ABSTRACT
Mangaba is a widely-consumed fruit in the Northeast of Brazil, which is usually exploited through extractivism. This fruit is rich in various nutrients, especially in vitamin C, with pleasant taste and aroma. The lyophilization process transforms these fruits into amorphous powders, which must be analyzed regarding their properties and hygroscopic trend. Thus, the objective of this study was to characterize and evaluate the physico-chemical properties of adsorption isotherms of the lyophilized ‘mangaba’ pulp powder, with addition of maltodextrin (DE 20). The pH, titratable acidity, soluble solids, ascorbic acid and water activity were analyzed. Regarding the isotherms, the mathematical models of GAB, BET, Oswin, and Henderson were used at temperatures of 25, 30, 35 and 40 °C. The obtained powder presented pH of 3.14, titratable acidity of 1.95 mg of citric acid 100g-1 of powder, soluble solid contents of 99 ºBrix, ascorbic acid content of 55.97 mg 100g-1 and water activity of 0.16. Henderson was the mathematical model that best fitted the data of the adsorption isotherms at the four evaluated temperatures, with average errors ranging from 5.76 to 9.70% and R2 from 0.9974 to 0.9995.
Key words:
Hancornia speciosa Gomes; sorption isotherm; hygroscopicity; dehydration
RESUMO
A mangaba é um fruto bastante consumido no Nordeste do Brasil e ainda explorado de maneira extrativista. Os frutos são ricos em vários nutrientes, notadamente em vitamina C, além de possuírem sabor e aroma agradáveis. A liofilização permite a obtenção de pós de natureza amorfa que necessitam de análise de suas propriedades e comportamento higroscópico. O objetivo do presente trabalho foi realizar a caracterização físico-química e avaliação das isotermas de adsorção do pó da polpa de mangaba liofilizada adicionada de maltodextrina (DE 20). Foram realizadas as análises de pH, acidez titulável, sólidos solúveis, ácido ascórbico e atividade de água. Em relação às isotermas, foram empregados os modelos matemáticos de GAB, BET, Oswin e Henderson, nas temperaturas de 25, 30, 35 e 40 ºC. O pó obtido apresentou pH de 3,14, acidez titulável de 1,95 mg 100g-1 de ácido cítrico, teor de sólidos solúveis de 99 ºBrix, teor ácido ascórbico de 55,97 mg 100g-1 e atividade de água de 0,16. O melhor ajuste de modelo matemático foi o de Henderson nas quatro temperaturas avaliadas, em que apresentou erros médios de 5,76 a 9,70% e R2 de 0,9974 a 0,9995.
Palavras-chave:
Hancornia speciosa Gomes; isoterma de sorção; higroscopicidade; desidratação
Introduction
‘Mangaba’ (Hancornia speciosa Gomes) is a Brazilian native tree, whose fruits have important properties, such as high contents of vitamin C and antioxidants. Thus, they are consumed as fresh fruits and also used as raw material for the production of various products (Soares et al., 2011Soares, F. P.; Paiva, R.; Alvarenga, A. A. de; Nery, F. C.; Vargas, D. P.; Silva, D. R. G. Taxa de multiplicação e efeito residual de diferentes fontes de citocinina no cultivo in vitro de Hancornia speciosa Gomes. Ciência e Agrotecnologia, v.35, p.152-157, 2011. http://dx.doi.org/10.1590/S1413-70542011000100019
http://dx.doi.org/10.1590/S1413-70542011...
).
‘Mangaba’ fruits are highly perishable and, according to Campos et al. (2011)Campos, R. P.; Knoch, B.; Hiane, P. A.; Ramos, M. I. L.; Ramos Filho, M. M. 1-MCP em mangaba armazenadas em temperatura ambiente e a 11 ºC. Revista Brasileira de Fruticultura, v.especial, p.206-212, 2011. http://dx.doi.org/10.1590/S0100-29452011000500024
http://dx.doi.org/10.1590/S0100-29452011...
, preservation methods are needed to increase their shelf life and maintain their quality. The fruit pulp industry uses drying methods, which concentrate the raw material components and allow the product to be stored for longer periods, even at room temperature (Silva et al., 2014Silva, M. P. da; Gomes, F. dos S.; Freire Júnior, M.; Cabral, L. M. C. Avaliação dos efeitos da radiação gama na conservação da qualidade da polpa da amora-preta (Rubus spp L.). Revista Brasileira de Fruticultura, v.36, p.620-627, 2014. http://dx.doi.org/10.1590/0100-2945-218/13
http://dx.doi.org/10.1590/0100-2945-218/...
).
According to Oikonomopoulou et al. (2011)Oikonomopoulou, V. P.; Krokida, M. K; Karathanos, V. K. The influence of freeze drying conditions on microstructural changes of food products. Procedia Food Science, v.1, p.647-654, 2011. http://dx.doi.org/10.1016/j.profoo.2011.09.097
http://dx.doi.org/10.1016/j.profoo.2011....
