Abstract
Morphological and chromosomal markers were used to infer the structure and genetic variability of a population of fish of the genus Astyanax, geographically isolated at sinkhole 2 of Vila Velha State Park, Paraná, Brazil. Two morphotypes types were observed, the standard phenotype I and phenotype II which showed an anatomical alteration probably due to an inbreeding process. Fluctuating asymmetry (FA) analysis of different characters showed low levels of morphological variation among the population from sinkhole 2 and in another population from the Tibagi river (Paraná, Brazil). The Astyanax karyotype was characterized in terms of chromosomal morphology, constitutive heterochromatin and nucleolar organizer regions. Males and females presented similar karyotypes (2n=48, 6M+18SM+14ST+10A) with no evidence of a sex chromosome system. One female from sinkhole 2 was a natural triploid with 2n=3x=72 chromosomes (9M+27SM+21ST+15A). The data are discussed regarding the maintenance of population structure and their evolutionary importance, our data suggesting that Astyanax from the Vila Velha State Park sinkhole 2 is a recently isolated population.
Fish; Astyanax sp.; fluctuating asymmetry; karyotype structure; triploidy; genetic conservation
ANIMAL GENETICS
RESEARCH ARTICLE
Population structure, fluctuating asymmetry and genetic variability in an endemic and highly isolated Astyanax fish population (Characidae)
Maria Claudia GrossI; Carlos Henrique SchneiderI; Mara Cristina de Almeida MatielloI; Maysa de Lima LeiteII; Luiz Antonio Carlos BertolloIII; Roberto Ferreira ArtoniI
IUniversidade Estadual de Ponta Grossa, Campus de Uvaranas, Departamento de Biologia Estrutural, Molecular e Genética, Ponta Grossa, PR, Brazil
IIUniversidade Estadual de Ponta Grossa, Campus de Uvaranas, Departamento de Biologia Geral, Ponta Grossa, PR, Brasil
IIIUniversidade Federal de São Carlos, Departamento de Genética e Evolução, São Carlos, SP, Brasil
Correspondence Correspondence to Roberto Ferreira Artoni Universidade Estadual de Ponta Grossa, Campus de Uvaranas, Departamento de Biologia Estrutural, Molecular e Genética 84030-900 Ponta Grossa, PR, Brasil E-mail: rfartoni@uepg.br
ABSTRACT
Morphological and chromosomal markers were used to infer the structure and genetic variability of a population of fish of the genus Astyanax, geographically isolated at sinkhole 2 of Vila Velha State Park, Paraná, Brazil. Two morphotypes types were observed, the standard phenotype I and phenotype II which showed an anatomical alteration probably due to an inbreeding process. Fluctuating asymmetry (FA) analysis of different characters showed low levels of morphological variation among the population from sinkhole 2 and in another population from the Tibagi river (Paraná, Brazil). The Astyanax karyotype was characterized in terms of chromosomal morphology, constitutive heterochromatin and nucleolar organizer regions. Males and females presented similar karyotypes (2n=48, 6M+18SM+14ST+10A) with no evidence of a sex chromosome system. One female from sinkhole 2 was a natural triploid with 2n=3x=72 chromosomes (9M+27SM+21ST+15A). The data are discussed regarding the maintenance of population structure and their evolutionary importance, our data suggesting that Astyanax from the Vila Velha State Park sinkhole 2 is a recently isolated population.
Key words: Fish, Astyanax sp., fluctuating asymmetry, karyotype structure, triploidy, genetic conservation.
Introduction
In South America, the Astyanax species is a very diversified and widely distributed group exhibiting taxonomic and systematic complexity (Gery, 1977; Garutti and Britski, 2000). These small Characidae live in varied freshwater environments, including under conditions of high stress such as those occurring in headwaters (LoweMcConnel, 1969), and can also form species complexes showing morphological and genetic variability (Caramaschi, 1986; Langeani, 1989; Moreira-Filho and Bertollo, 1991).
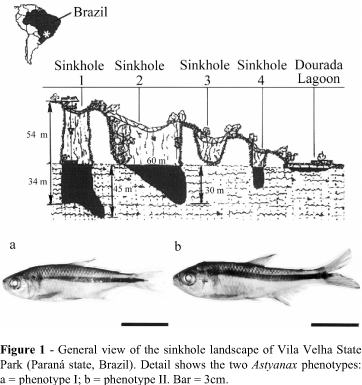
At Vila Velha State Park (Paraná state, Brazil), there is a rare form of sandstone landscape made up of deep cave or crater-like wells known as 'sinkholes' which have been formed by the action of the water in the water table (Soares, 1989) and which present a water column approximately 50m deep (Figure 1) where isolated populations of Astyanax can be found (Artoni and Almeida, 2001). The genera Astyanax also occurs in rivers in the Paraná region and since there is some chromosomal variability between the different riverine populations it is possible that the isolated sinkhole habitats could hold new species of the Astyanax complex (Artoni and Almeida, 2001; Matoso et al., 2002).
