ABSTRACT
Background:
Papiliotrema laurentii is one of several non-neoformans cryptococci that have rarely been associated with human infection, since it was previously considered saprophyte and thought to be non-pathogenic to humans. Nevertheless, increasing number of reports of human infection have emerged in recent years, mostly in oncologic patients.
Aim:
To report a case of a female patient with pyloric obstructive cancer with a catheter-related Papiliotrema laurentii blood stream infection and systematically review the available evidence on P. laurentii infection in humans.
Methods:
Retrieval of studies was based on Medical Subject Headings and Health Sciences Descriptors, which were combined using Boolean operators. Searches were run on the electronic databases Scopus, Web of Science, MEDLINE (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), Cochrane Library for Systematic Reviews and Opengray.eu. There was no language or date of publication restrictions. The reference lists of the studies retrieved were searched manually.
Results:
The search strategy retrieved 1703 references. In the final analysis, 31 references were included, with the description of 35 cases. Every patient but one had a previous co-morbidity - 48.4 % of patients had a neoplasm. Amphotericin B was the most used treatment and only a single case of resistance to it was reported. Most patients were cured of the infection.
Conclusion:
P. laurentii infection in humans is usually associated to neoplasia and multiple co-morbidities, and amphotericin B seems to be a reliable agent for treatment.
Keywords:
Cryptococcus; Papiliotrema; Catheter-related infections; Amphotericin B; Stomach neoplasms
Introduction
The advancement of Medicine brought new medications, therapeutics, invasive diagnostic methods and surgical approaches have in different pathologies. However, new obstacles emerge to defy our scientific knowledge. Rare pathogens until then unknown take advantage of health fragility in humans to cause infections with alarming proportions.
Cryptococcus spp. other than C. neoformans and C. gattii were previously considered to be saprophytes and non-pathogenic to humans; however, opportunistic infections associated with rare Cryptococcus spp., such as Cryptococcus laurentii and Cryptococcus albidus, have increased over the past four decades.11 Park SS, Lee H, Park WS, Hwang SH, Choi SI, Choi MH, et al. A case of disseminated infection with skin manifestation due to non-neoformans and non-gattii cryptococcus in a patient with refractory acute myeloid leukemia. Infect Chemother. 2017;49:142-5.Cryptococcus laurentii belongs to the phylum basidiomycota of the fungi and is an encapsulated saprobic yeast and can be widely isolated from various types of environments.22 Bhat V, Vira H, Khattry N, Toshniwal M. Cryptococcus laurentii diarrhea post hematopoietic stem cell transplant. Transpl Infect Dis. 2017;19:e12663. It is widely distributed throughout the world, including the Caribbean, Antarctic and the Himalayas and can be acquired from air, water, wood, soil, pigeon excrements as well as various foods, such as cheese, fruit, pork products, bean, and wine.33 Furman-Kuklinska K, Naumnik B, Mysliwiec M. Fungaemia due to cryptococcus laurentii as a complication of immunosuppressive therapy--a case report. Adv Med Sci. 2009;54:116-9. Since 2015, the species name Cryptococcus laurentii was replaced by Papiliotrema laurentii. This nomenclature was based on phylogenetic analyses based on the sequencing of seven genes and regions such as ITS rRNA gene, the D1/D2 domains of the large subunit (LSU or 26S) rRNA gene, the small subunit (SSU or 18S) rRNA gene, two subunits of RNA polymerase II (RPB1 and RPB2), translation elongation factor 1-α (TEF1) andcytochrome b (CYTB).
With increasing immunosuppression due to antineoplastic therapy, organ transplantation, catheter insertion, dialysis and other invasive diagnostic and therapeutic procedures, systemic fungal infections are observed more frequently.44 Krcméry VJ, Kunova A, Mardiak J. Nosocomial cryptococcus laurentii fungemia in a bone marrow transplant patient after prophylaxis with ketoconazole successfully treated with oral fluconazole. Infection. 1997;25:130. Non-neoformans cryptococci have been reported to cause infection in many organs. The bloodstream and central nervous system are the most common sites of non-neoformans cryptococcal infection.55 Khawcharoenporn T, Apisarnthanarax A, Kiratisin P, Mundy LM, Bailey TC. Evaluation of cryptococcus laurentii meningitis in a patient with hiv infection: A case report and review of the literature. Hawaii Med J. 2006;65:260-2. Due to the rarity of cases involving P. laurentii, a standard treatment has not yet been established. Commonly, amphotericin B with flucytosine is recommended.66 Neves RP, Lima Neto RG, Leite MC, Silva VK, Santos Fde A, Macedo DP. Cryptococcus laurentii fungaemia in a cervical cancer patient. Braz J Infect Dis. 2015;19:660-3.
