Abstracts
The present work investigated the efficiency of 0.9% saline solution and Phosphate Buffered Saline (PBS) in the preservation of goat preantral follicles in situ at different temperatures and incubation times. The ovarian pair of each animal was divided into 19 fragments. One ovarian fragment was taken randomly and fixed (control). The other 18 fragments were randomly distributed in tubes containing 0.9% saline solution or PBS at 4, 20 or 39 ºC for 4, 12 or 24 h. A total of 5,921 preantral follicles were examined. The quality of preantral follicles was evaluated by classical histology. The storage of ovarian fragments in 0.9% saline solution or PBS at 4 ºC did not reduce significantly the percentage of morphologically normal follicles when compared with the control, except after preservation in 0.9% saline solution for 24 h. The storage of ovarian fragments at 20 or 39°C reduced the percentage of normal preantral follicles when compared to the control, except after preservation in PBS at 20°C for 4 h. In conclusion, this study showed for the first time that goat preantral follicles can be stored in situ successfully at 4 ºC in 0.9% saline solution for 12 h and in PBS for 24 h, and at 20 ºC in PBS for 4 h.
Goat; Preantral follicle; Preservation; 0.9% saline solution; PBS
O presente trabalho investigou a eficiência da solução salina 0,9% e tampão fosfato salina (PBS) na conservação de folículos pré-antrais caprinos in situ a diferentes temperaturas e tempos de incubação. O par ovariano de cada animal foi dividido em 19 fragmentos. Um fragmento foi escolhido aleatoriamente e fixado (controle). Os outros 18 fragmentos foram distribuídos aleatoriamente em tubos contendo solução salina 0,9% ou PBS a 4, 20 ou 39 °C por 4, 12 ou 24 h. Um total de 5.921 folículos pré-antrais foram analisados. A qualidade dos folículos pré-antrais foi avaliada através de histologia clássica. A incubação de fragmentos ovarianos em solução salina 0,9% ou PBS a 4 ºC não reduziu significativamente a percentagem de folículos morfologicamente normais quando comparados com o controle, exceto após a conservação em solução salina 0,9% por 24 h. A incubação de fragmentos ovarianos a 20 ou 39°C reduziu a percentagem de folículos pré-antrais normais quando comparados com o controle, exceto após conservação em PBS a 20°C por 4 h. Em conclusão, este estudo mostrou pela primeira vez que folículos pré-antrais caprinos podem ser conservados in situ com sucesso a 4 ºC em solução salina 0,9% por 12 h e em PBS por 24 h, e a 20 ºC em PBS por 4 h.
Caprino; Folículo pré-antral; Conservação; Solução salina 0,9%; PBS
Effect of 0.9% saline solution and phosphate buffer saline at different temperatures and incubation times on the morphology of goat preantral follicles
Efeito da solução salina 0,9% e tampão fosfato salina em diferentes temperaturas e tempos de incubação sobre a morfologia de folículos pré-antrais caprinos
Regiane Rodrigues dos SantosI; José Roberto Viana SilvaI; Sonia Helena Furtado CostaI; Ana Paula Ribeiro RodriguesI; Raimundo Nonato Braga LôboII; José Ricardo de FigueiredoI
ILAMOFOPA, Faculdade de Ciências Veterinárias da UECE, Fortaleza - CE
IIDepartamento de Ciências Animal da UECE, Fortaleza - CE
Correspondence Correspondence to Regiane Rodrigues dos Santos Faculdade de Veterinária Universidade Estadual do Ceará Av. Paranjana, 1700 - Campus do Itaperi 60740-000 - Fortaleza - CE E-mail: regianers-20@yahoo.com
SUMMARY
The present work investigated the efficiency of 0.9% saline solution and Phosphate Buffered Saline (PBS) in the preservation of goat preantral follicles in situ at different temperatures and incubation times. The ovarian pair of each animal was divided into 19 fragments. One ovarian fragment was taken randomly and fixed (control). The other 18 fragments were randomly distributed in tubes containing 0.9% saline solution or PBS at 4, 20 or 39 ºC for 4, 12 or 24 h. A total of 5,921 preantral follicles were examined. The quality of preantral follicles was evaluated by classical histology. The storage of ovarian fragments in 0.9% saline solution or PBS at 4 ºC did not reduce significantly the percentage of morphologically normal follicles when compared with the control, except after preservation in 0.9% saline solution for 24 h. The storage of ovarian fragments at 20 or 39°C reduced the percentage of normal preantral follicles when compared to the control, except after preservation in PBS at 20°C for 4 h. In conclusion, this study showed for the first time that goat preantral follicles can be stored in situ successfully at 4 ºC in 0.9% saline solution for 12 h and in PBS for 24 h, and at 20 ºC in PBS for 4 h.