Isolated populations tend to reduce their genetic variability and, consequently, their ability to adapt to environmental variation, thus restricting their evolutionary options (Meffe and Caroll, 1994). Inbreeding predisposes small populations the population to a deleterious recessive allele effect which can manifest itself not only by precocious mortality but also by a reduction in fertility or growth rate which can lead to the extinction of the populations (Galbusera et al., 2000). An accurate measurement of genetic heterozygosity and the environmental stress on organisms can be assessed using the fluctuating asymmetry index (FA; Palmer, 1996), which Van Valen (1962) defined as the mean difference between two bilateral characteristics in a population.
Our study used external anatomy and karyotype characteristics to estimate the genetic variation and evolutionary behavior of the endemic Astyanax population of Vila Velha State Park sinkhole 2.
Material and Methods
Characterization of the study area and sampling
Vila Velha State Park is situated in the southern Brazilian state of Paraná at 25° 14' 09''S, 50° 00' 17''W and covers an area of 3,122 ha destined for environmental preservation and eco-tourism. The Vila Velha sinkholes (Figure 1) number 1, 2 and 3 occur at an altitude of 850m and are deep wells excavated from the sandstone bedrock by the action the water in the water table, sinkholes 1 and 2 being connected to the water table at a depth of more than 50m by only tiny fissures in the rocks without any defined or continuous large-scale connections, sinkhole 3 being presently dry (Soares, 1989). All the sinkholes drains into a 200m diameter lake called the Dourada Lagoon (Golden Lagoon), surrounded by a well-preserved riparian forest, and there is constant drainage of water into the Tibagi river (Maack, 1956).
For our study, the first of its kind in these sinkholes, 39 Astyanax specimens were caught at random in sinkhole 2 (Table I) and 37 further Astyanax specimens in the Tibagi river inside the Vila Velha Park, the riverine fish being only used in the morphological analysis (Table II). All specimens were identified and catalogued by Dr Oscar A. Shibatta (Zoology Museum of Londrina State University, Londrina, Paraná, Brazil).
Morphological characters
Based on morphological characters diagnostic for the genus Astyanax (e.g. continuity of the lateral line and the body height/body length ratio; Bristski et al., 1984) there were two main morphotypes, the standard phenotype (phenotype I) and phenotype II which presented some deformation of the spinal column in relation to phenotype I (Figure 1a, b), the chi-squared (c2) test being used to test the relationship between sex and phenotypes I and II.
Four bilateral characters (number of pectoral and pelvic fin rays, number of inferior jaw teeth and number of gill rakers on the right and left side of each specimen) were used to estimate the fluctuating asymmetry of the Astyanax specimens from both sinkhole 2 and the Tibagi river (Figure 2, Tables 1 and 2).
Fluctuating asymmetry (FA) analysis
The fluctuating asymmetry (FA) of the sinkhole 2 and Tibagi river Astyanax specimens was estimated using the formula of Palmer and Strobeck (1986):
where Ri and Li are the right (Ri) and left (Li) side morphological characteristic chosen for the study (number of pectoral and pelvic fin rays, number of inferior jaw teeth and number of gill rakers; Figure 2 and Tables 1 and 2). The FA frequency distribution for each character was analyzed comparatively for the two populations as the absolute difference between both sides for each individual in both populations using the Kolmogorov-Smirnov test at the 5% significance level.
Chromosomal markers
Thirty-five sinkhole 2 Astyanax specimens (10 males and 25 females; Table 1) were analyzed cytogenetically (Figure 3). Chromosome preparations were obtained by the air-drying method of Bertollo et al. (1978) and the constitutive heterochromatin detected using Sumner's (1972) method, silver nitrate (Ag) staining being used to visualize the nucleolar organizer regions (Ag-NORs) according to the method of Howell and Black (1980). The chromosomes were classified as metacentric (M), submetacentric (SM), subtelocentric (ST) or acrocentric (A) according to their morphology and arm ratios (Levan et al., 1964) and arranged in decreasing order of size in the karyotype.