The aim of this paper was to report a case of a female patient with pyloric obstructive cancer with catheter-related Papiliotrema laurentii bloodstream infection and systematically review the available evidence on P. laurentii infection in humans.
Case report
A 68-year old female patient, previously diagnosed with type 2 diabetes, arterial hypertension, non-alcoholic fatty liver disease, with a previous history of breast cancer which was treated with radical mastectomy, radiotherapy and chemotherapy a decade ago, sought care due to weight loss (20 % of total body mass - over 40 pounds), incoercible vomiting, weakness, hypoglicemia and upper abdominal pain.
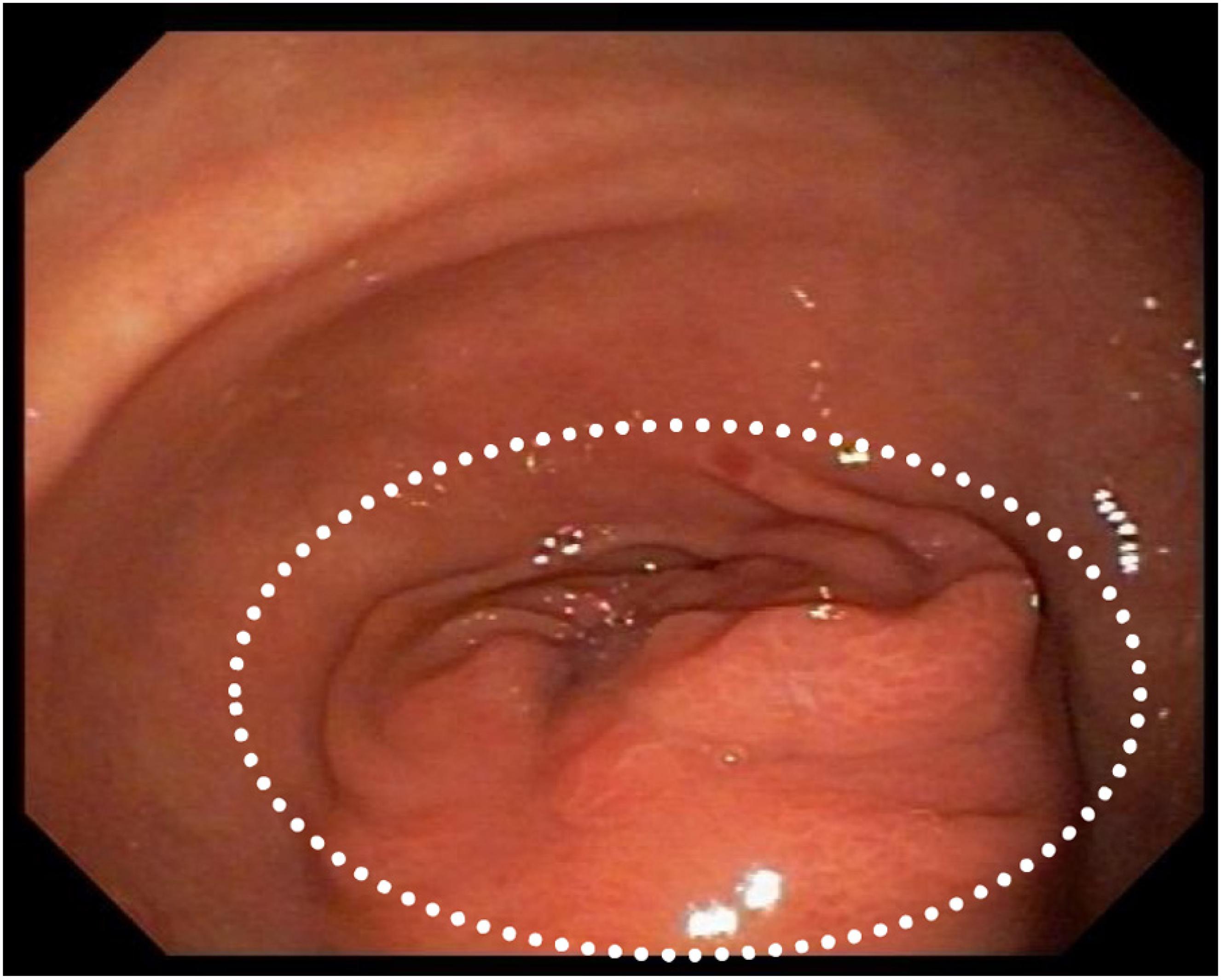
She was admitted to the hospital for investigation. CAT-scan showed gastric distension. Upper digestive endoscopy showed submucosal obstructive pyloric malignancy (Fig. 1), but superficial biopsies came back negative for cancer. Colonoscopy was incomplete due to inadequate colonic preparation - the patient vomited manitol. Magnetic resonance showed a pyloric-duodenal mass, suggestive of submucosal pyloric cancer. A two-week parenteral nutrition (PN) was initiated with the purpose of improving nutrition prior to surgery. Patient gained five pounds while on PN. In the 12th day of PN, the patient began to present fever. Blood cultures were drawn and ampicillin-sulbactam was initiated with little response.
