Abstract
The PSS genotypes of 596 F2 pigs produced by initial mating of Brazilian native boars commercial sows and were characterized by PCR-RFLP and their carcass and performance traits were evaluated. Among the 596 animals analyzed, 493 (82.72%) were characterized as NN and 103 (17.28%) as Nn. With respect to carcass traits, Nn animals presented higher (p < 0.05) right half carcass weight, left half carcass weight, loin depth and loin eye area, and lower shoulder backfat thickness, backfat thickness between last and next to last but one lumbar vertebrae and backfat thickness after last rib at 6.5 cm from the midline compared to NN animals. Nn animals also showed (p < 0.05) higher values for most of the cut yields, indicating higher cutting yields for animals carrying the n allele and lower values for bacon depth, confirming lower fat deposition in carcass. In addition, Nn animals presented (p < 0.05) lower values for the performance trait weight at 105 days of age. These results indicate that animals carrying the PSS gene generate leaner carcasses, higher cut yields, and that the effects of the gene can be observed even in divergent crosses.
carcass; performance; PSS gene; PCR-RFLP; pig
ANIMAL GENETICS
RESEARCH ARTICLE
Relationship between the Porcine Stress Syndrome gene and carcass and performance traits in F2 pigs resulting from divergent crosses
Guilherme de Oliveira BandI; Simone Eliza Facioni GuimarãesI; Paulo Sávio LopesI; Jane de Oliveira PeixotoI; Danielle Assis FariaI; Aldrin Vieira PiresI; Frederico de Castro FigueiredoI; Carlos Souza do NascimentoI; Lúcio Alberto de Miranda GomideII
IUniversidade Federal de Viçosa, Departamento de Zootecnia, Viçosa, MG, Brazil
IIUniversidade Federal de Viçosa, Departamento de Tecnologia de Alimentos, Viçosa, MG, Brazil
Correspondence Correspondence to Simone E.F. Guimarães Universidade Federal de Viçosa, Departamento de Zootecnia, Viçosa, MG, Brazil E-mail: sfacioni@ufv.br
ABSTRACT
The PSS genotypes of 596 F2 pigs produced by initial mating of Brazilian native boars commercial sows and were characterized by PCR-RFLP and their carcass and performance traits were evaluated. Among the 596 animals analyzed, 493 (82.72%) were characterized as NN and 103 (17.28%) as Nn. With respect to carcass traits, Nn animals presented higher (p < 0.05) right half carcass weight, left half carcass weight, loin depth and loin eye area, and lower shoulder backfat thickness, backfat thickness between last and next to last but one lumbar vertebrae and backfat thickness after last rib at 6.5 cm from the midline compared to NN animals. Nn animals also showed (p < 0.05) higher values for most of the cut yields, indicating higher cutting yields for animals carrying the n allele and lower values for bacon depth, confirming lower fat deposition in carcass. In addition, Nn animals presented (p < 0.05) lower values for the performance trait weight at 105 days of age. These results indicate that animals carrying the PSS gene generate leaner carcasses, higher cut yields, and that the effects of the gene can be observed even in divergent crosses.
Key words: carcass, performance, PSS gene, PCR-RFLP, pig.
Introduction
Since the identification of Porcine Stress Syndrome (PSS), several studies have been carried out comparing performance and carcass traits between PSS genotypes. Most of these studies used the halothane challenge test to separate reactor (nn) and non-reactor (NN and Nn) animals (Leach et al., 1996). The more thorough identification of the three PSS genotypes by molecular techniques has revived interest in their effects on carcass traits (Fisher et al., 2000c).
The PSS gene, also called halothane gene (Hal gene), encodes the precursor protein of the calcium release channel of skeletal muscle sarcoplasmic reticulum (Fujii et al., 1991). The Hal gene, in recessive homozygosis (nn), is associated with the development of PSS, and is related to mortality during transportation as well as occurrence of pale, soft and exudative (PSE) meat. When in heterozygosis (Nn), the Hal gene continues to be related to poor meat quality, but recessive homozygous (nn) animals gain more weight when compared to normal homozygous (NN) ones (Bastos et al., 2001; Santana et al., 1998).
Zhang et al. (1992), studying Pietran x Yorkshire crossed animals, attributed 1 to 10% of the phenotypic variation in meat yield and growth traits to the PSS gene locus. Other studies have indicated that Nn animals presented advantages in traits such as feed efficiency, carcass yield and lean meat content compared to dominant homozygous animals (NN); however, they showed a higher incidence of PSE meat (Leach et al., 1996; Fisher et al., 2000c), a source of economic loss in the swine industry.