Results
External morphology and sex ratio
Two morphological types were observed for the sinkhole 2 Astyanax (Figure 1), 16 specimens (4 males and 12 females) belonging to phenotype I while 19 (6 males and 13 females) belonged to phenotype II, but there was no significant association between sex and morphotype (c2-test, p=0.9572). The number of females exceeded the number of males by about 2.5 times, indicating a possible distortion of the sex ratio.
Fluctuating asymmetry
The fluctuating asymmetry (FA) of the sinkhole 2 Astyanax specimens did not differ significantly from that of Astyanax specimens from the Tibagi river, suggesting no significant morphological differences between the two populations according to the Kolmogorov-Smirnov test (P=0.1337 for the pectoral fin; 0.1111 for the pelvic fin, 0.0512 for the gill rakers and 0.2051 for the inferior jaw teeth).
Chromosomal variability
Both male and female sinkhole 2 Astyanax specimens had a diploid number of 2n = 48 composed of 3 metacentric, 9 submetacentric, 7 subtelocentric and 5 acrocentric pairs (Figure 3a) and there was no differentiation regarding heteromorphic sex chromosomes. One female specimen was a natural triploid with a chromosome complement of 2n = 3x = 72 (9M + 27 SM + 21 ST +15 A) (Figure 3b). As normally seen in other Characidae, the number 1 metacentric pair was the largest chromosome of the complement, indicating that this is a preserved characteristic of the Astyanax group.
The Ag-NORs were mainly located on chromosomal pairs 4 and 5 at the distal region of the short arm (Figure 3a, b, in the box). Conspicuous heterochromatic segments were present on the telomeric regions of the long arm of the subtelo-acrocentric chromosomes and less evident heterochromatin blocks also occurred on several other chromosomes (Figure 3c, d).
Discussion
Genetic variation, inbreeding effects and implications for species conservation
Populations with a high level of endogamy generally show depressed fitness, especially in respect to factors such as fertility, growth and survival (Leberg, 1990), lower levels of which damage the ability of a population to adapt to changes in their environment. For example, the South African cheetah Acinomyx jubatus jubatus has an uncommon allozyme loci and a complex major histocompatibilty monomorphism resulting from a severe bottleneck and inbreeding during its recent evolutionary history (O'Brien et al., 1983; 1985) and, in contrast with other felines, also presents a low level of fertility due the significant quantity (71%) of morphologically anomalous sperm produced by the male and a high infant mortality rate (O'brien et al., 1985). In addition, A. jubatus jubatus also has a high level of variation in the morphological characters of the facial area of the skull, which reinforces the theory that this feline has suffered developmental instability as consequence of inbreeding.
The sinkhole 2 Astyanax specimens showed some alteration in external morphology (Figure 1) against a low (but not statistically significant) level of asymmetry and karyotypic stability. For example, Phenotype II showed an alteration in the normal vertebral column not present in phenotype I (Figure 1), although no association was found between these two phenotypes and sex (c2-test, p = 0.9572), and it is probable that the anomalous phenotype II is a congenital deformation caused by inbreeding and the absence of gene flow between populations. Genetic problems such as loss of the genetic variability and low fertility rate due to inbreeding are common artificially-reared salmon (Oncorhynchus kisutch) stocks (Winkler et al., 1999) but these problems are uncommon in natural populations because of a large gene pool and a slight endogamic effect (Zakharov and Graham, 1992).
The review by Palmer and Strobeck (1986) shows that there is a high index of fluctuating asymmetry in various fish populations under strong environmental pressure. In spite of the exaggerated endemism of the sinkhole 2 Astyanax population and the probable morphological evidence for a possible loss of heterozygosity detected by us the level of fluctuating asymmetry detected within this population does not indicate that endogamy is having an effect on development. Although both the sample size and the morphological characters employed for fluctuating asymmetry analysis may have influenced the results, the possibility of a recent colonization of the sinkhole by Astyanax remains plausible. In addition, the effective size of the pioneer population is still unknown.
Karyotypic data did not show significant differences between phenotypes I and II Astyanax specimens, supporting the notion that this population is one taxonomic unit.
The sinkhole 2 Astyanax specimens showed a distortion in the sex ratio (2.5 females for each captured male), which seems to be a common occurrence in other Astyanax populations (Salvador and Moreira-Filho, 1992; Vicente et al., 1996) and is probably related to the behavioral and ecological strategies of this group of fish.