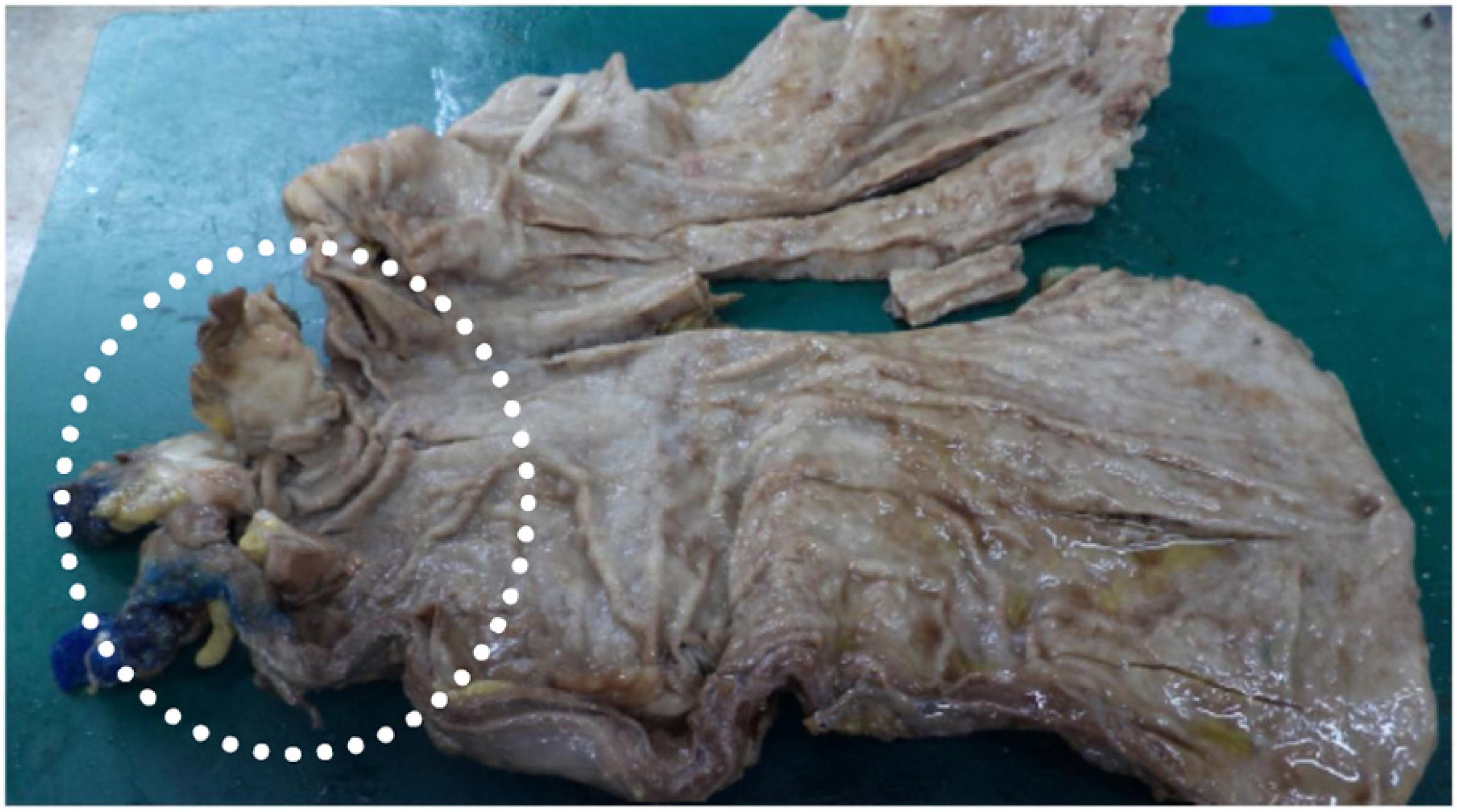
Five days thereafter, the patient presented with bacteremia. Catheter and peripheral cultures drawn during fever came back positive for Papiliotrema laurentii, with antifungigram pending. Identification of the isolate was performed on the Vitek 2 ( YST card - BioMérieux, Marcy l'Etoile, France) automated identification system, which reported P. laurentii. The concern regarding misdiagnosis by Vitek systems was minimized since the P. laurentii culture had been sent to two different laboratories and regrown for the antifungigram, which confirmed the first and second P. laurentii diagnosis with the same resistance profile. The isolate sent to the second lab was again identified by the same Vitek 2 (BioMérieux, Marcy l'Etoile, France) yeast identification card with testing staff blinded to previous Vitek 2 and antifungigram results. Xiao et al. cited that compared to the gold standard (identification of ITS- internal transcribed spacer), Vitek 2 can correctly identify 81.0 % of P. laurentii isolates.77 Xiao M, Fan X, Chen XX, Wang H, Zhang L, Xu ZP, et al. Misidentification of a Rare Species, Cryptococcus laurentii, by Commonly Used Commercial Biochemical Methods and Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Systems: Challenges for Clinical Mycology Laboratories. J Clin Microbiol. 2016;54(1):226-9. Fluconazole, piperacillin-tazobactam and vancomycin replaced ampicillin-sulbactam and the site of the catheter was switched. In five days, fever subdued and after 5-day negative control blood cultures, partial gastrectomy with Y-en-Roux gastroenteric anastomosis was performed (Fig. 2). With an adequate evolution, patient began to eat orally and PN was reduced gradually.
Five days after surgery, fever was again noted and catheter and peripheral cultures were positive for P. laurentii and Candida parapsilosis. The first was susceptible to flucytosine (intermediary), fluconazole, amphotericin B, voriconazole, and resistant to micafungin and caspofungin. The latter was susceptible to flucytosine, fluconazole, amphotericin B, voriconazole, micafungin and caspofungin. Transesophageal echocardiogram was negative for infective endocarditis. Vancomycin and piperacillin-tazobactam were already finished and patient was on monotherapy with fluconazole 800 mg once a day. Catheter site was switched again and amphotericin B 50 mg once a day was associated, with resolution of fever. After 14 days of combined therapy and negative peripheral cultures, patient was discharged with a dosage of 800 mg oral fluconazole daily. A post-operative control CAT-scan and an upper digestive endoscopy were performed, showing no signs of recurrence of the neoplasm (Fig. 3).