The objective of the present study was to evaluate carcass and performance traits and to determine their relationship with the PSS gene in a F2 population resulting from divergent crosses.
Material and Methods
The 596 genotyped F2 animals were produced by outbreed crossing of 18 commercial females, including 11 Landrace x Large White and 7 (Landrace x Large White x Pietrain) with two Brazilian native boars (Piau breed). Both boars and 11 parental females had the NN genotype. The F2 animals were reared and slaughtered at a live weight of 65.03 ± 5.51 kg at the Pig Breeding Farm, Department of Animal Science, Federal University of Viçosa, Viçosa, Minas Gerais State, Brazil.
Animals were deprived of food for 18 h before slaughter, with ad libidum access to fresh water. Afterwards they were electrically stunned (300V/5s) and bled by heart puncture under the animal's left armpit.
At slaughter the following carcass traits were evaluated: slaughter age (SA), right half carcass weight (RHCW), left half carcass weight (LHCW), carcass yield including feet and head (CY), carcass length according to the American carcass classification method (MLC), shoulder backfat thickness (SBT), midline backfat thickness after last rib (LR), midline backfat thickness between last and next to last but one lumbar vertebrae (LL), midline lower backfat thickness above the last lumbar vertebra (L), backfat thickness after last rib, 6.5 cm from the midline (P2), backfat thickness after last rib, 6.5 cm from the midline, equivalent to P2 (ETO), loin depth (LD), and loin eye area (LEA).
The following cut yields were also evaluated after 24 h of cooling at 4 °C: cold right half carcass weight (CRHCW), total ham weight (THW), skinless and fatless ham weight (HW), total shoulder weight (BSW), skinless and fatless shoulder weight (SFBSW), total picnic shoulder weight (PSW), skinless and fatless picnic shoulder weight (SFPSW), total loin (bone-in) weight (TLW), boneless loin weight (LW), bacon weight (BCW), bacon depth (BCD) and sirloin weight (SW). Evaluated performance traits were: birth weight (BW), live weights at 21 (W21), 77 (W77) and 105 (W105) days of age. Feed intake (FI), average daily gain (ADG), and feed:gain ratio (FG) were measured from 77 to 105 days of age.
Carcass traits and cut yields were determined by the carcass dissection technique described by Nascimento and Mota (2000) and Benevenuto Júnior (2001)
Genotypic analys were performed at the Laboratory of Animal Biotechnology, Department of Animal Science, Federal University of Viçosa.
DNA was salt extracted from white blood cells collected immediately after slaughter. Briefly, leukocytes were obtained from 10 mL blood collected in 0.5 mL 0.5% EDTA and centrifuged for 15 min at 958.69 g. The plasma was discarded and each pellet was stored in 1 mL NET 100 (100 mM NaCl, 10 mM Tris, pH 8.3, 100 mM EDTA, pH 8.0) at -20 °C for an indeterminate period of time. On the day of extraction, the samples were thawed at room temperature (about 25 °C). The cells were then washed twice in 1X PBS (3.825 g NaCl, 0.469 g NaH2PO4.H2O, 500 mL MilliQ water) and each microtube was filled with 2 mL hemolysis solution (10 mM Tris, pH 7.5, 5 mM MgCl2, 10 mM NaCl). The cells were then homogenized by vortexing, centrifuged at 958.69 g for 10 min in a refrigerated centrifuge at 4 °C and the supernatant was discarded. This step was repeated until a white pellet was obtained. The samples were then transferred to new labeled microtubes and the pellets were incubated at 55 °C with 200 mL proteinase K buffer (5X) (0.375 M NaCl, 0.12 M EDTA, pH 8.0), 20 mL proteinase (20 mg/mL), 26 mL 20% SDS (20 g SDS, 100 mL MilliQ water) and 744 mL distilled water, in a final volume of 1 mL, for 4-6 h or overnight. For DNA salt precipitation, each sample was divided into two additional microtubes, 110 mL 5 M NaCl was added, and each sample was homogenized manually for 15 s and then centrifuged at 15,338 g for 5 min at room temperature. The supernatant (DNA solution) was then transferred to a new microtube and 1 mL of absolute ethanol was added to each sample. The samples were resuspended and then centrifuged for 5 min at 15,338 g. The supernatant was discarded and the pellet was dried for 15 to 20 min. at room temperature. After drying, the pellet was resuspended in 100 to 200 mL TR (20 mM Tris, pH 8.3, 0.1 mM EDTA, pH 8.0) and incubated in a water bath at 37 °C for 30 min. Finally, the samples were stored at 4 °C for an indeterminate period of time.
