Abstract
This study aimed to explore: 1) DNA methylation in the promoter regions of Wilms tumor gene 1 (WT1), NK6 transcription factor related locus 1 gene (NKX6-1) and Deleted in bladder cancer 1 (DBC1) gene in cervical cancer tissues of Uygur women in Xinjiang, and 2) the correlation of gene methylation with the infection of HPV16/18 viruses. We detected HPV16/18 infection in 43 normal cervical tissues, 30 cervical intraepithelial neoplasia lesions (CIN) and 48 cervical cancer tissues with polymerase chain reaction (PCR) method. Methylation in the promoter regions of the WT1, NKX6-1 and DBC1 genes in the above-mentioned tissues was measured by methylation-specific PCR (MSP) and cloning sequencing. The expression level of these three genes was measured by real-time PCR (qPCR) in 10 methylation-positive cervical cancer tissues and 10 methylation-negative normal cervical tissues. We found that the infection of HPV16 in normal cervical tissues, CIN and cervical cancer tissues was 14.0, 36.7 and 66.7%, respectively. The infection of HPV18 was 0, 6.7 and 10.4%, respectively. The methylation rates of WT1, NKX6-1 and DBC1 genes were 7.0, 11.6 and 23.3% in normal cervical tissues, 36.7, 46.7 and 30.0% in CIN tissues, and 89.6, 77.1 and 85.4% in cervical cancer tissues. Furthermore, WT1, NKX6-1 and DBC1 genes were hypermethylated in the high-grade squamous intraepithelial lesion (CIN2, CIN3) and in the cervical cancer tissues with infection of HPV16/18 (both P< 0.05). The expression of WT1, NKX6-1 and DBC1 was significantly lower in the methylation-positive cervical cancer tissues than in methylation-negative normal cervical tissues. Our findings indicated that methylation in the promoter regions of WT1, NKX6-1 and DBC1 is correlated with cervical cancer tumorigenesis in Uygur women. The infection of HPV16/18 might be correlated with methylation in these genes. Gene inactivation caused by methylation might be related to the incidence and development of cervical cancer.
Keywords:
gene methylation; gene expression; HPV16/18; cervical cancer; Uygur women
Introduction
Cervical cancer is a common gynecologic malignancy with its incidence and mortality ranked third and fourth, respectively, in women malignant tumors (Ferlay et al., 2013Ferlay J, Soerjomataram I and Ervik M (2013) International Agency for Research on Cancer. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11.http://www.globocan.iarc.fr. (accessed December 12, 2012)
http://www.globocan.iarc.fr...
). A third of the world’s morbidity and mortality from cervical cancer is in China (Gao et al., 2007Gao Q, Wei CJ and Liu XX (2007) The screening and early diagnosis of cervical cancer. J Med Soc 20:41-42.). Xinjiang is a high-incidence region of cervical cancer in China, especially in its southern part. The incidence and mortality of cervical cancer are higher in Uygur than in Han women and other ethnic groups who live in the same region. Therefore, cervical cancer is a major threat to Uygur women’s health in Xinjiang (Pan et al., 2010Pan Z, Chen S, Pan X, Wang Z, Han H, Zheng W, Wang X, Li F, Qu S and Shao R (2010) Differential gene expression identified in Uygur women cervical squamous cell carcinoma by suppression subtractive hybridization. Neoplasma 57:123-128.). Human papillomavirus (HPV) infection is one of the most important factors related to cervical cancer (Huang et al., 2012Huang RL, Chang CC, Su PH, Chen YC, Liao YP, Wang HC, Yo YT, Chao TK, Huang HC, Lin CY, et al. (2012) Methylomic analysis identifies frequent DNA methylation of zinc finger protein 582 (ZNF582) in cervical neoplasms. PLoS One 7:e41060.) and its persistency is a prerequisite for cervical cancer and its precursor lesions (Moscicki et al., 2008Moscicki AB, Ma Y, Wibbelsman C, Powers A, Darragh TM, Farhat S, Shaber R and Shiboski S (2008) Risks for cervical intraepithelial neoplasia 3 among adolescents and young women with abnormal cytology. Obstet Gynecol 112:1335-1342.; Dempsey and Mendez, 2010Dempsey AF and Mendez D (2010) Examining future adolescent human papillomavirus vaccine uptake, with and without a school mandate. J Adolesc Health 47:242-248.). It has been suggested that epigenetic changes can also cause cervical cancer (Sova et al., 2006Sova P, Feng Q, Geiss G, Wood T, Strauss R, Rudolf V, Lieber A and Kiviat N (2006) Discovery of novel methylation biomarkers in cervical carcinoma by global demethylation and microarray analysis. Cancer Epidemiol Biomarkers Prev 15:114-123.).