Materials and methods
This study was carried out in accordance with the recommendations contained in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA-P) guidelines.88 Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. Bmj. 2015;350:G7647. Our systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO), maintained by York University, on 14 January 2019 [registration No. CRD42019122125 (www.crd.york.ac.uk/prospero/)].
Data sources
Studies were retrieved using the term “Cryptococcus laurentii”. Searches were run on the electronic databases Scopus, Web of Science, Medline (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), Cochrane Library for Systematic Reviews and Opengray.eu. There was no language or date of publication restrictions. The reference lists of the retrieved studies were submitted to manual search. Databases were searched January 2019.
Inclusion criteria and outcomes
Case report or case series studies were eligible for selection. If there was more than one study published using the same case, the most recent study was selected for analysis. Studies published only as abstracts were included, as long as the data available made data collection possible. The outcome measured was cure of the infection or death.
Study selection and data extraction
An initial screening of titles and abstracts was the first stage to select potentially relevant papers. The second step was the analysis of the full-length papers. Two independent reviewers extracted data using a standardized data extraction form after assessing and reaching consensus on eligible studies. The same reviewers separately assessed each study and extracted data about the characteristics of the subjects and the outcomes measured. A third reviewer was responsible for clearing divergences in study selection and data extraction.
Statistical analysis
Data was summarized using descriptive analysis - frequency and means.
Results
Systematic review
The search strategy retrieved 1703 references, 767 references were excluded because they were duplicates. After analyzing titles and abstracts, 900 references were excluded. Full texts were retrieved for 37 references. In the final analysis, 31 references were included, comprehending 35 cases. Flowchart illustrating the search strategy is shown in Fig. 4. Studies included were either a case report or a case series.