The sequence of the ryr-1 gene that contains the C ® T mutation responsible for triggering PSS (Fujii et al., 1991) was amplified by PCR-RFLP using the primers cited by O'Brien et al. (1993), which generated a product of 659 bp.
The amplification mixture contained 1 U of Taq DNA polymerase (Phoneutria), 0.2 mM of each primer (forward - 5'-TCCAGTTTGCCACAGGTCCTACCA-3' - and reverse - 5'-TTCACCGGAGTGGAGTCTCTGAG-T-3'), 2 mM MgCl2, 20 mM Tris, pH 8.3, 50 mM KCl, 0.2 mM dNTPs, and 25 ng genomic DNA in distinct tubes for each animal, in a final volume of 20 mL, following the standard protocol described by Fujii et al. (1991).
Samples were distributed into microtubes, each labeled with the number of the animal to be analyzed. These microtubes, containing the reagent mixture described above, were centrifuged at 7,826 g for 10 s to guarantee that the samples were at the bottom of each microtube. The microtubes were then added to a 96-sample tray of a MJ-Research PTC-100 thermocycler. The amplification program, modified from Fujii et al. (1991) and Houde et al. (1993), consisted of initial denaturation at 94 °C for 3 min and 35 cycles at 94 °C/45 s, 68 °C/1 min and 72 °C/1 min, and a final polymerization step at 72 °C for 5 min.
For mutation analysis of the previously amplified samples, the BsiHKA I restriction enzyme (New England Biolabs) was used. This enzyme cleaves the DNA 659-bp sequence containing the PSS mutation and generates fragments of 524 and 135 bp in normal homozygotes (NN), fragments of 524, 358, 166 and 135 bp in heterozygotes (Nn), and fragments of 358, 166 and 135 bp in mutant homozygous animals (nn). After digestion, the samples were analyzed on 8% silver nitrate-stained polyacrylamide gels and the animals were classified as normal homozygotes (NN), heterozygotes (Nn) and recessive homozygotes (nn) according to the size of the DNA fragments.
Statistical analysis of the association of the genotypes with the traits evaluated was performed using the SAS General Linear Models (SAS, 1997) program, according to the following model:
Yijklm = m + Gi + Sj + Lk + b(Cijklm -  )+ eijklm
)+ eijklm
where Yijkl = observed trait in l animal, of i genotype, j sex and k batch; m = general mean; Gi = genotype effect (NN or Nn); Sj = sex effect (1 = castrated male and 2 = female); Lk = batch effect (k = 1, 2, 3, 4 and 5); b = linear regression coefficient of trait as a function of the covariate; Cijkl = observed covariate value in l animal, of i genotype, j sex, k batch, and eijkl = random error.
For carcass traits and cut yields, carcass weight and cold right half carcass weight (CRHCW) were used as covariates, respectively. For traits FI, W105, ADG, and FG, W77 was used as covariate.
Results
The RFLP patterns observed were as expected. Animals homozygous for the mutation (nn) were characterized by bands of 358, 166 and 135 bp. Normal animals (NN) showed the 524-bp band and the complementary 135-bp band. Heterozygous animals showed bands of 524 and 358 bp and smaller bands of 166 and 1 35 bp.
The frequencies of the NN and Nn genotypes were 493 (82.72%) and 103 (17.28%) animals, respectively. Since only one nn animal was identified, it was not considered for analysis. These unusual frequencies in F2 crosses were found due to divergent mating patterns, in which parental boars were not carriers and the F1 generation was randomly mated despite their PSS genotype.
Average results for carcass traits and number of observations within each genotype are shown in Table 1. Traits showing significant differences between NN and Nn genotypes were RHCW, LHCW, SBT, LL, P2, LD and LEA. Higher (p < 0.05) RHCW, LHCW, LD and LEA and lower (p < 0,05) SBT, LL and P2 were observed for Nn animals, indicating a higher lean content and lower fat deposition.