Development and progression of cervical cancer is caused by a combination of virus, proto-oncogenes, tumor suppressor genes and immune factors. In developing countries, due to poor early diagnosis, precancerous lesions are not found in time to receive the best treatment, making the mortality of cervical cancer far higher than in developed countries (Parkin et al., 2000Parkin DM, Bray F, Ferlay J and Pisani P (2000) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94:153-156.). Additionally to DNA sequence changes (i.e. mutations and deletions), DNA methylation is suggested as a mechanism for cervical cancer by inactivating tumor suppressor genes (Buysschaert et al., 2008Buysschaert I, Schmidt T, Roncal C, Carmeliet P and Lambrechts D (2008) Genetics, epigenetics and pharmaco-(epi) genomics in angiogenesis. J Cell Mol Med 12:2533-2551.). Epigenetic changes can regulate gene expression and DNA methylation is an important component of the epigenetic modifications that cause cancer (Feinberg and Tycko, 2004Feinberg AP and Tycko B (2004) The history of cancer epigenetics. Nat Rev Cancer 4:143-153.). Previous studies have found that high methylation can cause suppressor gene inactivation in cancer tissues. The WT1, NKX6-1 and DBC1 genes in malignant tumor tissues are prone to high methylation (Grønbæk et al., 2008Grønbæk K, Ralfkiaer U, Dahl C, Hother C, Burns JS, Kassem M, Worm J, Ralfkiaer EM, Knudsen LM, Hokland P, et al. (2008) Frequent hypermethylation of DBC1 in malignant lymphoproliferative neoplasms. Modern Pathol 21:632-638., Bruno et al., 2012Bruno P, Gentile G, Mancini R, De Vitis C, Esposito MC, Scozzi D, Mastrangelo M, Ricci A, Mohsen I, Ciliberto G, et al. (2012) WT1 CpG islands methylation in human lung cancer: A pilot study. Biochem Biophys Res Commun 426:306-309., Shimazu et al., 2015Shimazu T, Asada K, Charvat H, Kusano C, Otake Y, Kakugawa Y, Watanabe H, Gotoda T, Ushijima T and Tsugane S (2015) Association of gastric cancer risk factors with DNA methylation levels in gastric mucosa of healthy Japanese: A cross-sectional study. Carcinogenesis 36:1291-1298.). Thus, gene methylation analysis combined with HPV infection detection can be used in the early diagnosis of cervical cancer.
The WT1 gene was first identified in kidney tumor on human chromosome 11p13. WT1 comprises ~5 kb and contains 10 exons; its mRNA spans ~2.9 kb, coding for the renal tumor protein (Wilms tumor protein), which has 449 amino acids (Breslow et al., 1993Breslow N, Olshan A, Beckwith JB and Green DM (1993) Epidemiology of Wilms tumor. Med Pediatr Oncol 21:172-181.). Breslow et al. (1993)Breslow N, Olshan A, Beckwith JB and Green DM (1993) Epidemiology of Wilms tumor. Med Pediatr Oncol 21:172-181. found that WT1 protein is a transcriptional regulation factor. It can activate or inhibit the expression of target genes, producing different biological effects. WT1 plays a role in regulating cell proliferation, growth, differentiation and apoptosis (Scharnhorst et al., 2001Scharnhorst V, van-der-Eb AJ and Jochemsen AG (2001) WT1 proteins: Functions in growth and differentiation. Gene 273:141-161.) and can be both a tumor suppressor and a carcinogenic inducer. Moreover, WT1 has been found hypermethylated in many tumors including glioblastoma, prostate cancer and ovarian cancer (Jacobs et al., 2013Jacobs DI, Mao Y, Fu A, Kelly WK and Zhu Y (2013) Dysregulated methylation at imprinted genes in prostate tumor tissue detected by Methylation microarray. BMC Urol 13:37.; Jiang et al., 2014Jiang Y, Chu Y, Tang W, Wan Y, Zhang L and Cheng W (2014) Transcription factor WT1 and promoter CpG hypomethylation coactivate HOX-A10 expression in ovarian cancer. Curr Pharm Des 20:167-1654.; Rankeillor et al., 2014Rankeillor KL, Cairns DA, Loughrey C, Short SC, Chumas P, Ismail A, Chakrabarty A, Lawler SE and Roberts P (2014) Methylation-specific multiplex ligation-dependent probe amplification identifies promoter methylation events associated with survival in glioblastoma. J Neurooncol 117:243-251.).
