HHV-6A/B has a tropism to thyrocytes which are permissive to virus replication.1313 Caselli E, Zatelli MC, Rizzo R, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8:e1002951. CD46 (also known as membrane cofactor protein or MCP) has been shown as the cellular receptor for HHV-6A entry,2626 Hoffmann A, Kirn E, Kuerten A, Sander C, Krueger GR, Ablashi DV. Active human herpesvirus-6 (HHV-6) infection associated with Kikuchi-Fujimoto disease and systemic lupus erythematosus (SLE). In Vivo. 1991;5:265-9, http://www.ncbi.nlm.nih.gov/pubmed/1654149. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/16541...
whereas CD134, a member of the TNF receptor superfamily, is the specific receptor that allows HHV-6B entry into cells.1818 Tang H, Serada S, Kawabata A, et al. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc Natl Acad Sci. 2013;110:9096-9. On the other hand, CD46 are expressed strongly on the cell surface of almost all benign and malignant thyroid follicular cells.2727 Yamakawa M, Yamada K, Tsuge T, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994;73:2808-17, http://www.ncbi.nlm.nih.gov/pubmed/7514955. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/75149...
Although HHV-6A/B share 90% identity in their nucleic acid sequence, they show distinct pathogenesis and cell tropism.
Some studies have reported the presence of HHV-6 DNA in PBMCs of healthy individuals.2828 Cone RW, Huang ML, Ashley R, Corey L. Human herpesvirus 6 DNA in peripheral blood cells and saliva from immunocompetent individuals. J Clin Microbiol. 1993;31:1262-7, http://www.ncbi.nlm.nih.gov/pubmed/8388889. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/83888...
29 Cuende JI, Ruiz J, Civeira MP, Prieto J. High prevalence of HHV-6 DNA in peripheral blood mononuclear cells of healthy individuals detected by nested-PCR. J Med Virol. 1994;43:115-8, http://www.ncbi.nlm.nih.gov/pubmed/8083658. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/80836...
-3030 Géraudie B, Charrier M, Bonnafous P, et al. Quantitation of human herpesvirus-6A, -6B and -7 DNAs in whole blood, mononuclear and polymorphonuclear cell fractions from healthy blood donors. J Clin Virol. 2012;53:151-5. But, the detection of HHV-6 DNA by PCR in PBMCs, the site where the virus establishes latency, does not discriminate between active and latent/chronic persistent infection. However, viral load is a preferred indicator of active infection.3131 Gautheret-Dejean A, Manichanh C, Thien-Ah-Koon F, et al. Development of a real-time polymerase chain reaction assay for the diagnosis of human herpesvirus-6 infection and application to bone marrow transplant patients. J Virol Methods. 2002;100(1-2):27-35, http://www.ncbi.nlm.nih.gov/pubmed/11742650. Accessed March 13, 2019.
http://www.ncbi.nlm.nih.gov/pubmed/11742...
Alternatively, detection of HHV-6 DNA by PCR in the serum sample is a useful indicator of active infection. However, HHV-6 DNA is not always present in plasma when the virus is active in a distant lymphoid tissue or organ.3232 Achour A, Boutolleau D, Slim A, Agut H, Gautheret-Dejean A. Human herpesvirus-6 (HHV-6) DNA in plasma reflects the presence of infected blood cells rather than circulating viral particles. J Clin Virol. 2007;38:280-5.
Even though there was no significant correlation between the presence of anti-HHV-6 antibody in serum samples among our experimental groups (p = 0.3), the percentage of infected patients with HT and non-autoimmune thyroiditis diseases positive for HHV-6 antibody was higher than among normal subjects. Overall, 67% of HT patients with positive anti-HHV-6 antibody harbored HHV-6 DNA variant A in serum samples.
To clarify the association and possible role of HHV-6 as a trigger of HT, further studies are required. Several mechanisms have been suggested in which HHV-6 infections might induce an autoimmune response. However, quantification of HHV-6 viral loads in whole blood or serum samples of HT patients in association with measuring of anti-TPO antibody titer will support the role of HHV-6 active infection as a potential trigger of thyroid disease.
Conclusion
HHV-6A DNA was detected in serum samples of patients with Hashimoto's thyroiditis but not in serum samples of euthyroid patients, supporting the association of HHV-6A infection and disease progression. In addition, the results of this study support other findings that HHV-6A infection might contribute to Hashimoto's thyroiditis development.
Compliance with ethical standards
-
Ethical statementThe study was approved by the Ethics Committee of Shiraz University of Medical Sciences and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
-
Informed consentInformed consent was obtained from all individual participants included in the study.
-
Funding sourcesThis research was supported by Shiraz University of Medical Sciences grant number 1396-01-45-14603. There was no further funding from other sources to report for this submission.
Acknowledgment
The authors wish to thank Ms. Kristin Loomis, president & executive director at the HHV-6 Foundation for her invaluable assistance in editing this manuscript.
References
-
1Noureldine SI, Tufano RP. Association of Hashimoto's thyroiditis and thyroid cancer. Curr Opin Oncol. 2015;27:21-5.
-
2Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Roliński J. Immune disorders in Hashimoto's thyroiditis: what do we know so far?. J Immunol Res. 2015;2015:979167.
-
3Shukla SK, Singh G, Ahmad S, Pant P. Infections, genetic and environmental factors in pathogenesis of autoimmune thyroid diseases. Microb Pathog. 2018;116:279-88.
-
4Mori K, Yoshida K. Viral infection in induction of Hashimoto's thyroiditis: a key player or just a bystander?. Curr Opin Endocrinol Diabetes Obes. 2010;17:418-24.
-
5Ablashi D, Agut H, Alvarez-Lafuente R, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014;159:863-70.