Karyotypic features of the Astyanax populations studied
The sinkhole 2 Astyanax had a diploid chromosome number of 2n = 48, with a preferential distribution of the constitutive heterochromatin in the telomeric region of the acrocentric chromosomes (Figure 3c, d). This heterochromatin distribution seems to be quite common among the Astyanax populations which live in the different Vila Velha sinkholes, this same karyotypic pattern having been detected in sinkhole 1 (Matoso et al., 2002) and Tibagi river (Artoni and Almeida, 2001) Astyanax specimens. This karyotype homogeneity may indicate that the sinkhole and riverine populations became isolated only recently and that insufficient evolutionary time has elapsed for the fixation of chromosomal rearrangements. In addition, the genomic and mitochondrial DNA of these populations are very similar with only a small divergence occurring in the sinkhole 2 Astyanax population, which also supports the recent colonization hypothesis (Matoso, 2002).
Karyotypic divergence is a common feature of Astyanax populations with, for example, Astyanax scabripinnis populations from different populations from regions of the Piracuama river (São Paulo state, Brazil) which are at different altitudes showing the same diploid chromosome number (2n = 50) but exhibiting conspicuous differentiation in regard to chromosome types, with geographical accidents such as waterfalls appearing to have served to isolate different populations (Souza and Moreira-Filho, 1995). According Lowe-McConnel (1969), some neotropical fish species can be isolated at the headwaters of river basins through physical, chemical and even biotic barriers. Indeed, works on the taxonomy, distribution and cytogenetics of the ichthyofauna of headwaters have reinforced this proposition (Caramaschi, 1986; Langeani, 1989; Moreira-Filho and Bertollo, 1991).
Natural triploidy is a relatively common event in neotropical fish, especially in members of the genus Astyanax (Fauaz et al., 1994), with, according to Centofante et al. (2001), about 14 species/populations from different neotropical fish families presenting triploid specimens. Although the fertilization of a 2n egg by a haploid sperm is one of the most probable origins of a triploid zygote (Cuellar and Uyeno, 1972; Valenti, 1975) an unreduced egg can also be formed by the retention of the second polar body through thermal shock, fish living at higher altitudes (which is the case for the Vila Velha sinkholes), especially in headwater regions where the temperature can drop suddenly, being more susceptible to this type of event. This hypothesis supports the de novo formation of natural triploids in different fish populations due to sporadic occurrences related to adverse environmental conditions. The triploid sinkhole 2 Astyanax specimens showed three typical Ag-NORs, indicating that all NOR sites were transcriptionally active and the absence of a differential regulatory process for these genes.
In conclusion, our data, including the karyotype characteristics, indicate that this interesting model of enclosed Astyanax populations from the Vila Velha sinkholes shows evidence of inbreeding depression and loss of heterozygosity due to the particular conditions verified in the sinkholes.
Acknowledgments
The authors thank Dr Wagner F. Molina (Universidade Federal do Rio Grande do Norte, Natal, Brazil) for his valuable suggestions. We also thank Instituto Ambiental do Paraná (IAP), Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis (IBAMA) and Paranaturismo for permission to capture fish and the Second Detachment of Military Firemen of Ponta Grossa-PA (Destacamento Militar do Corpo de Bombeiros de Ponta Grossa) for its support in the fish capture at sinkhole 2. This work was also supported by the Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Received: May 7, 2003; Accepted: May 5, 2004
Associate Editor: Fausto Foresti
- Artoni RF and Almeida MC (2001) A singular diversidade dos peixes dos Campos Gerais: uma visão genética para abordagem conservacionista da região. In: Ditzel CHM and Sahr CLL (eds) Espaço e Cultura: Ponta Grossa e os Campos Gerais. Editora da UEPG, Ponta Grossa, Brazil, pp 505-518.
- Bertollo LAC, Takahashi CS and Moreira-Filho O (1978) Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Brazil J Genet 1:103-120.
- Britski HA, Sato Y and Rosa ABS (1984) Manual de identificação de peixes da região de Três Marias: Com chaves de identificação para os peixes da Bacia do São Francisco. Brasilia, Distrito Federal. Câmara dos Deputados, Coordenação de Publicações CODEVASF, Divisão de Piscicultura e Pesca. 143 pp.
- Caramaschi EP (1986) Distribuição da ictiofauna de riachos das bacias do Tietê e do Paranapanema, junto ao divisor de águas (Botucatu, SP). PhD Thesis, Universidade Federal de São Carlos, São Paulo.
- Centofante L, Bertollo LAC and Moreira-Filho, O (2001) Comparative cytogenetics among sympatric species of Characidium (Pisces, Characiformes). Diversity analysis with the description of a ZW sex chromosome system and natural triploidy. Caryologia 54(3):253-260.
- Cuellar D and Uyeno T (1972) Triploidy in rainbow trout. Cytogenetics 11:508-515.
- Fauaz G, Vicente VE and Moreira-Filho O (1994) Natural triploidy and B chromosomes in the neotropical fish genus Astyanax (Characidae). Brazil J Genet 2:157-163.