The NKX6-1 gene is located in human chromosome 4q21.2-q22, its coding region comprises ~4.9 kb with three exons. This gene codes for a protein of 367 amino acids (Inoue et al., 1997Inoue H, Rudnick A, German MS, Veile R, Donis-Keller H and Permutt MA (1997) Isolation, characterization, and chromosomal mapping of the human NKX6-1 gene (NKX6A), a new pancreatic islet homeobox gene. Genomics 40:367-370.). NKX6-1, which was identified initially in rodents, is a specific transcription factor for islet beta cells and is crucial for their differentiation in the pancreas.
The DBC1 gene is located in human chromosome 9q32-33 (Habuchi et al., 1998Habuchi T, Luscombe M, Elder PA and Knowles MA (1998) Structure and methylation-based silencing of a gene (DBCCR1) within a candidate Bladder cancer tumor suppressor region at 9q32-q33. Genomics 48:277-288.); The DBC1 protein is a member of the RHO atypical family, which contains small GTP enzymes. DBC1 loses heterozygosity in many cancers and is a new gene with hypermethylation status in malignant tumor tissues. It has been shown that DBC1 gene expression increases cell death in bladder cancer cell line (Wright et al., 2004Wright KO, Messing EM and Reeder JE (2004) DBCCR1 mediates death in cultured bladder tumor cells. Oncogene 23:82-90.) and inhibits the growth of non-small cell lung cancer (Izumi et al., 2005Izumi H, Inoue J, Yokoi S, Hosoda H, Shibata T, Sunamori M, Hirohashi S, Inazawa J and Imoto I (2005) Frequent silencing of DBC1 is by genetic or epigenetic mechanisms in non-small cell lung cancers. Hum Mol Genet 14:997-1007.).
We investigated the relationship between gene methylation and infection of HPV16 and HPV18 in cervical cancer. We aimed to understand the expression of WT1, NKX6-1 and DBC1 in the cervical cancer of Uygur women in Xinjiang, and the potential of methylation markers for the screening of cervical cancer.
Materials and Methods
Sample collection
Forty-three normal cervical tissues, 30 cervical intraepithelial neoplasia lesions (CIN) and 48 cervical cancer tissues were collected at the First and Third Affiliated Hospital, School of Medicine, Shihezi University, and the First People’s Hospital of Kashgar. All samples were fresh biopsy tissues from Uygur women who had no radiation nor chemotherapy treatment. All samples were examined by at least two pathologists. Ethical approval for this study was granted by the hospitals with informed consent from patients and their families. The samples were stored in -80 °C freezer.
DNA extraction and HPV detection
Genomic DNA was extracted with TIANamp FFPE DNA Kit DP331-02 Kit (TIANGEN, Beijing), checked by agarose gel (0.7%) electrophoresis for quality and stored at -20 °C. All samples were assessed for high-risk HPV16/18 by PCR with specific primers (Table 1).
Bisulfite conversion and methylation-specific PCR (MSP)
The genomic DNA (1 μg) was bisulfite-modified using CpGenomeTM DNA Modification Kit (S7820, CHEMICON, American) according to the manufacturer’s recommendations and dissolved in 30 μL of nuclease-free water. The methylation and non-methylation primers and their optimal annealing temperatures for WT1, NKX6-1 and DBC1 are listed in Table 1. In vitro methylated DNA (IVD) was used as the positive control.
Cloning sequencing of MSP products
Four microliters of PCR product was used to link with T vector by pEASY-T1 Cloning kit (TransGen Biotech, Beijing) according to the manufacturer’s instruction. E. coli DH5α competent cells and LB agar plates coated with ampicillin (AMP), IPTG and X-gal were used in the transformation. Colonies were grown at 37 °C for 12-16 h. Positive white colonies for methylated and unmethylated WT1, NKX6-1 and DBC1 genes were selected and the plasmids were extracted. PCR further confirmed the colonies, and gene sequence analysis confirmed the MSP of the gene fragments.
RNA extraction and RT-qPCR
Total RNA was prepared with Trizol (Invitrogen) following the manufacturer’s instruction. cDNA was produced from 1 μg of RNA using the RevertAid First Strand cDNA Synthesis Kit (K1622, Thermo, American). Gene expression was analyzed by real-time PCR (qPCR) with the QuantiFast SYBR Green PCR Kit (QIAGEN). The primers used are listed in Table 1. β-actin was used as the internal control.
Statistical analysis
SPSS 17.0 software was used for statistical analysis. Methylation in the promoter regions of WT1, NKX6-1 and DBC1 was analyzed with chi-square test. The respective mRNA levels in cervical cancer tissues and normal cervical tissues were analyzed by Student’s t-test. P< 0.05 was considered statistically significant.