, the lyophilization method removes the water from fruits through sublimation, applying vacuum and low temperature, which result in products with high rehydration capacity. This drying method allows to maintain the organoleptic characteristics of the fruits after dehydration.
The evaluation of sorption isotherms to define the hygroscopic equilibrium of products is important to understand the hygroscopic trend under different conditions of temperature and air humidity, predict chemical, enzymatic and microbiological reactions, and develop appropriate packaging for storage (Oliveira et al., 2011Oliveira, V. S. de; Afonso, M. R. A.; Costa, J. M. C. da. Physico chemical and hygroscopic behavior of sapodilla lyophilized. Revista Ciência Agronômica, v.42, p.342-348, 2011. http://dx.doi.org/10.1590/S1806-66902011000200012
http://dx.doi.org/10.1590/S1806-66902011...
).
Several mathematical models are used to determine the sorption isotherms; however, according to Moreira et al. (2013)Moreira, T. B.; Rocha, E. M. F. F.; Afonso, M. R.; Costa, J. M. C. da. Comportamento das isotermas de adsorção do pó da polpa de manga liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.17, p.1093-1098, 2013. http://dx.doi.org/10.1590/S1415-43662013001000011
http://dx.doi.org/10.1590/S1415-43662013...
, the accuracies of these models depend on specific ranges of water activity or types of food. Therefore, the use of only one equation model for isotherm analysis of various types of foods is not possible due to specific conditions of water activity and food composition.
Thus, the main objectives of this study were to obtain the ‘mangaba’ pulp powder, characterize its physicochemical properties and evaluate the hygroscopic trend of the lyophilized mangaba pulp powder through adsorption isotherms, aiming to find the best storage conditions for this product.
Material and Methods
Frozen pulps of ‘mangaba’ were acquired from a pulp industry in Natal, Rio Grande do Norte, Brazil, taken to the Laboratory of Food Drying and Quality Control of the Federal University of Ceará, and stored at -18°C.
These pulps were combined with maltodextrin at 30% (dextrose equivalent of 20), placed on stainless steel trays and stored in an ultra-freezer (CL90-40V, Terroni) for freezing during 24 h. They were then dehydrated, during 24 h in a lyophilizer (L101, Liotop). The obtained product was stored in laminated polyethylene packages.
The lyophilized ‘mangaba’ pulp powder was physicochemically characterized. The pH, titratable acidity and soluble solids were analyzed according to the method described by the Adolfo Lutz Institute (IAL, 2008IAL - Instituto Adolfo Lutz. Normas analíticas do Instituto Adolfo Lutz: Métodos químicos e físicos para análise de alimentos. 4.ed. São Paulo: IAL, 2008. 1020p.). The ascorbic acid was determined according to the method described by Strohecker & Henninger (1967)Strohecker, R.; Henning, H. M. Análises de vitaminas: Métodos comprobados. 1.ed. Madrid: Paz Montalvo, 1967. 428p., and the water activity was measured in a water activity meter (4TEV, Aqualab). All analyses were performed with three replications.
The moisture adsorption isotherm was determined through the statistic gravimetric method described by Greespan (1977)Greespan, L. Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National of Standards A. Physics and Chemistry, v.81, p.89-96, 1977., using saturated salt solutions such as CH3COOK, K2CO3, NaBr, SnCl2, KCl, and BaCl2 (Table 1) in glass cells, at 21 ± 2 ºC.
Aluminum crucibles with approximately 0.2 g of each sample, weighed with three replications, were placed into cells containing the saturated solutions, where they remained for 3 days to reach equilibrium, i.e., until no weight change could be detected by weighing on the analytical balance (B-TEC-210A, Tecnal).
Weight measurements were performed every 24 h until reaching the equilibrium condition. Subsequently, the water activity was measured in the Aqualab 4TEV, at four different temperatures (25, 30, 35 and 40 ºC). The crucibles containing the samples were then placed into a vacuum oven at 70 °C to evaluate the final moisture content of the samples.
The moisture equilibrium (X0) was calculated by the difference between the sample weights at equilibrium and dry conditions, using the Eq. 1.
where:
X0 - moisture equilibrium (g of water per g of dry solids);
Meq - sample weight at equilibrium (g); and,
Ms - sample dry weight (g).
The experimental data of the adsorption isotherms were adjusted to the mathematical models GAB, BET, Henderson, and Oswin, as described in Eqs. 2 to 5:
- GAB
- BET
- Henderson
- Oswin
where:
aw - water activity;
Xm - moisture content in the molecular monolayer (g of water per g of dry solids);
X0 - moisture equilibrium (g of water per g of dry solids);
C - BET constant, related to the sorption heat of the molecular layer; and,
a, b, and K - are parameters of adjustment.
The statistical program Statistica 7.0 was used to fit the data to the models. The error values (E) were calculated according to the Eq. 6 (Kurozawa et al., 2005Kurozawa, L. E.; El-Aouar, A. A.; Murr, F. E. Obtenção de isotermas de dessorção de cogumelo in natura e desidratado osmoticamente. Ciência e Tecnologia de Alimentos, v.25, p.828-834, 2005. http://dx.doi.org/10.1590/S0101-20612005000400033
http://dx.doi.org/10.1590/S0101-20612005...