Cases from India, Slovakia, USA and Italy were the most common (19.3 %, 12.9 %, 9.7 % and 9.7 %, respectively). A total of 35 patients were included, corresponding to 17 male and 12 female (the sex of six patients was not informed). Age ranged from a 6-day-old neonate to 88 years old (mean age was 40.3 years). The most common clinical presentation was fever (25 %); 16.1 % were related to catheter infection; 67.7 % had positive blood cultures (54.8 %) or of cerebrospinal fluid (12.9 %).
Only one patient was found to have no previous co-morbidity. Twenty-three patients were immunosuppressed (considering both immunologic disorders and/or use of immunosuppressive agents). Neoplasias were described in 48.4 % of the patients.
Resistance profile of P. laurentii was reported for most cases; one case showed resistance to fluconazole and flucytosine and another to amphotericin B. Amphotericin B was the first choice of treatment for 51.6 % of the patients, followed by fluconazole in 35.5 % of the cases. Fluconazole was the choice for maintenance treatment for a longer period. Cure was achieved in 82.8 % of the patients included on this study after proper treatment. These results are summarized in Table 1.
Discussion
P. laurentii has a high degree of interspecies heterogeneity and has been divided into phylogenetic groups I and II. Physiologic and biochemical characteristics of the species in the complex are similar. Nevertheless, the species in phylogenetic group I, such as Cryptococcus flavescens and Cryptococcus aureus, can be distinguished from phylogenetic group II by their combination of assimilation patterns of D-glucosamine, Nacetyl-D-glucosamine, DL-lactic acid, 1,2-propanediol and sodium nitrite and vitamin requirements.3434 Tashima M, Sugita T, Shinoda T, Nakase T. Three new combinations from the Cryptococcus laurentii complex: Cryptococcus aureus, Cryptococcus carnescens and Cryptococcus peneaus. Int J Syst Evol Microbiol. 2003;53:1187-94.Cryptococcus neoformans and P. laurentii share many common traits and structures - the hemolytic capacity of P. laurentii is an intrinsic characteristic that optimizes its infective capacity and increases its growth in blood.3535 Ferreira-Paim K, Andrade-Silva L, Mora DJ, Lages-Silva E, Pedrosa AL, da Silva PR, et al. Antifungal susceptibility, enzymatic activity, PCR-fingerprinting and ITS sequencing of environmental Cryptococcus laurentii isolates from Uberaba, Minas Gerais, Brazil. Mycopathologia. 2012;174(1):41-52.
The likelihood of cryptococcal infection is highly increased in patients with impaired cell-mediated immunity, including lymphoproliferative disorders, HIV infection (CD4 counts < 100 cells/µl) and hematologic malignancies.3636 Perfect JR. Cryptococcus neoformans. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 2005. p. 2997–3009. Other risk factors are: use of steroid or chemotherapy,3737 Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;16:837-74. organ transplantation, impaired humoral immunity such as hyper-IgM syndrome,3838 Iseki M, Anzo M, Yamashita N, Matsuo N. Hyper-IgM immunodeficiency with disseminated cryptococcosis. Acta Paediatr. 1994;83:780-2. non-HIV lymphopenia,3939 Dev D, Basran GS, Slater D. Consider: HIV negative immunodeficiency in cryptococcosis. BMJ. 1994;308:1436. invasive devices4040 Mocani H, Murphy AV, Beattie TJ, McAllister TA. Fungal peritonitis in children on continuous ambolatory peritoneal dialysis. Scot Med J. 1989;34:494-6. and direct or indirect exposures to pigeon excreta.4141 da Cunha T, Lusins J. Cryptococcus albidus meningitis. South Med J. 1973;66:1230. From our analysis, the presence of invasive catheters, immunosuppression and neoplasms were significant risk factors associated to P. laurentii infection.
P. laurentii has been reported to cause infections in many organ systems.4242 Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Non-neoformans Cryptococcal Infections: a Systematic Review. Infection. 2007;35:51-8. The bloodstream and central nervous system were the most common sites of infection, although some other sites such as keratitis4343 Ritterband DC, Seedor JA, Shah MK, Waheed S, Schorr I. A unique case of cryptococcus laurentii keratitis spread by a rigid gas permeable contact lens in a patient with onychomycosis. Cornea. 1998;17(1):115-8. have been reported. Fever was the most common clinical finding, present in most cases. Choices and duration of treatment for P. laurentii infections depended on the anatomical involvement, host-immune status, and severity of infection. Recommendations regarding treatment for infections are limited, due to the small number of empirically treated cases and the absence of controlled trial data. Amphotericin B alone was used for most treatments, with a high rate of cure (80 %). The most used regimen was an induction period of 14 days followed by maintenance fluconazole, with a cure rate of 75 %. Nonetheless, 10 patients were treated with monotherapy with fluconazole, with a cure rate of 90 %.