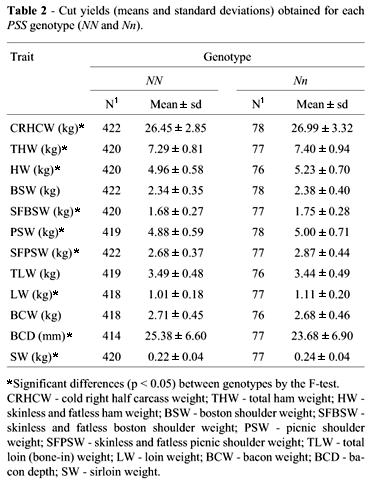
Average results and the number of observations for cut yields within each genotype are shown in Table 2. Significant differences between NN and Nn animals were observed for CRHCW, THW, HW, SFBSW, PSW, SFPSW, LW and SW. Nn animals showed higher (p < 0.05) values for all these cut yields compared to NN animals, confirming the carcass trait results, indicating a higher lean mean content in Nn animals. For BCD Nn animals presented a significantly (p < 0.05) lower value, confirming their lean carcass trait.
Average results and the number of observations for each performance trait within each genotype are shown in Table 3. Among the performance traits evaluated, only weight at 105 days of age was significantly different (p < 0.05) between NN and Nn genotypes, with higher values being observed for NN animals.
Discussion
In contrast to some authors (Eggert et al., 1996; McPhee and Trout, 1995), Nn animals showed better carcass traits compared to NN animals, with higher (p < 0.05) RHCW, LHCW, LD and LEA and lower (p < 0.05) SBT, LL and P2 values, thus confirming the effect of the PSS gene in terms of higher lean meat content and lower fat deposition (Fisher et al., 2000a, 2000b; Leach et al., 1996; Lundstrom et al., 1995.). However, Bastos et al. (2001) did not find significant differences in hot carcass weight, backfat thickness, loin depth or lean meat content among the three genotypes (NN, Nn and nn) when studying animals of the Large White, Landrace, Duroc and Pietrain breeds. The results of the present study differ from those described by those authors in terms of hot carcass weight and loin depth, which were higher in Nn than in NN animals (p < 0.05).
Hamilton et al. (2000), Fisher et al. (2000a), Lundstrom et al. (1995) and McPhee and Trout (1995) found shorter carcasses in Nn animals compared to NN animals, in contrast to the present study in which no significant difference in MLC traits was observed between genotypes (p > 0.05). However, Miller et al. (1999) also observed no difference in carcass length between NN and Nn genotypes. These authors suggested that this gene might have been removed from pig populations, since their results indicated only a small effect of the PSS gene on lean meat content.
Lundstrom et al. (1995), studying F2 crosses between European wild pigs and Large White animals, found no significant difference in growth rate between PSS genotypes, as also observed in the present study.
Higher (p < 0.05) skinless and fatless cut yields (HW, SFBSW and SFPSW) in Nn than in NN animals were also reported in the literature (Fisher et al., 2000a; Leach et al., 1996; Zhang et al., 1992). However, in contrast to Fisher et al. (2000b) Nn animals also showed (p < 0.05) lower BCD than NN animals. However, Fisher et al. (2000b) has slaughtered heavier (86 kg) pigs. In this case the lack of difference in BCD between NN and Nn animals in their experiment may have been due to the fact that their Nn animals had already attained a growth phase of fast fat deposition which could diminish the difference in fat depth between these two genotypes. Lower BCD, associated with no difference in BCW between the two genotypes, suggests higher lean meat content in Nn animals with a live weight up to 65 kg.
The lack of statistical differences (p > 0.05) in average daily gain between NN and Nn animals disagrees with the results reported by Zhang et al. (1992) and McPhee et al. (1994), and is supported by Leach et al. (1996), Miller et al. (1999) and Jin et al. (2002). On the other hand, the lack of a difference (p > 0.05) in the feed:gain ratio between NN and Nn animals disagrees with Leach et al. (1996), but agrees with the results reported by McPhee et al. (1994) and Miller et al. (1999). Taken together, the lack of statistical differences in average daily gain and the feed:gain ratio indicates that the n allele does not influence these traits in pigs fed up to 65 kg of live weight. The lack of a difference (p > 0.05) in the W21 trait between NN and Nn animals confirms the results of Jin et al. (2002).
In the present study, the PSS gene had a positive effect on lean meat deposition and cut yields, but not on performance traits of pigs grown up to 65 kg of live weight. In addition, the effect of the PSS gene was even observed in animals resulting from divergent crossings, thus confirming studies regarding the major effects of this gene.
Acknowledgments
The authors wish to thank FAPEMIG, CNPq and CAPES for financial support.
Received: December 5, 2003; Accepted: September 28, 2004.
Associate Editor: Pedro Franklin Barbosa
- Bastos RG, Federezzi J, Deschamps JC, Cardellino RA and Dellagostin OA (2001) Efeito do gene do estresse suíno sobre características de quantidade e qualidade de carcaça. Revista Brasileira de Zootecnia 30:37-40.