):
where:
E - mean relative error;
Mi - value experimentally obtained;
Mpi - value obtained by the model; and,
n - number of experimental data.
Results and Discussion
The characterization of the lyophilized ‘mangaba’ pulp powder with 30% of maltodextrin is shown in Table 2. The lyophilized ‘mangaba’ pulp powder has acid characteristic, which contributes to the stability of the product. Santos et al. (2012)Santos, J. T. S.; Costa, F. S. C.; Soares, D. S. C.; Campos, A. F. P.; Carnelossi, M. A. G.; Nunes, T. P.; Oliveira Júnior, A. M. Avaliação de mangaba liofilizada através de parâmetros físico-químicos. Scientia Plena, v.8, p.1-5, 2012. physico-chemically characterized lyophilized ‘mangaba’ pulp and found pH values near 3.01, even though no drying adjuvant was added. These figures are important to maintain the quality of the product, since according to Breda et al. (2012)Breda, C. A.; Sanjinez-Argandoña, E. J.; Correia, C. de A. Shelf life of powdered Campomanesia adamantium pulp in controlled environments. Food Chemistry, v.135, p.2960-2964, 2012. http://dx.doi.org/10.1016/j.foodchem.2012.07.029
http://dx.doi.org/10.1016/j.foodchem.201...
, pH values from 5 to 7 cause enzymatic browning in fruits.
The titratable acidity found in the present study (1.95 mg 100g-1) is consistent with acidic characteristics that allow the storage of the product. This parameter represents the content of organic acids in the fruit (Soares et al., 2014Soares, D. C.; Santos, J. T. S.; Costa, D. G.; Abud, A. K. S.; Nunes, T. P.; Figueiredo, A. V. D.; Oliveira Júnior, A. M. de. Evaluation of Hancornia speciosa Gomes lyophilization at different stages of maturation. International Journal of Biological, Veterinary, Agricultural and Food Engineering, v.8, p.144-118, 2014.). Lima et al. (2015)Lima, J. P. de.; Rodrigues, L. F.; Monteiro, A. G. D. P.; Boas, E. V. de B. V. Climacteric pattern of mangaba fruit (Hancornia speciosa Gomes) and its responses to temperature. Scientia Horticulturae, v.197, p.399-403, 2015. http://dx.doi.org/10.1016/j.scienta.2015.09.059
http://dx.doi.org/10.1016/j.scienta.2015...
found titratable acidity values in ‘mangaba’ fruits of 1.06 mg 100g-1.
The lyophilized ‘mangaba’ pulp powder presented soluble solid contents greater than those of other dehydrated products. Santos et al. (2014)Santos, A. A. C. dos; Florêncio, A. K. G. D.; Rocha, É. M. de. F. F.; Costa, J. M. C. da. Avaliação físico-química e comportamento higroscópico de goiaba em pó obtida por spray-dryer. Revista Ciência Agronômica, v.20, p.508-514, 2014. http://dx.doi.org/10.1590/S1806-66902014000300010
http://dx.doi.org/10.1590/S1806-66902014...
found soluble solid contents of 93 ºBrix in atomized guava powder, while Oliveira et al. (2014b)Oliveira, G. S.; Costa, J. M. C. da; Afonso, M. R. A. Caracterização e comportamento higroscópico do pó da polpa de cajá liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.18, p.1059-1064, 2014b. http://dx.doi.org/10.1590/1807-1929/agriambi.v18n10p1059-1064
http://dx.doi.org/10.1590/1807-1929/agri...
found 92.67 ºBrix in lyophilized mombin (Spondias mombin L.) pulp powder. Sousa et al. (2015)Sousa, K. dos S. M. de; Figueirêdo, R. M. F. de; Queiroz, A. J. de M.; Fernandes, T. K. da S. Production and characterization of atemoya pulp powder. Revista Brasileira de Fruticultura, v.37, p.718-728, 2015. http://dx.doi.org/10.1590/0100-2945-135/14
http://dx.doi.org/10.1590/0100-2945-135/...
compared the soluble solid contents of ‘atemoya’ (Annona cherimola Mill × A. squamosa L.) powder with the ones in the fruit pulp and found greater soluble solid contents in the powder due to the addition of a drying adjuvant. Soluble solids in fruits are related to compounds that influence the acceptance by the consumers, such as the taste of the product (Santos et al., 2012Santos, J. T. S.; Costa, F. S. C.; Soares, D. S. C.; Campos, A. F. P.; Carnelossi, M. A. G.; Nunes, T. P.; Oliveira Júnior, A. M. Avaliação de mangaba liofilizada através de parâmetros físico-químicos. Scientia Plena, v.8, p.1-5, 2012.).