-
6Abidi MZ, Hari P, Chen M, et al. Virus detection in the cerebrospinal fluid of hematopoietic stem cell transplant recipients is associated with poor patient outcomes: a CIBMTR contemporary longitudinal study. Bone Marrow Transpl. 2019;January, http://dx.doi.org/10.1038/s41409-019-0457-9
» http://dx.doi.org/10.1038/s41409-019-0457-9 -
7Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-76.
-
8Readhead B, Haure-Mirande J-V, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99, 64–82.e7.
-
9Marci R, Gentili V, Bortolotti D, et al. Presence of HHV-6A in endometrial epithelial cells from women with primary unexplained infertility. PLoS One. 2016;11:e0158304.
-
10Voumvourakis KI, Kitsos DK, Tsiodras S, Petrikkos G, Stamboulis E. Human herpesvirus 6 infection as a trigger of multiple sclerosis. Mayo Clin Proc. 2010;85:1023-30.
-
11Behzad-Behbahani A, Mikaeili MH, Entezam M, et al. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. J Microbiol Immunol Infect. 2011;44:247-51.
-
12Broccolo F, Drago F, Paolino S, et al. Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J Clin Virol. 2009;46:43-6.
-
13Caselli E, Zatelli MC, Rizzo R, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8:e1002951.
-
14Ward KN, Thiruchelvam AD, Couto-Parada X. Unexpected occasional persistence of high levels of HHV-6 DNA in sera: detection of variants A and B. J Med Virol. 2005;76:563-70.
-
15Eliassen E, Di Luca D, Rizzo R, Barao I. The interplay between natural killer cells and human herpesvirus-6. Viruses. 2017;9:367.
-
16Jasirwan C, Furusawa Y, Tang H, Maeki T, Mori Y. Human herpesvirus-6A gQ1 and gQ2 are critical for human CD46 usage. Microbiol Immunol. 2014;58:22-30.
-
17Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817-27, http://www.ncbi.nlm.nih.gov/pubmed/10619434 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/10619434 -
18Tang H, Serada S, Kawabata A, et al. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc Natl Acad Sci. 2013;110:9096-9.
-
19Caselli E, D'Accolti M, Soffritti I, et al. HHV-6A in vitro infection of thyrocytes and T cells alters the expression of miRNA associated to autoimmune thyroiditis. Virol J. 2017;14:3.
-
20Alvarez-Lafuente R, Martín-Estefanía C, de Las Heras V, et al. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch Neurol. 2002;59:929-33, http://www.ncbi.nlm.nih.gov/pubmed/12056928 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/12056928 -
21Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150:605-18, http://www.ncbi.nlm.nih.gov/pubmed/15132715 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/15132715 -
22Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5.
-
23Janegova A, Janega P, Rychly B, Kuracinova K, Babal P. Rola infekcji wirusem Epstein-Barr'a w rozwoju autoimmunologicznych chorób tarczycy. Endokrynol Pol. 2015;66:132-6.
-
24Dittfeld A, Gwizdek K, Michalski M, Wojnicz R. A possible link between the Epstein-Barr virus infection and autoimmune thyroid disorders. Cent Eur J Immunol. 2016;3:297-301.
-
25Sultanova A, Cistjakovs M, Gravelsina S, et al. Association of active human herpesvirus-6 (HHV-6) infection with autoimmune thyroid gland diseases. Clin Microbiol Infect. 2017;23, 50.e1-50.e5.
-
26Hoffmann A, Kirn E, Kuerten A, Sander C, Krueger GR, Ablashi DV. Active human herpesvirus-6 (HHV-6) infection associated with Kikuchi-Fujimoto disease and systemic lupus erythematosus (SLE). In Vivo. 1991;5:265-9, http://www.ncbi.nlm.nih.gov/pubmed/1654149 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/1654149 -
27Yamakawa M, Yamada K, Tsuge T, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994;73:2808-17, http://www.ncbi.nlm.nih.gov/pubmed/7514955 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/7514955 -
28Cone RW, Huang ML, Ashley R, Corey L. Human herpesvirus 6 DNA in peripheral blood cells and saliva from immunocompetent individuals. J Clin Microbiol. 1993;31:1262-7, http://www.ncbi.nlm.nih.gov/pubmed/8388889 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/8388889 -
29Cuende JI, Ruiz J, Civeira MP, Prieto J. High prevalence of HHV-6 DNA in peripheral blood mononuclear cells of healthy individuals detected by nested-PCR. J Med Virol. 1994;43:115-8, http://www.ncbi.nlm.nih.gov/pubmed/8083658 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/8083658 -
30Géraudie B, Charrier M, Bonnafous P, et al. Quantitation of human herpesvirus-6A, -6B and -7 DNAs in whole blood, mononuclear and polymorphonuclear cell fractions from healthy blood donors. J Clin Virol. 2012;53:151-5.
-
31Gautheret-Dejean A, Manichanh C, Thien-Ah-Koon F, et al. Development of a real-time polymerase chain reaction assay for the diagnosis of human herpesvirus-6 infection and application to bone marrow transplant patients. J Virol Methods. 2002;100(1-2):27-35, http://www.ncbi.nlm.nih.gov/pubmed/11742650 Accessed March 13, 2019.
» http://www.ncbi.nlm.nih.gov/pubmed/11742650 -
32Achour A, Boutolleau D, Slim A, Agut H, Gautheret-Dejean A. Human herpesvirus-6 (HHV-6) DNA in plasma reflects the presence of infected blood cells rather than circulating viral particles. J Clin Virol. 2007;38:280-5.
Publication Dates
-
Publication in this collection
14 Feb 2020 -
Date of issue
Nov-Dec 2019
History
-
Received
8 July 2019 -
Accepted
17 Oct 2019