Results
Infection of HPV16/18 in cervical tissues
We found that six of the 43 normal cervical tissues, 11 of the 30 CIN lesions and 32 of the 48 cervical cancer tissues were infected with HPV16. HPV18 infection was not found in normal cervical tissues but was found in two of the 30 CIN lesions and five of the 48 cervical cancer tissues. The positive cases of HPV16 infection in CIN1, CIN2, CIN3 were 1, 4 and 6, respectively. The positive cases of HPV18 infection in the above tissues were 0, 1, 1 (Table 2). There was only one tumor sample exclusively positive for HPV18 infection; the other four HPV18 positive samples were also co-infected by HPV16 (Table 3). The difference in HPV16 infection rate among normal, CIN and cervical cancer tissues was statistically significant (P< 0.01). However, the difference in HPV18 infection rate among those tissues was not statistically significant.
Methylation of WT1, NKX6-1 and DBC1
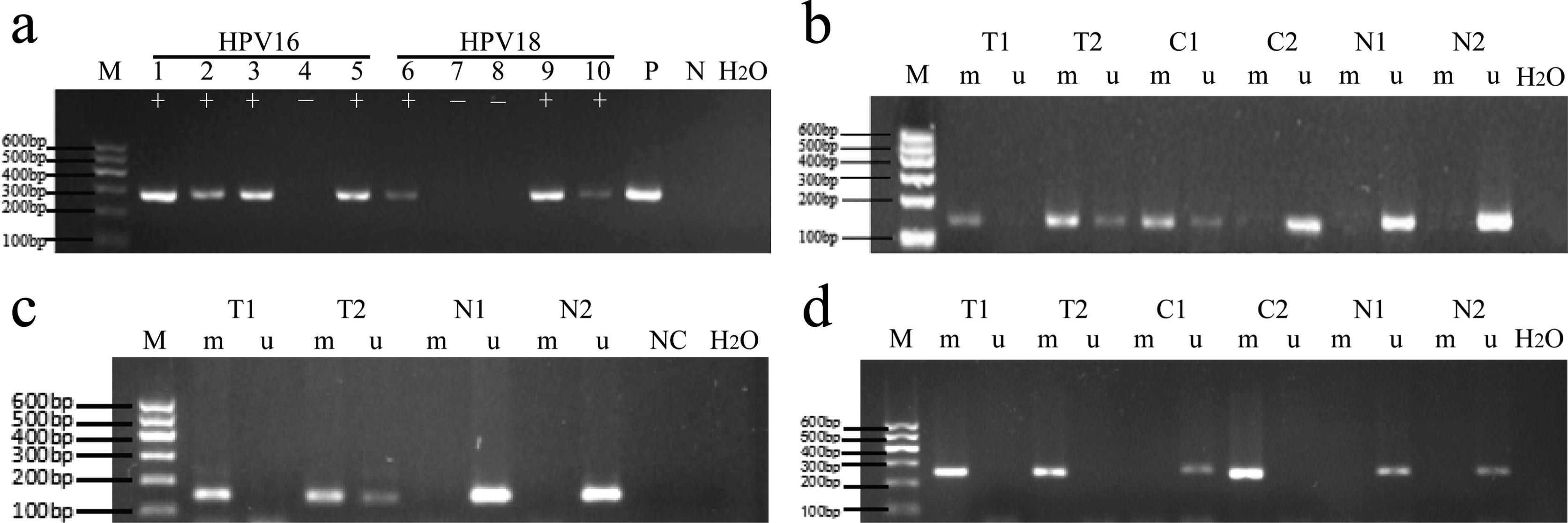
The methylation rate of WT1 in normal cervical tissues, CIN tissues and cervical cancer tissues (Tables 4 and 5) was 7.0, 36.7 and 89.6%, respectively. The methylation rate of NKX6-1 gene in these tissues was 11.6, 46.7 and 77.1%. The methylation rate of DBC1 gene in these tissues was 23.3%, 30% and 85.4%. The corresponding results of agarose gel electrophoresis are shown in Figure 1.
Infection with high-risk human papillomavirus (hr-HPV) and methylation of WT1, NKX6-1 and DBC1 genes in different stages of cervical lesions by agarose gel electrophoresis. (A) HPV16/18 infection; (B) WT1 methylation; (C) NKX6-1 methylation; (D) DBC1 methylation. M: marker (100 ~ 600 bp); lanes 1-5: HPV16 virus PCR products; lanes 6-10: HPV18 virus PCR products; P: positive control, N: negative control; +: positive, -: negative. M: methylation-specific PCR products; U: unmethylation-specific PCR products; T: cervical cancer tissue; C: cervical intraepithelial neoplasia lesions; N: normal cervical tissue.