The ascorbic acid content was lower than that found by Soares et al. (2014)Soares, D. C.; Santos, J. T. S.; Costa, D. G.; Abud, A. K. S.; Nunes, T. P.; Figueiredo, A. V. D.; Oliveira Júnior, A. M. de. Evaluation of Hancornia speciosa Gomes lyophilization at different stages of maturation. International Journal of Biological, Veterinary, Agricultural and Food Engineering, v.8, p.144-118, 2014. (188.72 mg 100g-1) in lyophilized ‘mangaba’ without addition of adjuvants. Oliveira et al. (2014b)Oliveira, G. S.; Costa, J. M. C. da; Afonso, M. R. A. Caracterização e comportamento higroscópico do pó da polpa de cajá liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.18, p.1059-1064, 2014b. http://dx.doi.org/10.1590/1807-1929/agriambi.v18n10p1059-1064
http://dx.doi.org/10.1590/1807-1929/agri...
found 90.46 mg of ascorbic acid per 100 g of lyophilized mombin pulp powder with 17% of maltodextrin, and attributed these low values to the addition of a drying adjuvant. Despite the addition of drying adjuvant, the values of ascorbic acid found in 100 g of the lyophilized ‘mangaba’ pulp powder were greater than the daily intake value recommended by the Brazilian Health Regulatory Agency (Brasil, 2005Brasil. Agência Nacional de Vigilância Sanitária. Resolução RDC n.269, de 22 de setembro de 2005, que dispõe sobre o Regulamento Técnico sobre a ingestão diária recomendada (IDR) de proteína, vitaminas e minerais, Diário Oficial da República Federativa do Brasil, Brasília, DF, 2005.), which is 45 mg. Moreira et al. (2013)Moreira, T. B.; Rocha, E. M. F. F.; Afonso, M. R.; Costa, J. M. C. da. Comportamento das isotermas de adsorção do pó da polpa de manga liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.17, p.1093-1098, 2013. http://dx.doi.org/10.1590/S1415-43662013001000011
http://dx.doi.org/10.1590/S1415-43662013...
found 54.47 mg of ascorbic acid per 100 g of lyophilized mango pulp.
The values of water activity in the lyophilized ‘mangaba’ pulp powder were low. According to Soares et al. (2014)Soares, D. C.; Santos, J. T. S.; Costa, D. G.; Abud, A. K. S.; Nunes, T. P.; Figueiredo, A. V. D.; Oliveira Júnior, A. M. de. Evaluation of Hancornia speciosa Gomes lyophilization at different stages of maturation. International Journal of Biological, Veterinary, Agricultural and Food Engineering, v.8, p.144-118, 2014., the water activity values of the ‘mangaba’ fresh pulp are within 0.97 and 0.98. The dehydration process of the pulp and the addition of maltodextrin in the pulp powder reduce the water activity and increase the soluble solids (Sousa et al., 2015Sousa, K. dos S. M. de; Figueirêdo, R. M. F. de; Queiroz, A. J. de M.; Fernandes, T. K. da S. Production and characterization of atemoya pulp powder. Revista Brasileira de Fruticultura, v.37, p.718-728, 2015. http://dx.doi.org/10.1590/0100-2945-135/14
http://dx.doi.org/10.1590/0100-2945-135/...
). Dieb et al. (2015)Dieb, J. T.; Gurgel, C. M.; Dantas, T. P.; Medeiros, M. D. F. de. Secagem da polpa de graviola pelo processo foam-mat e avaliação sensorial do produto obtido. Tecnologia & Informação, v.2, p.24-31, 2015. found water activity values between 0.25 and 0.32 and reported low water activity values in dehydrated soursop (Annona muricata L.).
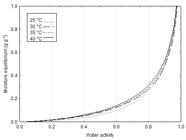
The moisture equilibrium (X0) of the evaluated isotherms increased with the increasing water activity (Figure 1). The same trend was found by Moreira et al. (2013)Moreira, T. B.; Rocha, E. M. F. F.; Afonso, M. R.; Costa, J. M. C. da. Comportamento das isotermas de adsorção do pó da polpa de manga liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.17, p.1093-1098, 2013. http://dx.doi.org/10.1590/S1415-43662013001000011
http://dx.doi.org/10.1590/S1415-43662013...
in an experiment with lyophilized mango.
This trend is a characteristic of amorphous powders, such as the ‘mangaba’ pulp, after subjected to lyophilization with the addition of maltodextrin (Muzaffar & Kumar, 2016Muzaffar, K.; Kumar, P. Moisture sorption isotherms and storage study of spray dried tamarind pulp powder. Powder Technology, v.291, p.322-327, 2016. http://dx.doi.org/10.1016/j.powtec.2015.12.046
http://dx.doi.org/10.1016/j.powtec.2015....