Keywords: Goat. Preantral follicle. Preservation. 0.9% saline solution. PBS.
RESUMO
O presente trabalho investigou a eficiência da solução salina 0,9% e tampão fosfato salina (PBS) na conservação de folículos pré-antrais caprinos in situ a diferentes temperaturas e tempos de incubação. O par ovariano de cada animal foi dividido em 19 fragmentos. Um fragmento foi escolhido aleatoriamente e fixado (controle). Os outros 18 fragmentos foram distribuídos aleatoriamente em tubos contendo solução salina 0,9% ou PBS a 4, 20 ou 39 °C por 4, 12 ou 24 h. Um total de 5.921 folículos pré-antrais foram analisados. A qualidade dos folículos pré-antrais foi avaliada através de histologia clássica. A incubação de fragmentos ovarianos em solução salina 0,9% ou PBS a 4 ºC não reduziu significativamente a percentagem de folículos morfologicamente normais quando comparados com o controle, exceto após a conservação em solução salina 0,9% por 24 h. A incubação de fragmentos ovarianos a 20 ou 39°C reduziu a percentagem de folículos pré-antrais normais quando comparados com o controle, exceto após conservação em PBS a 20°C por 4 h. Em conclusão, este estudo mostrou pela primeira vez que folículos pré-antrais caprinos podem ser conservados in situ com sucesso a 4 ºC em solução salina 0,9% por 12 h e em PBS por 24 h, e a 20 ºC em PBS por 4 h.
Palavras-chave: Caprino. Folículo pré-antral. Conservação. Solução salina 0,9%. PBS.
INTRODUCTION
At birth, a large population of follicles constitutes the mammalian ovary. However, the great majority of these follicles do not reach ovulation, but rather die by the atresia during their growth and maturation, resulting in few viable oocytes produced during the reproductive lifespan of the female (4). A new biotechnique called Manipulation of Oocytes Enclosed in Preantral Follicles has been developed to save preantral follicles from the ovaries before becoming atretic and culture them in vitro up to maturation stages by preventing follicular atresia (10).
A limiting factor for farm animals for the success of this biotechnique would be the quality of preantral follicles after removal and during transportation of the ovaries, since the ovarian donor of preantral follicles for in vitro studies is commonly far from reproduction laboratories. Thus, the preservation of preantral follicles in situ during transportation to laboratories is very important when using preantral follicles in protocols of cryopreservation and/or in vitro culture.
The 0.9% saline solution is used as a preservation medium for complex-oocyte-cumulus (COC) transport from cow (21), llama (6) and sow (26). The PBS (Phosphate Buffer Saline) is largely used for transportation of embryos from sheep (22), cows (19), mice (20), sows (25) and rabbits (23) as well as for COC transport from cows (17), sows (18) and cats (15). However, the effect of both solutions on the preservation of goat preantral follicles enclosed in ovarian tissue is unknown.
This study was carried out to evaluate the effect of 0.9% saline solution and PBS on the morphology of goat preantral follicles in situ, at different temperatures and for different preservation periods, with a long-term perspective of harvesting oocytes from the preantral follicle pool.