- Benevenuto Júnior AA (2001) Avaliação de rendimento de carcaça e de qualidade da carne de suínos comerciais, de raça nativa e cruzados. Master Thesis, Universidade Federal de Viçosa, Viçosa.
- Eggert JM, Sheiss EB, Scinckel AP, Forrest JC, Grants AL, Mills SE and Watkins BA (1996) Effects of the halothane gene on muscle quality and carcass composition of pigs. http://www.pasture.ecn.purdue.edu/~epados/far.../swine/draftinfo/ genetic/pubs/eggert2 (accessed on 05/18/1999).
- Fisher P, Mellett FD and Hoffman LC (2000a) Halothane genotype and pork quality. 1. Carcass and meat quality traits from the three halothane genotypes. Meat Science 54:97-105.
- Fisher P, Mellett FD and Hoffman LC (2000b) Halothane genotype and pork quality. 2. Cured meat products from the three halothane genotypes. Meat Science 54:107-111.
- Fisher P, Mellett FD and Hoffman LC (2000c) Halothane genotype and pork quality. 3. Comminuted meat products derived from the three halothane genotypes. Meat Science 54:113-117.
- Fujii J, Otsu K, Zorzato F, De Leon S, Khanna VK, Weiler JE, O'Brien PJ and Maclennan DH (1991) Identification of a mutation in the porcine ryanodine receptor associated with malignant hyperthermia. Science 253:448-451.
- Hamilton DN, Ellis M, Miller KD, McKeith FK and Parret DF (2000) The effect of the Halothane and Rendement Napole genes on carcass and meat quality traits of pigs. Journal of Animal Science 78:2862-2867.
- Houde A, Pommier SA and Roy R (1993) Detection of the ryanodine receptor mutation associated with malignant hyperthermia in purebred swine populations. Journal of Animal Science 71:1414-1418.
- Jin HJ, Kim CD, Chung HY, Yeon SH, Cho CY, Lee JH, Kim IC, Ryu IS, Yoo CH and Kim CI (2002) The effects of Porcine Stress Syndrome (PSS) genotype on growth and litter size. 7th World Congress on Genetics Applied to Livestock Production, August 19-23, Montpellier, France.
- Leach LM, Ellis M, Sutton DS, McKeith FK and Wilson ER (1996) The growth performance, carcass traits, and meat quality of halothane carrier and negative pigs. Journal of Animal Science 74:934-943.
- Lundstrom K, Karlsson A, Hakansson J, Hansson I, Johansson M, Andersson and Andersson K (1995) Production, carcass, and meat quality traits of F2-breed crosses between European Wild Pigs and domestic pigs including halothane gene carriers. Animal Science 61:325-331.
- McPhee CP, Daniels LJ, Kramer HL, Macbeth GM and Noble JW (1994) The effects of selection for lean growth and the halothane allele on growth performance and mortality of pigs in a tropical environment. Livestock Production Science 38:117-123.
- McPhee CP and Trout GR (1995) The effects of selection for lean growth, and the halothane allele on carcass and meat quality of pigs transported long and short distances for slaughter. Livestock Production Science 42:55-62.
- Miller D, Ellis M and McKeith FWE (1999) Influence of sire line and halothane genotype on growth performance, carcass and meat quality traits in pigs. http://www.ansci.uiuc.edu/ porknet/ (accessed on 06/04/1999).
- Nascimento JD and Mota EO (2000) Dissecção de carcaças de suínos na Agroceres PIC. Informe Técnico AGROCERES PIC, 5 pp.
- O'Brien PJ, Shen H, Cory CR and Zhang X (1993) Use of a DNA-based test for the mutation associated with Porcine Stress Syndrome (malignant hyperthermia) in 10,000 breeding swine. JAVMA 203:842-851.
- Santana BAA, Borges GSN, Franco MM, Bernardeli K, Nunes ALP, Antunes RC, Borges M and Goulart LR (1998) Aplicação da genotipagem do gene halotano ao melhoramento genético suíno. Anais II Simpósio Nacional de Melhoramento Animal, Uberaba, MG, p 453.
- SAS (1997) STAT User's Guide. Statistical Analysis System. Cary, NC.
- Zhang W, Kuhlers DL and Rempel E (1992) Halothane gene and swine performance. J Anim Sci 70:1307-1313.
Correspondence to
Publication Dates
-
Publication in this collection
08 Sept 2005 -
Date of issue
Mar 2005
History
-
Accepted
28 Sept 2004 -
Received
05 Dec 2003