Cloning and sequencing of the MSP products showed that after bisulfite modification the methylated CpG in C sites did not change, whereas the unmethylated C sites changed to the base of T (Figure 2). We analyzed statistically the relationship of the methylation rates of WT1, NKX6-1 and DBC1 with patient age and the staging of the International Federation of Gynecology and Obstetrics (FIGO), in 48 cervical cancer tissues; there was no statistically significant difference (Table 6).
Sequencing of MSP products. Methylated C in CpG loci remained unchanged whereas unmethylated C residues were modified into T (partial modifications do not change into T). A, B: methylation in the promoter region of the WT1 gene; C, D: methylation in the promoter region of the NKX6-1 gene; E, F: methylation in the promoter region of the DBC1 gene. Left lane: methylation products; right lane: unmethylated products; arrows indicate CpG loci.
Correlation of promoter region methylation with clinical factors of cervical cancer patients.
Correlation between the methylation status of WT1, NKX6-1 and DBC1 and HPV16/18 infection
In the 20 high-grade squamous intraepithelial lesions (CIN2, CIN3) and the 48 cervical cancer tissue samples, the methylation rates of WT1 and DBC1 in the HPV16/18 positive group were significantly higher than those in the HPV16/18 negative group (P< 0.05). The methylation of NKX6-1, however, showed no significant difference between the two groups (Table 7).
Promoter of gene methylation and HPV16/18 infection distribution in CIN2, CIN3 and cervical cancer tissue.
Diagnostic performance of HPV16/18 infection and methylation in the promoter regions of WT1, NKX6-1 and DBC1
We tested and compared the sensitivity, specificity, positive predictive value and negative predictive value of gene methylation and HPV16/18 infection in normal tissue, low-grade squamous epithelial lesions (CIN1), high-grade squamous intraepithelial lesions (CIN2 and CIN3) and cervical cancer tissues. For the diagnosis of cervical cancer, methylation in the promoter region of WT1 showed a higher specificity (94.3%), sensitivity (79.4%) and positive predictive value (94.7%) than methylation in the promoter regions of NKX6-1 (88.7%, 73.5% and 89.3%) and DBC1 (79.2%, 70.6% and 80.0%). For HPV16/18 infection, the specificity and sensitivity were 84.9% and 61.8%, and the positive predictive value and the negative predictive value were 84% and 63.4%. Methylation had a higher sensitivity than HPV16/18 infection. Furthermore, the specificity and sensitivity of the combined methylation analysis were 81.1 and 86.7%, respectively (Table 8).