MATERIAL AND METHOD
Source of ovaries
Ovaries (n=10) from 5 adult mixed breed goats were collected at a local slaughterhouse. The ovaries were stripped of surrounding fat tissue and ligaments, washed in 70% alcohol, then twice in 0.9% saline solution and processed as described bellow.
Experimental protocol
In the slaughterhouse, the ovarian pair from each animals was divided into 19 fragments of approximately 9 mm3 consisting of the complete ovarian cortex of the two ovaries. Then, one ovarian fragment was taken randomly and immediately fixed for histology (control - time zero). The other 18 ovarian fragments were randomly distributed into tubes containing 2 mL of 0.9% saline solution or PBS at 4, 20 or 39oC and stored for 4, 12 or 24 h. The temperatures were maintained using thermoflasks filled with water at 4, 20 and 39o C. For each treatment, variables such as temperature, osmosis (micro-osmometer Type 13/13 PR-Autocal, Roebling, Berlin) and pH (pH-meter PM608, Analion, SP, Brazil) of the solutions were monitored at the beginning and at the end of the treatments. Each treatment was repeated five times.
Qualitative analysis of goat preantral follicles enclosed in ovarian tissue
At the end of the treatments the ovarian fragments were processed as follows to evaluate the morphology of the goat preantral follicles enclosed in small fragments of ovarian tissue. The ovarian fragments from each treatment, including the control, were fixed individually in Carnoy for 12 h. Subsequently, the ovarian fragments were dehydrated in a graded series of ethanol, clarified with xylol and embedded in paraffin wax. The tissue was sectioned serially at a thickness of 7 mm, and every 5th section was stained using standard protocols with PAS-Hematoxilyn. Sections were examined by light microscopy (Leica) and the preantral follicles were counted and evaluated in the section where the nucleus of the oocyte was visible.
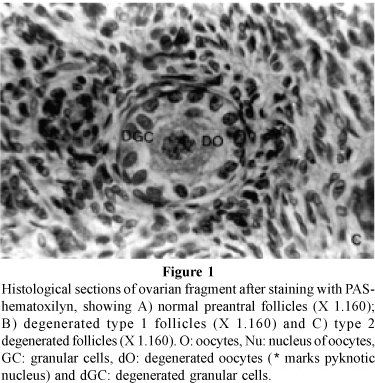
Preantral follicles were evaluated based on the integrity of the basement membrane, presence or absence of pycnotic bodies and integrity of the oocyte. Based on these parameters, preantral follicles were classified as morphologically normal (containing a healthy oocyte and well organized granular cells; Fig. 1A), degenerated type 1 follicles (containing an oocyte with pycnotic nuclei and, sometimes had a retracted oocyte, but normal granular cells; Fig. 1B) and degenerated type 2 follicles (containing a shrunken oocyte with pycnotic nuclei and disorganized granular cells; Fig. 1C). These three classifications were assigned on a basis of atresia observed in the control and/or combined with changes that occurred as a result of storage. Criteria for granular cells and oocyte degeneration were identified using ovaries fixed at time zero (control).
Statistical analysis
All data were submitted to ANOVA using a factorial design (media effects, time, temperature and interactions between factors). The data were transformed in ((x+1)3). The means were compared by the Tukey test. For each treatment, the data of normal degenerated preantral follicle from five ovarian fragments were pooled. The effect of the medium, temperatures and preservation time on the percentage of morphologically normal preantral follicles in relation to the control was analyzed by a Chi-square test. The mean pH values between the control and other treatments were compared using the Fisher PLSD test (Stat View for Macintosh). Values were considered statistically significant when P < 0.05.
RESULTS
Qualitative analysis of goat preantral follicles in the control and after in situ storage
In this study, a total of 5,921 preantral follicles were analyzed. Figure 2 shows the percentage of normal preantral follicles stored in 0.9% saline solution and in PBS. The percentage of normal follicles after storage at 4o C in 0.9% saline solution for 12 h or in PBS for 24 h was not affected when compared to the Control Time zero (P>0.05). In contrast, storage of preantral follicles at 20 and 39 ºC in both solutions, at all incubation times tested, decreased (P<0.05) the follicular viability when compared with controls (P<0.05), except when the preservation was performed in PBS, at 20oC for 4 h (P>0.05).