- Galbusera P, Lens L, Schnenck T, Waiyaki E and Matthysen E (2000) Genetic variability and gene flow in the globally, critically-endangered Taita thrush. Conservation Genetics 1:45-55.
- Garutti V and Britski HA (2000) Descrição de uma espécie nova de Astyanax (Teleostei: Characidae) da bacia do alto rio Paraná e considerações sobre as demais espécies do gênero na bacia. Comum Mus Ciên Tecnol 13:65-68.
- Gery J (1977) Characoids of the World. Neptune City, New Jersey. T.F.H. Publications, 672 pp.
- Howell WM and Black DA (1980) Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 36:1014-1915.
- Langeani F (1989) Ictiofauna do alto curso do rio Tietê (SP): Taxonomia. Master Thesis, Universidade de São Paulo, São Paulo.
- Leberg PL (1990) Influence of genetic variability on population growth: Implications for conservation. J Fish Biol 37:193-195.
- Levan A, Fredga K and Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201-220.
- Lowe-McConnel RH (1969) Speciation in tropical fresh water fishes. Biol J Linnean Soc 1:51-75.
- Maack R (1956) Fenômenos carstiformes de natureza climática e estrutural nas regiões de arenitos do Estado do Paraná. Arquivos de Biologia e Tecnologia 11:151-162.
- Matoso DA (2002) Análise da diversidade genética e estrutura populacional em Astyanax sp. do Parque Estadual de Vila Velha e cabeceira do rio Tibagi. Master Thesis, Universidade Federal de São Carlos, São Paulo.
- Matoso DA, Vicari MR, Almeida MC, Shibatta AO, Moreira-Filho O, Bertollo LAC and Artoni RF (2002) Karyotypic studies in the characidae fish, genus Astyanax An endemic and highly isolated populations of Astyanax sp. Cytologia 67:123-128.
- Meffe GK and Caroll R (1994) Genetics: Conservation of diversity within species. In: Sinauer Associates (ed) Principles of conservation biology. Sunderland, MA, pp 143-178.
- Moreira-Filho O and Bertollo LAC (1991) Astyanax scabripinnis (Pisces, Characidae): A species complex. Brazil J Genet 2:331-357.
- O'Brien SJ, Goldman D, Merril CR, Bush M and Wildt DE (1983) The cheetan is depauperate in biochemical genetic variation. Science 221:459-462.
- O'Brien SJ, Roelke ME, Marker L, Newman A, Winkler CA, Meltzer D, Colly L, Evermann JF, Bush M and Wildt DE (1985) A genetic basis for species vulnerability in the cheetah. Science 227:1428-1434.
- Palmer AR (1996) Waltzing with asymmetry. Is fluctuating asymmetry a powerful new tool of biologist or just an alluring new dance step? BioScience 46:518-532.
- Palmer AR and Strobeck C (1986) Fluctuating asymmetry: Measurement, analysis, patterns. Annu Rev Ecol Syst 17:75-86.
- Salvador LB and Moreira-Filho OM (1992) B chromosomes in Astyanax scabripinnis (Pisces, Characidae). Heredity 69:50-56.
- Soares O (1989) Furnas nos Campos Gerais, Paraná. Curitiba, Paraná. Scientia et Labor, 82 pp.
- Souza IL and Moreira-Filho OM (1995) Cytogenetic diversity in the Astyanax scabripinnis (Pisces, Characidae) complex. II. Different cytotypes living in sympatry. Cytologia 60:273-281.
- Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304-306.
- Valenti RJ (1975) Induced poliploidy in Tilapia aurea (Steindachner) by means of temperature shock treatment. J Fish Biol 7:519-528.
- Van Valen L (1962) A study of fluctuating asymmetry. Evolution 16:125-142.
- Vicente VE, Moreira-Filho OM and Camacho JPM (1996) Sex-ratio distortion associated to the presence of a B chromosome in Astyanax scabripinnis (Pisces, Characidae). Cytogenet Cell Genet 74:70-75.
- Zakharov VM and Graham JH (1992) Developmental stability in natural populations. Acta Zool Fennica 191:1-201.
- Winkler FM, Bartley D and Díaz NF (1999) Genetic differences among year classes in a hatchery population of coho salmon (Oncorhynchus kisutch (Walbaum, 1792)) in Chile. Aquaculture 173:425-433.
Correspondence to
Publication Dates
-
Publication in this collection
14 Jan 2005 -
Date of issue
2004
History
-
Accepted
05 May 2004 -
Received
07 May 2003