Sensitivity and PPV to detect CIN2, CIN3 or cancer, and NPV and specificity for normal or CIN1.
Gene expression
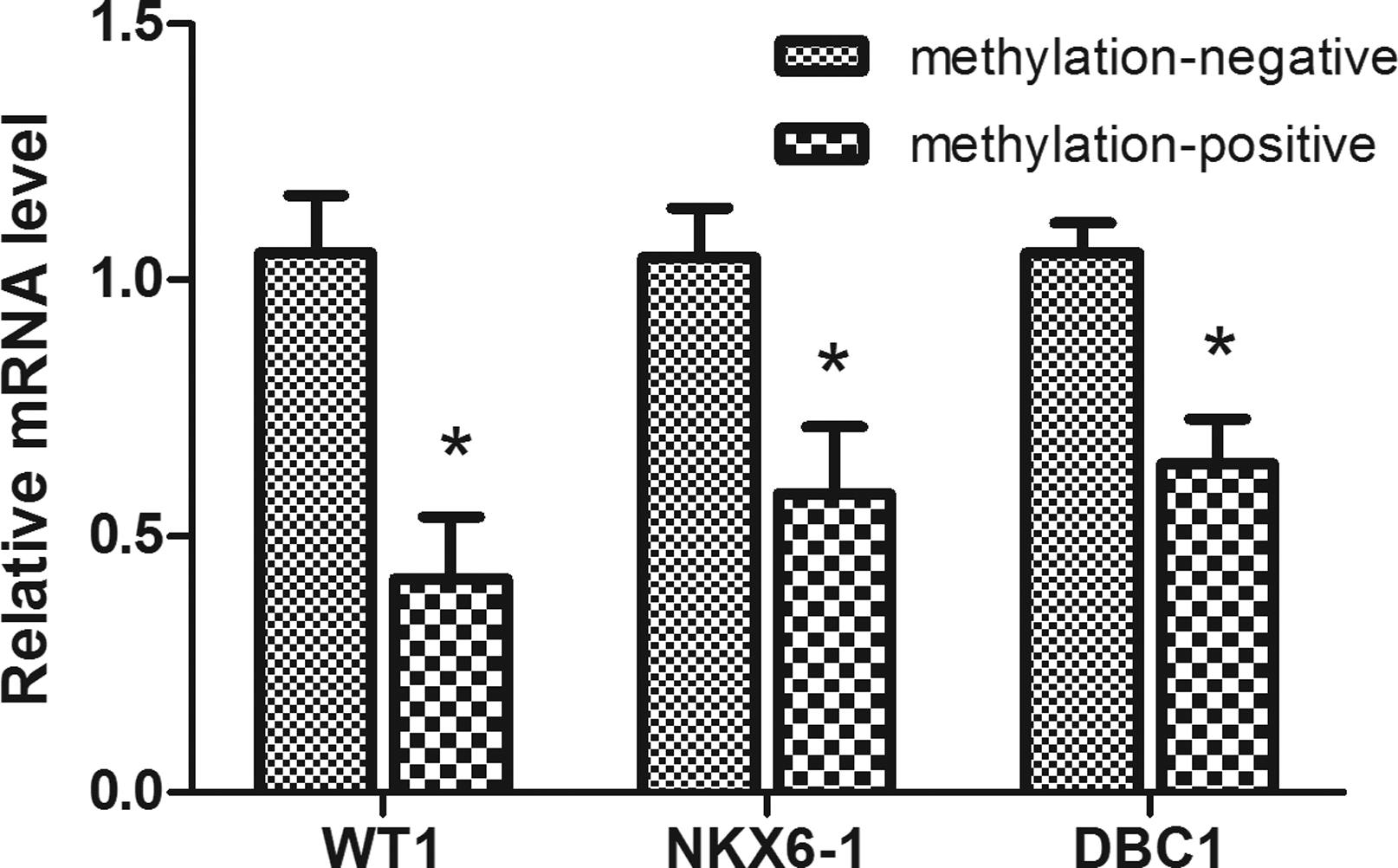
The transcript levels of WT1, NKX6-1 and DBC1 in methylation-positive tissues were 0.416±0.387, 0.582±0.415, and 0.642±0.272, respectively. In methylation-negative tissues, the expression levels of these genes were 1.053±0.349, 1.043±0.308, and 1.052±0.187. The expression levels in methylation-positive cases were significantly lower than in the methylation-negative cases (Figure 3).
Expression of the WT1, NKX6-1 and DBC1 genes in 10 methylation-positive tissues and 10 methylation-negative tissues. β-actin served as internal control. Note: * statistically significant compared with methylation-negative group, P< 0.05.
Discussion
Cervical cancer is a preventable and treatable disease with early diagnosis and treatment. Active treatment can effectively alleviate the disease and increase the survival rate of patients. By understanding the mechanisms of cervical cancer, we hope to identify potential biomarkers for its early diagnosis.
Persistent infection with high-risk human papilloma virus (hr-HPV) is an important factor in cervical cancer incidence (Ribeiro et al., 2015Ribeiro AA, Costa MC, Alves RR, Villa LL, Saddi VA, Carneiro MA, Zeferino LC and Rabelo-Santos SH (2015) HPV infection and cervical neoplasia: associated risk factors. Infect Agent Cancer 10:16.). However, HPV virus alone cannot cause cervical cancer. Due to individual immune defenses, most HPV infections can be removed in two years without causing any clinical symptoms and physical discomfort. The infection rate of the high-risk HPVl6 in China is 79.6% and is significantly higher than in other countries (Lo et al., 2002Lo KW, Wong YF, Chan MK, Li JC, Poon JS, Wang VW, Zhu SN, Zhang TM, He ZG, Wu QL, et a1. (2002) Prevalence of human papillomavirus in cervical cancer: A multicenter study in China. Int J Cancer 100:327-331.). HPV16 has the highest infection rate, followed by HPV18, HPV58 and HPV52 (Davies et al., 2001Davies P, Kornegay J and Iftner T (2001) Current methods of testing for human papillomavirus. Best Pract Res Clin Obstet Gynaecol 15:677-700.). Zuo et al. (2014)Zuo Q, Zheng W, Zhang J, Pan Z, Liu Y, Long H, Fan P, Guo C, Li F and Shao R (2014) Methylation in the promoters of HS3ST2 and CCNA1 genes is associated with cervical cancer in Uygur women in Xinjiang. Int J Biol Markers 29:e354-e362. proposed that cervical cancer in Uygur women in Xinjiang is correlated with multiple HPV infections.