There was no effect of incubation time on the percentage of morphologically normal preantral follicles on the preservation of follicles in PBS at 4oC. However, in the fragments stored at 4oC in saline solution there was a decrease (P<0.05) in the percentage of morphologically normal follicles when stored for 24 h. Similar results were observed with the use of PBS and saline solution at 20 ºC and 39 oC with the increase of the incubation time from 4 to 12 h and from 12 to 24 h (P<0.05).
With regard the effect of temperature at the same incubation time, the results showed that for the saline solution there was an effect (P<0.05) of temperature on the percentage of morphologically normal preantral follicles at all times tested, with a progressive reduction in the percentage of morphologically normal follicles with the increase of temperature from 4 to 39 ºC . Similar results were obtained for the PBS except for the storage time of 4 h, at which the temperature of 20 ºC did not decrease follicular viability when compared with storage at 4 ºC.
Comparisons were made between saline solution and PBS at the same temperature and incubation time. Although no significant difference in the percentage of normal preantral follicles between these solutions was detected at 4 ºC for 12h, a greater (P<0.05) percentage of morphologically normal follicles was observed in PBS for all incubation times tested at 4 ºC for 24 h, 20 ºC for 4 and 12 h, and at 39 ºC for 4 h.
Distribution of follicular degeneration type in the control and after incubation
Fig. 3 shows the distribution of degenerated type 1 and 2 preantral follicles in the control and after storage in the different treatments, in 0.9% saline solution (Fig. 3A) and in PBS (Fig. 3B). There was a predominance (P<0.05) of type 1 degeneration in the fragments stored in both solutions at 4 ºC, at all incubation times, and at 20 ºC in PBS for 4 h. In contrast, at 39 ºC a predominance (P<0.05) of degenerated type 2 follicles was observed, except in the fragments stored in PBS for 4 h. Compared with the controls, a greater (P<0.05) percentage of degenerated type 1 follicles was observed at 20 ºC in saline solution and PBS for 12 and 24 h. A greater (P<0.05) percentage of degenerated type 2 follicles compared with controls was observed in follicles kept in saline solution at 4 ºC after 24 h, and 20 and 39 ºC at all incubation times and in PBS at 20 and 39ºC at all incubation times.
Changes in pH and osmolarity of 0.9% saline solution and PBS with preservation time
The mean values of pH and osmosis in the fresh media (before the addition of the fragment) respective to saline solution and PBS were 7.0+0.11 and 292+3.32 mOsm/L and 7.2+0.08 and 294+3.25 mOsm/L. The storage of ovarian fragments in situ at 4oC did not result in significant pH or osmosis changes in either solution. However, there was a significant decrease and increase in pH (6.8+0.32 - saline solution; 6.9+0.02 - PBS) and osmosis (318+3.08 - saline solution; 315+3,65 - PBS) at 39oC in the test solutions compared to the fresh media, respectively.
DISCUSSION
The present study shows that goat preantral follicles can be stored successfully in situ for a long time in saline solution or PBS at low temperature.
These results may be due to the fact that this temperature provided lower rates of cellular metabolism, consequently minimizing the metabolic need and, thus, increasing the resistance of follicles to the absence of nutrients and oxygen. The oxygen consumption required for energy production can be substantially reduced by means of hypothermia. Studies involving goat preantral follicle storage in vitro are not currently available. However, cat oocytes from antral follicles stored for 24 h at 4oC are capable of reaching metaphase II and produce blastocysts after in vitro fertilization27. The temperature of 4oC has been successfully used in the preservation of domestic cat ovaries for 48 h 28 and cow ovaries for 24 h for blastocyst production in vitro 24. On the other hand, Yang et al.29 reported that the storage of bovine ovaries at 4oC for 24 h resulted in a very low cleavage rate and blastocyst percentage. This may be due to the distinct preservation methodology used by these authors.