). The moisture equilibrium also increased with the increasing temperatures. This increase was also observed by Ribeiro et al. (2016)Ribeiro, L. C.; Costa, J. M. C. da; Afonso, M. R. A. Hygroscopic behavior of lyophilized acerola pulp powder. Revista Brasileira de Engenharia Agrícola e Ambiental, v.20, p.269-274, 2016. http://dx.doi.org/10.1590/1807-1929/agriambi.v20n3p269-274
http://dx.doi.org/10.1590/1807-1929/agri...
in lyophilized west indian cherry (Malpighia glabra Millsp.) pulp powder, which is rich in sugars and has high tendency to absorb water at higher temperatures. According to Oliveira et al. (2011)Oliveira, V. S. de; Afonso, M. R. A.; Costa, J. M. C. da. Physico chemical and hygroscopic behavior of sapodilla lyophilized. Revista Ciência Agronômica, v.42, p.342-348, 2011. http://dx.doi.org/10.1590/S1806-66902011000200012
http://dx.doi.org/10.1590/S1806-66902011...
, besides the content of sugars, the porosity and soluble compounds of lyophilized foods also contribute to increasing their water content.
Powders are susceptible to different physical changes according to the relative humidity of their environment. The lyophilized ‘mangaba’ pulp powder showed a high moisture gain from a relative humidity of 80% (Figure 1). Therefore, its stability is maintained up to this value, which is the limit value to store this product without degradation caused by physical reactions.
The obtained isotherm had a type III curve, with a "J" shape (Hébrard et al., 2003Hébrard, A.; Oulahna, D.; Galet, L.; Cuq, B.; Abecassis, J.; Fages, J. Hydration properties of durum wheat semolina: Influence of particle size. Powder Technology, v.130, p.211-218, 2003. http://dx.doi.org/10.1016/S0032-5910(02)00268-1
http://dx.doi.org/10.1016/S0032-5910(02)...
) and an exponential trend. This type of curve is common in foods that have significant levels of soluble compounds, such as sugars (Lavoyer et al., 2013Lavoyer, F. C. G.; Gabas, A. L.; Oliveira, W. P.; Telis-Romero, J. Study of adsorption isotherms of green coconut pulp. Food Science and Technology, v.33, p.68-74, 2013. http://dx.doi.org/10.1590/S0101-20612013005000017
http://dx.doi.org/10.1590/S0101-20612013...
), although most of the foods show type II curve (sigmoid). A type III curve was also found by Oliveira et al. (2014a)Oliveira, D. M.; Clemente, E.; Costa, J. M. C. da. Hygroscopic behavior and degree of caking of grugru palm (Acrocomia aculeata) powder. Journal of Food Science and Technology, v.51, p.2783-2789, 2014a. https://doi.org/10.1007/s13197-012-0814-9
https://doi.org/10.1007/s13197-012-0814-...
in atomized ‘macauba’ (Acrocomia aculeata (Jacq.) Lodd. Ex Mart.) pulp powder, and by Santana et al. (2015)Santana, R. F. de; Oliveira Neto, E. R. de; Santos, A. V.; Soares, C. M. F.; Lima, Á. S.; Cardoso, J. C. Water sorption isotherm and glass transition temperature of freeze-dried Syzygium cumini fruit (jambolan). Journal of Thermal Analysis and Calorimetry, v.120, p.519-524, 2015. https://doi.org/10.1007/s10973-014-4014-x
https://doi.org/10.1007/s10973-014-4014-...
in lyophilized ‘jambolan’ (Syzygium cumini Lamarck) pulp.
The results obtained with the applied mathematical models are shown in Table 3. The GAB model at 40 °C and Henderson at all evaluated temperatures showed relative errors (E) lower than 10%, which are suitable values for the isotherm analysis (Lomauro et al., 1985Lomauro, C. J.; Bakshi, A. S.; Labuza, T. P. Evaluation of food moisture sorption isotherm equations. Part I: Fruit, vegetable and meat products. Lebensmittel- Wissenschaft & Technologie, v.18, p.111-117, 1985. http://dx.doi.org/10.1111/j.1365-2621.1985.tb13411.x
http://dx.doi.org/10.1111/j.1365-2621.19...
). The other relative errors were between 10.43 and 20.55%, which are considered high; thus, the use of these models is inadequate to predict the isotherms, regardless of their coefficients of determination (R2).
The moisture content in the monolayer (Xm) of the GAB and BET models had no well-defined trend with the increasing temperatures. This result may be due to the high relative errors (E), which hindered the use of these parameters to assess the hygroscopic trend of the lyophilized ‘mangaba’ pulp powder.
The moisture content in the monolayer (Xm) is the moisture content that ensures minimal loss of product quality for a longer period at a certain temperature, with minimal deteriorating reaction rate below this value, except for the oxidation of unsaturated fats (Goula et al., 2008Goula, A. M.; Karapantsios, T. D.; Achilias, D. S.; Adamopoulos, K. G. Water sorption isotherms and glass transition temperature of spray dried tomato pulp. Journal of Food Engineering, v.85, p.73-83, 2008. http://dx.doi.org/10.1016/j.jfoodeng.2007.07.015
http://dx.doi.org/10.1016/j.jfoodeng.200...
).