A joint clinical guideline published in 2013 by the European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group (ESCMID) and the European Confederation of Medical Mycology (ECMM) for the diagnosis and management of rare invasive yeast infections is the available consensus on how to manage these infections. For non-neoformans and non-gattii Cryptococcus infections it is recommended the use of amphotericin B with or without flucytosine for the induction of CNS and severe infections or fluconazole in a dose over 400 mg daily if demonstrated in-vitro sensitivity. For non-CNS and non-severe infections, 400 mg of daily fluconazole can be used for induction and maintenance treatment, reserving amphotericin B for less azole susceptible species. Due to intrinsic resistance, echinocandins are not recommended.4444 Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl 3):76-98.
Susceptibility testing was reported for only 13 isolates, including our isolate. One was found to be resistant in vitro to amphotericin B and other to fluconazole. Although our isolate was not resistant in vitro to fluconazole, monotherapy failed, justifying a switch to amphotericin B followed by maintenance therapy with fluconazole. Clinical correlations between susceptibility testing results and treatment outcome are lacking.1919 Johnson LB, Bradley SF, Kauhan CA. Fungaemia due to cryptococcus laurentii and a review of non-neoformans cryptococcaemia. Mycoses. 1998;41:277-80.
The cure rate of the infection was 82.8 %, and the most effective drug was amphotericin B, used in 44.8 % of the cured cases. Although this infection generally occurs in patients with multiple co-morbidities, it does not appear to be very severe, with a high response rate to commonly used therapy for resistant yeast.2222 Krcmery VJ, Oravcovab E, Spanikc S, Mrazova-Studenaa M, Truplc J, Kunovac A, et al. Nosocomial breakthrough fungaemia during antifungal prophylaxis or empirical antifungal therapy in 41 cancer patients receiving antineoplastic chemotherapy: Analysis of aetiology risk factors and outcome. J Antimicrob Chemother. 1998;41:373-80.,4545 Kovacicova G, Lovaszova M, Hanzen J, Roidova A, Mateicka F, Lesay M, et al. Persistent fungemia - Risk factors And outcome in 40 episodes. J Chemother. 2001;13(4):429-33.
A very important concern regarding our reported case must be brought into attention: there is a report of misdiagnosis by Vitek systems, confounding candida species, such as C. parapsilosis, with P. laurentii.77 Xiao M, Fan X, Chen XX, Wang H, Zhang L, Xu ZP, et al. Misidentification of a Rare Species, Cryptococcus laurentii, by Commonly Used Commercial Biochemical Methods and Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Systems: Challenges for Clinical Mycology Laboratories. J Clin Microbiol. 2016;54(1):226-9. We do not believe this was the case, since the culture had to be sent to a different lab and regrown for the antifungigram, which confirmed the first and second C. laurentii diagnosis with the same resistance profile and the C. parapsilosis diagnosis in a different system.
In conclusion, P. laurentii, generally considered a non-infective saprobe, may cause relevant fungemia and other infections, especially in immunocompromised and oncologic patients. Central catheters seem to be a particular risk factor for fungemia with this yeast. The main clinical manifestation is fever, blood cultures are useful for diagnosis, and induction treatment with amphotericin B followed by maintenance fluconazole seems to achieve a significant success rate.
References
-
1Park SS, Lee H, Park WS, Hwang SH, Choi SI, Choi MH, et al. A case of disseminated infection with skin manifestation due to non-neoformans and non-gattii cryptococcus in a patient with refractory acute myeloid leukemia. Infect Chemother. 2017;49:142-5.
-
2Bhat V, Vira H, Khattry N, Toshniwal M. Cryptococcus laurentii diarrhea post hematopoietic stem cell transplant. Transpl Infect Dis. 2017;19:e12663.
-
3Furman-Kuklinska K, Naumnik B, Mysliwiec M. Fungaemia due to cryptococcus laurentii as a complication of immunosuppressive therapy--a case report. Adv Med Sci. 2009;54:116-9.
-
4Krcméry VJ, Kunova A, Mardiak J. Nosocomial cryptococcus laurentii fungemia in a bone marrow transplant patient after prophylaxis with ketoconazole successfully treated with oral fluconazole. Infection. 1997;25:130.
-
5Khawcharoenporn T, Apisarnthanarax A, Kiratisin P, Mundy LM, Bailey TC. Evaluation of cryptococcus laurentii meningitis in a patient with hiv infection: A case report and review of the literature. Hawaii Med J. 2006;65:260-2.