Infection of HPV16 and other HPV types accounts for 97% of the multiple infections in cervical cancer (Sohrabi et al., 2017Sohrabi A, Hajia M, Jamali F and Kharazi F (2017) Is incidence of multiple HPV genotypes rising in genital infections? J Infect Public Health 16:S1876-0341(17)30041-2.). Our results showed that HPV16 infection rates were 14.0, 36.7 and 66.7%, respectively, in the normal cervical tissues, CIN and cervical cancer tissues. Pairwise comparisons showed that the difference in HPV16 infection was statistically significant among these three groups (P< 0.01). The HPV18 infection rates were 0, 6.7 and 10.4% in the same groups, with no significant difference. Our results showed that HPV16/18 infection rates in the tested tissues were gradually increasing along with the degree of pathological changes (Tables 2 and 3). In the present study, we tested only HPV16 and HPV18, although there are more than 20 other types of high risk HPV.
WT1 is a new gene with hypermethylation status in malignant tumors (Rauscher 3rd, 1993Rauscher 3rd FJ (1993) The WT1 Wilms tumor gene product: A developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J 7:896-903.). We found that the methylation rate of the WT1 promoter region in the analyzed tissues gradually and significantly increased along with the development of the disease. Our results indicate that methylation in the WT1 promoter region increases significantly in cervical cancer and high-grade squamous intraepithelial lesions in comparison to normal cervical tissues of Uygur women in Xinjiang. Our results are consistent with previous findings from other regions and ethnic groups (Zhang et al., 2012Zhang XR, Chen D and Tian XY (2012) Quantitative test of methylation suppressor gene locus in significance for early diagnosis of cervical cancer. Chin J Clin Physicians 6:8028-8032.). The methylation rate in the promoter region of NKX6-1 increased from the normal cervical tissues to CIN and cervical cancer tissues, which is consistent with other studies (Lai et al., 2008Lai HC, Lin YW, Huang TH, Yan P, Huang RL, Wang HC, Liu J, Chan MW, Chu TY, Sun CA, et al. (2008) Identification of novel DNA methylation markers in cervical cancer. Int J Cancer 123:161-167.). The methylation rate of the promoter of DBC1 also increased from normal cervical tissues to CIN tissues and the cervical cancer tissues. However, the methylation rates of these genes in cervical cancer tissues were not significantly correlated with age and FIGO stages.
Schlecht et al. (2015)Schlecht NF, Ben-Dayan M, Anayannis N, Lleras RA, Thomas C, Wang Y, Smith RV, Burk RD, Harris TM, Childs G, et al. (2015) Epigenetic changes in the CDKN2A locus are associated with differential expression of P16INK4A and P14ARF in HPV-positive oropharyngeal squamous cell carcinoma. Cancer Med 4:342-353. showed that abnormal methylation was associated with HPV infection. Henken et al. (2007)Henken FE, Wilting SM, Overmeer RM, van Rietschoten JG, Nygren AO, Errami A, Schouten JP, Meijer CJ, Snijders PJ and Steenbergen RD (2007) Sequential gene promoter methylation during HPV-induced cervical carcinogenesis. Br J Cancer 97:1457-1464. proposed that HPV infection could cause epigenetic reconstruction of a host cell in the process of malignant transformation, resulting in HPV phenotype in cervical cancer tissues. Leonard et al. (2012)Leonard SM, Wei W, Collins SI, Pereira M, Diyaf A, Constandinou-Williams C, Young LS, Roberts S and Woodman CB (2012) Oncogenic human papillomavirus imposes an instructive pattern of DNA methylation changes which parallel the natural history of cervical HPV infection in young women. Carcinogenesis 33:1286-1293. proposed that HPV could also induce changes in DNA methylation transferase activity. Whether methylation in the promoter regions of WT1, NKX6-1 and DBC1 is associated with HPV infection is unclear. We found that methylation in the promoter regions of WT1 and DBC1 genes is associated with HPV16/18 infection in cervical cancer tissues of Uygur women in Xinjiang. However, methylation in the promoter region of NKX6-1 gene was not associated with HPV 16/18 infection in cervical cancer tissues. Thus, the methylation of NKX6-1 and HPV16/18 infection appear to be independent factors in the development of cervical cancer. For the diagnosis of cervical cancer, we tested and compared the sensitivity, specificity, positive predictive value and negative predictive value of gene methylation and HPV16/18 infection. Sensitivity is the number of positive HPV16 or HPV18 divided by the number of CIN2, CIN3 and tumor samples (42/68). Specificity is the number of negative HPV16 and HPV18 divided by the number of normal samples (37/43). Gene methylation detection was also calculated according to this method. Our results showed that methylation in the promoter regions of WT1 and NKX6-1 had higher sensitivity, specificity, positive predictive value and negative predictive value than HPV16/18 infection. In addition, the combined methylation analysis of WT1, NKX6-1 and DBC1 had a higher sensitivity than individual genes. Considering that screening for gene methylation of cervical lesions is more reliable than detection of HPV16/18 infection, the probability of misdiagnosis by gene methylation is greatly reduced. Therefore, gene methylation provides a more reliable molecular marker for the diagnosis of cervical cancer of Uygur women.