This study shows that when the preantral follicles were stored in 0.9% saline solution at 4oC for 24 h, or at 20o or 39oC, in both solutions, at all incubation times (except at 20oC for 4 h in PBS), there was a significant decrease in the percentage of normal preantral follicles when compared with control. The normal (39oC) or subnormal (20oC) metabolism associated with low oxygen tension in vitro could result in a higher rate of follicular degeneration found in the treatments where the ovarian fragments were stored at 20oC and 39oC. Jennings et al. 4 suggested that changes in the cellular membrane permeability, induced by lack of oxygen, caused changes at a level of intracellular Na+, K+ and Cl-, that associated with changes in the distribution of Ca2+ and increase of intracellular water, may lead to increased cellular volume and consequently cellular degeneration. The results found in the literature are controversial about antral follicles storage at temperatures between 15oC and 39oC. Oocytes from antral follicles, aspirated after storing bovine ovaries for 12 h between 15oC and 25oC, did not show reduced capacity to develop to blastocysts29, 21. However, Azambuja et al.2 reported that the fertilization, cleavage and embryonic development rates are higher in oocytes maintained at 39o C in comparison to those cooled at 20oC or 10oC. Under our conditions, the higher degeneration rates were observed at 39°C, which were associated with a decrease and increase in pH and osmosis, respectively, in relation to the fresh media. This might be due to the fact that increasing cellular metabolism has accelerated the consumption of media nutrients, as well as exceeding the buffering power of PBS. The resulting drop in pH could have caused cellular injuries, with the release of intracellular contents and a consequent increase in osmosis.
In spite of the fact that the saline solution exhibited a comparable efficiency for the storage of goat preantral follicles at 4 ºC for 12 h, the percentage of morphologically normal follicles was significantly greater in the PBS. The 0.9% saline solution is easier to obtain, to prepare and is cheaper, but has a poor composition. The PBS is a buffer solution, and is rich in nutrients. According Eppig8, pyruvate, one of the compounds of PBS, can supply the energy required in the earliest stages of oocyte growth, maturation and cleaving eggs. In our preservation conditions, the preantral follicles had two nutrient sources, i.e., their own energetic sources and the nutrients from the preservation medium. Thus we can suggest that the goat preantral follicles were able to survive with their own energetic sources at 4oC for 12 h, since the media showed similar effectiveness in the preservation of preantral follicles enclosed in ovarian tissue. However, with the increase of incubation time and temperature, media composition became a very important factor for the maintenance of follicular viability. The good results obtained with PBS are probably due to the composition of this medium. However, the use of a richer medium is very important for maintenance of the viability of preantral follicles stored at higher temperatures and for a longer period.
In the histological analysis, a classical method in the detection of follicular atresia1, 5, 11,13, there was a predominance of degenerated type 1 preantral follicles in the control (time zero) as well as in the fragments stored at 4oC, at all incubation times, in both solutions, and at 20oC in 0.9% saline solution for 12 and 24 h or in PBS for 4 h. According to Wood et al.28, the first index of degeneration in preantral follicles is oocyte degeneration, whereas the first indicator in antral follicles is usually related to the granular cells. Similar results using fresh ovaries were obtained studying cow 9, rat 12, and goat preantral follicles 3, 16. Wood et al.28 obtained similar results after preservation of cat follicles in PBS for more than 48 h. According Driancourt & Thuel (7) the oocyte is the first cell within the follicle to be affected by atresia, and whether oocyte atresia is related to oocyte defects or to an improper dialogue between the oocyte and its surrounding granular cells remains unclear. In contrast, in the treatments where the ovarian fragments were preserved at 39oC in saline solution and in PBS, at all incubation times (except in PBS for 4 h), type 2 degenerated follicles predominated, i.e., degeneration in both oocyte and granular cells. This indicates that the degeneration occurred to the same extent in the oocyte and in granular cells when follicles were preserved at higher temperatures for a long period. There was also great individual variation of the follicles, regarding sensitivity to undergo atresia, indicating resistance of some follicles to degeneration, because even at high temperatures for long periods, a small number of follicles remained morphologically normal.