-
6Neves RP, Lima Neto RG, Leite MC, Silva VK, Santos Fde A, Macedo DP. Cryptococcus laurentii fungaemia in a cervical cancer patient. Braz J Infect Dis. 2015;19:660-3.
-
7Xiao M, Fan X, Chen XX, Wang H, Zhang L, Xu ZP, et al. Misidentification of a Rare Species, Cryptococcus laurentii, by Commonly Used Commercial Biochemical Methods and Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Systems: Challenges for Clinical Mycology Laboratories. J Clin Microbiol. 2016;54(1):226-9.
-
8Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. Bmj. 2015;350:G7647.
-
9Asano M, Mizutani M, Nagahara Y, Inagaki K, Kariya T, Masamoto D, et al. Successful treatment of cryptococcus laurentii peritonitis in a patient on peritoneal dialysis. Intern Med. 2015;54:941-4.
-
10Averbuch D, Boekhout T, Falk R, Engelhard D, Shapiro M, Block C, et al. Fungemia in a cancer patient caused by fluconazole-resistant cryptococcus laurentii. Med Mycol. 2002;40:479-84.
-
11Banerjee P, Haider M, Trehan V, Mishra B, Thakur A, Dogra V, et al. Cryptococcus laurentii fungemia Indian. J Med Microbiol. 2013;31:75-89.
-
12Bauters TGM, Swinne D, Boekhout T, Noens L, Nelis HJ. Repeated isolation of cryptococcus laurentii from the oropharynx of an immunocompromized patient. Mycopathologia. 2002;153:133-5.
-
13Calista F, Tomei F, Assalone P, Traficante D, Di Pilla G, Pepe C, et al. Cryptococcus laurentii diarrhea in a neoplastic patient. Case rep Oncol Med. 2015;2015:216458.
-
14Cheng M-W, AY-J Wu, Liu C-P, Lim K-H, Weng S-L, Tseng H-K. Cryptococcemia in an elderly woman with retroperitoneal diffuse large b-cell lymphoma after rituximab-containing chemotherapy. International Journal of Gerontology. 2016;10:112-6.
-
15Conti F, Spinelli F, Colafrancesco S, Truglia S, Ceccarelli F, Fattapposta F, et al. Acute longitudinal myelitis following cryptococcus laurentii pneumonia in a patient with systemic lupus erythematosus. Lupus. 2015;24:94-7.
-
16Ding CH, Kamarudin N. Non-neoformans cryptococcemia in a patient with hodgkin's lymphoma. Asian Journal of Pharmaceutical and Clinical Research. 2018;11:7-8.
-
17Gupta M, Mishra AK, Singh SK. Cryptococcus laurentii fungemia in a low birth weight preterm neonate: India. Journal of infection and public health. 2018;11:896-7.
-
18James M, Arias A, Roselli E, Tirado M, Ecllavarria P. Meningitis por cryptococcus laurentii caso clinico en una paciente con linfopenia cd4 idiopática: reporte de un caso. Centro Médico. 2000;45:138-40.
-
19Johnson LB, Bradley SF, Kauhan CA. Fungaemia due to cryptococcus laurentii and a review of non-neoformans cryptococcaemia. Mycoses. 1998;41:277-80.
-
20Kamalam A, Yesudian P, Thambiah AS. Cutaneous infection by cryptococcus laurentii. BJD. 1977;97:221-3.
-
21Kordossis T, Avlami A, Velegraki A, Stefanou I, Georgakopoulos G, Papalambrou C, et al. First report of cryptococcus laurentii meningitis and a fatal case of cryptococcus albidus cryptococcaemia in aids patients. Medical Mycology. 1998;36:335-44.
-
22Krcmery VJ, Oravcovab E, Spanikc S, Mrazova-Studenaa M, Truplc J, Kunovac A, et al. Nosocomial breakthrough fungaemia during antifungal prophylaxis or empirical antifungal therapy in 41 cancer patients receiving antineoplastic chemotherapy: Analysis of aetiology risk factors and outcome. J Antimicrob Chemother. 1998;41:373-80.
-
23Kunova A, Krcmery V. Fungaemia due to thermophilic cryptococci: 3 cases of cryptococcus laurentii bloodstream infections in cancer patients receiving antifungals. Scand J Infect. 1999;31:328.
-
24Lynch J, Schaberg D, Kissne r D, Kauffman C. Cryptococcus laurentii lung abscess. Am Rev Resp Dis. 1981;123:135-43.