The parameter K of the GAB model showed a trend of stabilization, with values below 1. These values indicate an appropriate physical aspect of the material, without infinite sorption (Chirife et al., 1992Chirife, J.; Timmermann, E. O.; Iglesias, H. A.; Boquet, R. Some features of the parameter k of the GAB equation as applied to sorption isotherms of selected food materials. Journal of Food Engineering, v.15, p.75-82, 1992. http://dx.doi.org/10.1016/0260-8774(92)90041-4
http://dx.doi.org/10.1016/0260-8774(92)9...
).
Oliveira et al. (2014b)Oliveira, G. S.; Costa, J. M. C. da; Afonso, M. R. A. Caracterização e comportamento higroscópico do pó da polpa de cajá liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.18, p.1059-1064, 2014b. http://dx.doi.org/10.1590/1807-1929/agriambi.v18n10p1059-1064
http://dx.doi.org/10.1590/1807-1929/agri...
found lower K values with the addition of maltodextrin to lyophilized mombin pulp, which reduced the interaction strength between the water vapor and the solid matrix, compared with the material with no maltodextrin. This result may explain the lower values of this parameter found in the present work. The parameters required for the Henderson (a > 0 and b < 1) and Oswin (a > 0 and b between 0 and 1) models (Blahovec, 2004Blahovec, J. Sorption isotherms in materials of biological origin mathematical and physical approach. Journal of Food Engineering, v.65, p.489-495, 2004. http://dx.doi.org/10.1016/j.jfoodeng.2004.02.012
http://dx.doi.org/10.1016/j.jfoodeng.200...
) were met; however, the Oswin model showed high relative errors and, thus, this model is not recommended.
Henderson was the best model to describe the hygroscopic trend of the lyophilized ‘mangaba’ pulp powder, with the parameters "a" and "b" within the standard values, relative errors lower than 10%, and coefficients of determination (R2) near 1. According to Oliveira et al. (2014aOliveira, D. M.; Clemente, E.; Costa, J. M. C. da. Hygroscopic behavior and degree of caking of grugru palm (Acrocomia aculeata) powder. Journal of Food Science and Technology, v.51, p.2783-2789, 2014a. https://doi.org/10.1007/s13197-012-0814-9
https://doi.org/10.1007/s13197-012-0814-...
), the Henderson model was one of the best models to predict the isotherm of atomized ‘macauba’ powder with 8% maltodextrin and water activity between 0.3 and 0.7. The Henderson model was also reported as the best model to predict isotherms in other works, such as those carried out by Mil et al. (2014)Mil, E.; Rodrigues, S.; Afonso, M. R. A.; Costa, J. M. C. da. Mathematical modeling for isotherms of mango pulp powder, obtained by atomization. Journal of Encapsulation and Adsorption Sciences, v.4, p.8-14, 2014. http://dx.doi.org/10.4236/jeas.2014.41002
http://dx.doi.org/10.4236/jeas.2014.4100...
on atomized mango pulp powder and by Santos et al. (2014)Santos, A. A. C. dos; Florêncio, A. K. G. D.; Rocha, É. M. de. F. F.; Costa, J. M. C. da. Avaliação físico-química e comportamento higroscópico de goiaba em pó obtida por spray-dryer. Revista Ciência Agronômica, v.20, p.508-514, 2014. http://dx.doi.org/10.1590/S1806-66902014000300010
http://dx.doi.org/10.1590/S1806-66902014...
on atomized guava pulp powder.
Conclusions
-
The lyophilized ‘mangaba’ pulp powder presented low water activity and high content of soluble solids.
-
The lyophilized ‘mangaba’ pulp powder presented low pH and titratable acidity, showing the stability and acidic characteristic of the product. Considering its ascorbic acid content, the lyophilized ‘mangaba’ pulp powder is a source of vitamin C.
-
Henderson mathematical model best fitted to the data of the adsorption isotherms at the four temperatures evaluated, with low relative errors and high coefficient of determination.
Literature Cited
- Blahovec, J. Sorption isotherms in materials of biological origin mathematical and physical approach. Journal of Food Engineering, v.65, p.489-495, 2004. http://dx.doi.org/10.1016/j.jfoodeng.2004.02.012
» http://dx.doi.org/10.1016/j.jfoodeng.2004.02.012 - Brasil. Agência Nacional de Vigilância Sanitária. Resolução RDC n.269, de 22 de setembro de 2005, que dispõe sobre o Regulamento Técnico sobre a ingestão diária recomendada (IDR) de proteína, vitaminas e minerais, Diário Oficial da República Federativa do Brasil, Brasília, DF, 2005.