CONCLUSIONS
In conclusion, this study showed that goat preantral follicles could be successfully preserved at 4oC for 12 h in 0.9% saline solution and in PBS. However, with the increase of the incubation time and/or of the temperature, a richer medium such as PBS is recommended. The preservation of goat preantral follicles in situ during transport to specialized laboratories, under the conditions recommended in this paper, could be important in assuring good quality oocytes for use in culture and/or cryopreservation, enhancing the efficiency of animal reproduction in the future.
ACKNOWLEDGEMENTS
The authors thank Vicente José de Figueiredo Freitas for technical support.
Received: 15/04/2002
Accepted: 31/07/2002
-
1ARIYARATNA, H.B.S.; GUNAWARDANA, V.K. Morphology and morphometry of ovarian follicles in the goat. Small Ruminant Research, v.26, p.123-129, 1997
-
2AZAMBUJA, R.M.; KRAEMER, D.C.; WESHUSIN, M.E. Effect of low temperatures on in vitro matured bovine oocytes. Theriogenology, v.49, p.1155-1164, 1998.
-
3BEZERRA, M.B.; RONDINA, D.; OLIVEIRA, L.C.; LIMA, A.K.F.; CECCHI, R.; LUCCI, C.M.; GIORGETTI, A.; FIGUEIREDO, J.R. Aspectos quantitativos e qualitativos da foliculogênese na fase pré-natal na espécie caprina. Ciência Animal, v.8, p.30-41, 1998.
-
4CARROLL, J.; WHITTINGHAM, D.G.; WOOD, M. J.; TELFER, E.; GOSDEN, R.G. Extraovarian production of mature viable mouse oocytes from frozen primary follicles. Journal of Reproduction and Fertility, v.90, p.321-327, 1990.
-
5CARVALHO, F.C.A.; LUCCI, C.M.; SILVA, J.R.V.;ANDRADE, E.R.; BÁO, S.N.; FIGUEIREDO, J.R. Effect of Braun-Collins and Saline solutions at different temperatures and incubation times on the quality of goat preantral follicles preserved in situ. Animal Reproduction Science, v.66, p.195-20, 2001.
-
6DEL CAMPO, M.R.; DEL CAMPO, C.H.; DONOSO, M.X.; BERLAND, M.; MAPLETOFT, R.J. In vitro fertilization and development of llama oocytes using epididymal spermatozoa and ovductal cell co-culture. Theriogenology, v.41, p.1219-1229, 1994.
-
7DRIANCOURT, M.A.; THUEL, B. Control of oocyte growth and maturation by follicular cells and molecules present in follicular fluid: A review. Reproduction, Nutrition and Development, v.38, p.345-362, 1998.
-
8EPPIG, J.J. Analysis of Mouse Oogenesis In vitro Oocyte isolation and Utilization of Exogenous Energy Sources by Growing Oocytes. J. Exp. Zoology, v.198, p.375-382, 1976.
-
9ERICKSON, G.F. An analysis of follicle development and ovum maturation. Seminars in Reproductive Endocrinology, v.3, p.233-254, 1986.
-
10FIGUEIREDO, J.R.; RODRIGUES, A.P.R.; GONÇALVES, P.B.D. State of art of the manipulation of oocytes enclosed in preantral follicles. Embryo Transfer Newsletter, v.18, p.11-15, 2000.
-
11FORTUNE, J.E.; CUSHMAN, R.A.; WAHL, C.M.; KITO, S. The primordial to primary follicle transition. Molecular and Cellular Endocrinology, v.163, p.53-20, 2000.
-
12HIRSHFIELD, A.N. Size frequency analysis of atresia in cycling rats. Biolology of Reproduction, v.38, p.1181-88, 1988.
-
13HUAMNIN, Z; YONG, Z. In vitro development of caprine ovarian follicles. Theriogenology, v.54, p.641-650, 2000.