-
25Manfredi R, Fulgaro C, Sabbatani S, Legnani G, Fasulo G. Emergence of amphotericin b-resistant cryptococcus laurentii meningoencephalitis shortly after treatment for cryptococcus neoformans meningitis in a patient with aids. Aids Patient Care STDS. 2006;20:227-32.
-
26Martinez E, Torres-Guerrero E, Cortes E, Tejada D, Arenas R. Cryptococcus laurentii infection in a patient with cutaneous leishmaniasis. Int J dermatol. 2017;56:e56-7.
-
27Mittal N, Vatsa S, Minz A. Fatal meningitis by cryptococcus laurentii in a post-partum woman: A manifestation of immune reconstitution inflammatory syndrome. Indian J Med Microbiol. 2015;33:590-3.
-
28Molina-Leyva A, Ruiz-Carrascosa JC, Leyva-Garcia A, Husein-Elahmed H. Cutaneous cryptococcus laurentii infection in an immunocompetent child. Int J Infect Dis. 2013;17, e1232-1233.
-
29Rodríguez DA, Pinilla AP. Infección asociada a catéter central por cryptococcus laurentii en niño críticamente enfermo: A propósito de un caso y revisión del tema. Infection. 2012;16:72-4.
-
30Shankar EM, Kumarasamy N, Bella D, Renuka S, Kownhar H, Suniti S, et al. Pneumonia and pleural effusion due to cryptococcus laurentii in a clinically proven case of aids. Can Resp J. 2006;13:275-8.
-
31Simon G, Simon G, Erdos M, Marodi L. Invasive cryptococcus laurentii disease in a nine-year-old boy with x-linked hyper-immunoglobulin m syndrome. Pediatr Infect Dis J. 2005;24:935-42.
-
32Sinnott J, Rodnite J, Emmanuel P, Campos A. Cryptococcus laurentii infection complicating peritoneal dialysis. Ped Infect Dis J. 1989;8:803-8.
-
33Vlchkova-Lashkoska M, Kamberova S, Starova A, Goleva L. Cutaneous cryptococcus laurentii infection in a human immunodeficiency virus-negative subject. J Eur Acad Dermatol Venereol. 2004;18:99-100.
-
34Tashima M, Sugita T, Shinoda T, Nakase T. Three new combinations from the Cryptococcus laurentii complex: Cryptococcus aureus, Cryptococcus carnescens and Cryptococcus peneaus. Int J Syst Evol Microbiol. 2003;53:1187-94.
-
35Ferreira-Paim K, Andrade-Silva L, Mora DJ, Lages-Silva E, Pedrosa AL, da Silva PR, et al. Antifungal susceptibility, enzymatic activity, PCR-fingerprinting and ITS sequencing of environmental Cryptococcus laurentii isolates from Uberaba, Minas Gerais, Brazil. Mycopathologia. 2012;174(1):41-52.
-
36Perfect JR. Cryptococcus neoformans. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 2005. p. 2997–3009.
-
37Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;16:837-74.
-
38Iseki M, Anzo M, Yamashita N, Matsuo N. Hyper-IgM immunodeficiency with disseminated cryptococcosis. Acta Paediatr. 1994;83:780-2.
-
39Dev D, Basran GS, Slater D. Consider: HIV negative immunodeficiency in cryptococcosis. BMJ. 1994;308:1436.
-
40Mocani H, Murphy AV, Beattie TJ, McAllister TA. Fungal peritonitis in children on continuous ambolatory peritoneal dialysis. Scot Med J. 1989;34:494-6.
-
41da Cunha T, Lusins J. Cryptococcus albidus meningitis. South Med J. 1973;66:1230.
-
42Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Non-neoformans Cryptococcal Infections: a Systematic Review. Infection. 2007;35:51-8.
-
43Ritterband DC, Seedor JA, Shah MK, Waheed S, Schorr I. A unique case of cryptococcus laurentii keratitis spread by a rigid gas permeable contact lens in a patient with onychomycosis. Cornea. 1998;17(1):115-8.
-
44Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl 3):76-98.
-
45Kovacicova G, Lovaszova M, Hanzen J, Roidova A, Mateicka F, Lesay M, et al. Persistent fungemia - Risk factors And outcome in 40 episodes. J Chemother. 2001;13(4):429-33.
Publication Dates
-
Publication in this collection
14 Feb 2020 -
Date of issue
Nov-Dec 2019
History
-
Received
2 June 2019 -
Accepted
20 Oct 2019