- Breda, C. A.; Sanjinez-Argandoña, E. J.; Correia, C. de A. Shelf life of powdered Campomanesia adamantium pulp in controlled environments. Food Chemistry, v.135, p.2960-2964, 2012. http://dx.doi.org/10.1016/j.foodchem.2012.07.029
» http://dx.doi.org/10.1016/j.foodchem.2012.07.029 - Campos, R. P.; Knoch, B.; Hiane, P. A.; Ramos, M. I. L.; Ramos Filho, M. M. 1-MCP em mangaba armazenadas em temperatura ambiente e a 11 ºC. Revista Brasileira de Fruticultura, v.especial, p.206-212, 2011. http://dx.doi.org/10.1590/S0100-29452011000500024
» http://dx.doi.org/10.1590/S0100-29452011000500024 - Chirife, J.; Timmermann, E. O.; Iglesias, H. A.; Boquet, R. Some features of the parameter k of the GAB equation as applied to sorption isotherms of selected food materials. Journal of Food Engineering, v.15, p.75-82, 1992. http://dx.doi.org/10.1016/0260-8774(92)90041-4
» http://dx.doi.org/10.1016/0260-8774(92)90041-4 - Dieb, J. T.; Gurgel, C. M.; Dantas, T. P.; Medeiros, M. D. F. de. Secagem da polpa de graviola pelo processo foam-mat e avaliação sensorial do produto obtido. Tecnologia & Informação, v.2, p.24-31, 2015.
- Goula, A. M.; Karapantsios, T. D.; Achilias, D. S.; Adamopoulos, K. G. Water sorption isotherms and glass transition temperature of spray dried tomato pulp. Journal of Food Engineering, v.85, p.73-83, 2008. http://dx.doi.org/10.1016/j.jfoodeng.2007.07.015
» http://dx.doi.org/10.1016/j.jfoodeng.2007.07.015 - Greespan, L. Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National of Standards A. Physics and Chemistry, v.81, p.89-96, 1977.
- Hébrard, A.; Oulahna, D.; Galet, L.; Cuq, B.; Abecassis, J.; Fages, J. Hydration properties of durum wheat semolina: Influence of particle size. Powder Technology, v.130, p.211-218, 2003. http://dx.doi.org/10.1016/S0032-5910(02)00268-1
» http://dx.doi.org/10.1016/S0032-5910(02)00268-1 - IAL - Instituto Adolfo Lutz. Normas analíticas do Instituto Adolfo Lutz: Métodos químicos e físicos para análise de alimentos. 4.ed. São Paulo: IAL, 2008. 1020p.
- Kurozawa, L. E.; El-Aouar, A. A.; Murr, F. E. Obtenção de isotermas de dessorção de cogumelo in natura e desidratado osmoticamente. Ciência e Tecnologia de Alimentos, v.25, p.828-834, 2005. http://dx.doi.org/10.1590/S0101-20612005000400033
» http://dx.doi.org/10.1590/S0101-20612005000400033 - Lavoyer, F. C. G.; Gabas, A. L.; Oliveira, W. P.; Telis-Romero, J. Study of adsorption isotherms of green coconut pulp. Food Science and Technology, v.33, p.68-74, 2013. http://dx.doi.org/10.1590/S0101-20612013005000017
» http://dx.doi.org/10.1590/S0101-20612013005000017 - Lima, J. P. de.; Rodrigues, L. F.; Monteiro, A. G. D. P.; Boas, E. V. de B. V. Climacteric pattern of mangaba fruit (Hancornia speciosa Gomes) and its responses to temperature. Scientia Horticulturae, v.197, p.399-403, 2015. http://dx.doi.org/10.1016/j.scienta.2015.09.059
» http://dx.doi.org/10.1016/j.scienta.2015.09.059 - Lomauro, C. J.; Bakshi, A. S.; Labuza, T. P. Evaluation of food moisture sorption isotherm equations. Part I: Fruit, vegetable and meat products. Lebensmittel- Wissenschaft & Technologie, v.18, p.111-117, 1985. http://dx.doi.org/10.1111/j.1365-2621.1985.tb13411.x
» http://dx.doi.org/10.1111/j.1365-2621.1985.tb13411.x - Mil, E.; Rodrigues, S.; Afonso, M. R. A.; Costa, J. M. C. da. Mathematical modeling for isotherms of mango pulp powder, obtained by atomization. Journal of Encapsulation and Adsorption Sciences, v.4, p.8-14, 2014. http://dx.doi.org/10.4236/jeas.2014.41002
» http://dx.doi.org/10.4236/jeas.2014.41002 - Moreira, T. B.; Rocha, E. M. F. F.; Afonso, M. R.; Costa, J. M. C. da. Comportamento das isotermas de adsorção do pó da polpa de manga liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.17, p.1093-1098, 2013. http://dx.doi.org/10.1590/S1415-43662013001000011
» http://dx.doi.org/10.1590/S1415-43662013001000011 - Muzaffar, K.; Kumar, P. Moisture sorption isotherms and storage study of spray dried tamarind pulp powder. Powder Technology, v.291, p.322-327, 2016. http://dx.doi.org/10.1016/j.powtec.2015.12.046