-
14JENNINGS, R.B.; GANOTE, C.E.; REIMER, K.R. Ischemic tissue injury. American Journal Pathology, v.81, p.179-197, 1975.
-
15JEWGENOW, K.; PENFOLD, L. M.; MEYER, H. H. D.; WILDT, D. E. Viability of small preantral follicles from domestic cats after cryoprotectant exposure and cryopreservation. Journal of Reproduction and Fertility, v.112, p.39-47, 1998.
-
16LUCCI, C.M.; AMORIM, C.A.; RODRIGUES, A.P.R.; FIGUEIREDO, J.R; BÁO, S.N.; SILVA, J.R.V.; GONÇALVES, P.B.D. Study of preantral follicle population in situ and after mechanical isolation from caprine ovaries at different reproductive stages. Animal Reproduction Science, v.56, p.223-236, 1999.
-
17MARTINO, A.; SONGSASEN, N.; LEIBO, S.P. Development into Blastocysts of Bovine Oocytes by Ultra-Rapide colling. Biology of Reproduction, v.54, p.1059, 1996.
-
18MATTIOLI, M.; GALEATI, G.; BACCI, M.L.; SEREN, E. Follicular factors influence oocyte fertilizability by modulation intercellular cooperation between cumulus cells and oocyte. Gamete Research, v.21, p.223-232, 1988.
-
19NIEMANN, H. Cryopreservation of ova and embryos from livestock: current status and research needs. Theriogenology, v.1, p.109-124, 1991.
-
20NUREDDIN, A.; EPSAROS, E.; KIESSLING, A.A. Purines inhibit the development of mouse embryos in vitro Journal of Reproduction and Fertility, v.90, p.455-464, 1990.
-
21SCHERNTHANER, W.; SCHMOLL, F.; BREM, G.; SCHELLANDER, K. Storing bovine ovaries for 24 hours between 15 and 21oC does not influence in vitro production of blastocists. Theriogenology, v.47, p.297, 1997.
-
22SMITH, C.L.; PETER, A.T.; PUGH, D.G. Reproduction in llamas and alpacas: A review. Theriogenology, v.41, p.573-592, 1994.
-
23SMORAG, Z.; GAJDA, B.; WIECZOREK, B.; JURA, J. Stage-dependent viability of vitrified rabbit embryos. Theriogenology, v.6, p.1227-1231, 1989.
-
24SOLANO, R.; ARMAS, R.; PUPO, C.A.; CASTRO, F.O. Short-term preservation of intrafollicular oocytes at 4 oC. Theriogenology, v.41, p.299, 1994.
-
25WEBER, P.K.; YOUNGS, C.R. Investigation of cryoprotectant toxicity to porcine embryos. Theriogenology, v.41, p.1291-1298, 1994.
-
26WESTHOF, G.; WESTHOF, K.F.; BRAENDLE, W.L.; DIZERECA, G.S. Differential steroid secretion and gonadotropin response by individual tertiary porcine follicle in vitro: Possible physiologic role of atretic follicles. Biology of Reproduction, v.44, p.461-468, 1991.
-
27WOLFE, B.A.; WILDT, D.E. Development to blastocysts of domestic cat oocytes matured and fertilized in vitro after prolonged cold storage. Journal of Reproduction and Fertility, v.106, p.135-141, 1996.
-
28WOOD, T.C.; MONTALI, R.J.; WILDT, D. Follicle-oocyte atresia and temporal taphonomy in cold-stored domestic cat ovaries. Molecular Reproduciton and Development, v.46, p.190-200, 1997.
-
29YANG, N.S.; LU, K.H.; GORDON, I. In vitro fertilization (IVF) and culture (IVC) of bovine oocytes from stored ovaries. Theriogenology, v.33, p.352, 1990.
Publication Dates
-
Publication in this collection
06 June 2003 -
Date of issue
2002
History
-
Received
15 Apr 2002 -
Accepted
31 July 2002