» http://dx.doi.org/10.1016/j.powtec.2015.12.046 - Oikonomopoulou, V. P.; Krokida, M. K; Karathanos, V. K. The influence of freeze drying conditions on microstructural changes of food products. Procedia Food Science, v.1, p.647-654, 2011. http://dx.doi.org/10.1016/j.profoo.2011.09.097
» http://dx.doi.org/10.1016/j.profoo.2011.09.097 - Oliveira, D. M.; Clemente, E.; Costa, J. M. C. da. Hygroscopic behavior and degree of caking of grugru palm (Acrocomia aculeata) powder. Journal of Food Science and Technology, v.51, p.2783-2789, 2014a. https://doi.org/10.1007/s13197-012-0814-9
» https://doi.org/10.1007/s13197-012-0814-9 - Oliveira, G. S.; Costa, J. M. C. da; Afonso, M. R. A. Caracterização e comportamento higroscópico do pó da polpa de cajá liofilizada. Revista Brasileira de Engenharia Agrícola e Ambiental, v.18, p.1059-1064, 2014b. http://dx.doi.org/10.1590/1807-1929/agriambi.v18n10p1059-1064
» http://dx.doi.org/10.1590/1807-1929/agriambi.v18n10p1059-1064 - Oliveira, V. S. de; Afonso, M. R. A.; Costa, J. M. C. da. Physico chemical and hygroscopic behavior of sapodilla lyophilized. Revista Ciência Agronômica, v.42, p.342-348, 2011. http://dx.doi.org/10.1590/S1806-66902011000200012
» http://dx.doi.org/10.1590/S1806-66902011000200012 - Ribeiro, L. C.; Costa, J. M. C. da; Afonso, M. R. A. Hygroscopic behavior of lyophilized acerola pulp powder. Revista Brasileira de Engenharia Agrícola e Ambiental, v.20, p.269-274, 2016. http://dx.doi.org/10.1590/1807-1929/agriambi.v20n3p269-274
» http://dx.doi.org/10.1590/1807-1929/agriambi.v20n3p269-274 - Santana, R. F. de; Oliveira Neto, E. R. de; Santos, A. V.; Soares, C. M. F.; Lima, Á. S.; Cardoso, J. C. Water sorption isotherm and glass transition temperature of freeze-dried Syzygium cumini fruit (jambolan). Journal of Thermal Analysis and Calorimetry, v.120, p.519-524, 2015. https://doi.org/10.1007/s10973-014-4014-x
» https://doi.org/10.1007/s10973-014-4014-x - Santos, A. A. C. dos; Florêncio, A. K. G. D.; Rocha, É. M. de. F. F.; Costa, J. M. C. da. Avaliação físico-química e comportamento higroscópico de goiaba em pó obtida por spray-dryer. Revista Ciência Agronômica, v.20, p.508-514, 2014. http://dx.doi.org/10.1590/S1806-66902014000300010
» http://dx.doi.org/10.1590/S1806-66902014000300010 - Santos, J. T. S.; Costa, F. S. C.; Soares, D. S. C.; Campos, A. F. P.; Carnelossi, M. A. G.; Nunes, T. P.; Oliveira Júnior, A. M. Avaliação de mangaba liofilizada através de parâmetros físico-químicos. Scientia Plena, v.8, p.1-5, 2012.
- Silva, M. P. da; Gomes, F. dos S.; Freire Júnior, M.; Cabral, L. M. C. Avaliação dos efeitos da radiação gama na conservação da qualidade da polpa da amora-preta (Rubus spp L.). Revista Brasileira de Fruticultura, v.36, p.620-627, 2014. http://dx.doi.org/10.1590/0100-2945-218/13
» http://dx.doi.org/10.1590/0100-2945-218/13 - Soares, D. C.; Santos, J. T. S.; Costa, D. G.; Abud, A. K. S.; Nunes, T. P.; Figueiredo, A. V. D.; Oliveira Júnior, A. M. de. Evaluation of Hancornia speciosa Gomes lyophilization at different stages of maturation. International Journal of Biological, Veterinary, Agricultural and Food Engineering, v.8, p.144-118, 2014.
- Soares, F. P.; Paiva, R.; Alvarenga, A. A. de; Nery, F. C.; Vargas, D. P.; Silva, D. R. G. Taxa de multiplicação e efeito residual de diferentes fontes de citocinina no cultivo in vitro de Hancornia speciosa Gomes. Ciência e Agrotecnologia, v.35, p.152-157, 2011. http://dx.doi.org/10.1590/S1413-70542011000100019
» http://dx.doi.org/10.1590/S1413-70542011000100019 - Sousa, K. dos S. M. de; Figueirêdo, R. M. F. de; Queiroz, A. J. de M.; Fernandes, T. K. da S. Production and characterization of atemoya pulp powder. Revista Brasileira de Fruticultura, v.37, p.718-728, 2015. http://dx.doi.org/10.1590/0100-2945-135/14
» http://dx.doi.org/10.1590/0100-2945-135/14 - Strohecker, R.; Henning, H. M. Análises de vitaminas: Métodos comprobados. 1.ed. Madrid: Paz Montalvo, 1967. 428p.
Publication Dates
-
Publication in this collection
May 2017
History
-
Received
20 Sept 2016 -
Accepted
13 Jan 2017