The expression of WT1, NKX6-1 and DBC1 genes in methylation-positive cervical cancer tissues was significantly lower than in methylation-negative normal cervical tissues. Thus, gene methylation may lead to gene inactivation and play a role in the genesis and development of cervical cancer.
In conclusion, Uygur women in Xinjiang are a high-risk population for cervical cancer. It is important to understand cervical cancer pathogenesis and develop suitable diagnosis and treatment strategies. Cytological diagnosis of cervical cancer usually requires specimens collected by surgery or biopsy. However, the sensitivity of cytological diagnosis is low (Nanda et al., 2000Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD and Matchar DB (2000) Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 132:810-819.) and there are different standards (Yang et al., 2009Yang N, Eijsink JJ, Lendvai A, Volders HH, Klip H, Buikema HJ, van Hemel BM, Schuuring E, van der Zee AG and Wisman GB (2009) Methylation markers for CCNA1 and C13ORF18 are strongly associated with high-grade cervical intraepithelial neoplasia and cervical cancer in cervical scrapings. Cancer Epidemiol Biomarkers Prev 18:3000-3007.). Gene methylation is a convenient marker for early diagnosis and screening of tumors. We showed in this study that methylation rate in the promoter regions of the WT1, NKX6-1 and DBC1 genes were higher in cancer than in normal tissues and the expression of these genes was lower in cervical cancer of Uygur women than in the methylation-negative normal cervical group. These three genes may be suitable molecular markers for diagnosis of cervical cancer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers U1503125, 30860302 and 30660193), the International Science and Technology Collaboration Projector of Xinjiang Production and Construction Corps (grant numbers 2013BC003), National Science and Technology Supporting Project (The Twelfth Five-Year-Researching Project) (grant numbers 2013BAI05B0503), The Scientific Research and Innovation Project of Shihezi University (grant numbers gxjs2013-zdgg05), The Fund from Ministry of Education of China in the Year of 2011 for Promotion of Research Collaboration with America and Oceania Region in Scientific Research and Cultivation (No. [2011]1056), High Level Talent of Scientific Research for Starting Project of Special Grant of Shihezi University (RCZX201333). RS acknowledges the funding support from the Australia-China Science & Research Fund (ACSRF00980).
References
- Breslow N, Olshan A, Beckwith JB and Green DM (1993) Epidemiology of Wilms tumor. Med Pediatr Oncol 21:172-181.
- Bruno P, Gentile G, Mancini R, De Vitis C, Esposito MC, Scozzi D, Mastrangelo M, Ricci A, Mohsen I, Ciliberto G, et al. (2012) WT1 CpG islands methylation in human lung cancer: A pilot study. Biochem Biophys Res Commun 426:306-309.
- Buysschaert I, Schmidt T, Roncal C, Carmeliet P and Lambrechts D (2008) Genetics, epigenetics and pharmaco-(epi) genomics in angiogenesis. J Cell Mol Med 12:2533-2551.
- Davies P, Kornegay J and Iftner T (2001) Current methods of testing for human papillomavirus. Best Pract Res Clin Obstet Gynaecol 15:677-700.
- Dempsey AF and Mendez D (2010) Examining future adolescent human papillomavirus vaccine uptake, with and without a school mandate. J Adolesc Health 47:242-248.
- Feinberg AP and Tycko B (2004) The history of cancer epigenetics. Nat Rev Cancer 4:143-153.
- Gao Q, Wei CJ and Liu XX (2007) The screening and early diagnosis of cervical cancer. J Med Soc 20:41-42.
- Grønbæk K, Ralfkiaer U, Dahl C, Hother C, Burns JS, Kassem M, Worm J, Ralfkiaer EM, Knudsen LM, Hokland P, et al. (2008) Frequent hypermethylation of DBC1 in malignant lymphoproliferative neoplasms. Modern Pathol 21:632-638.
- Habuchi T, Luscombe M, Elder PA and Knowles MA (1998) Structure and methylation-based silencing of a gene (DBCCR1) within a candidate Bladder cancer tumor suppressor region at 9q32-q33. Genomics 48:277-288.
- Henken FE, Wilting SM, Overmeer RM, van Rietschoten JG, Nygren AO, Errami A, Schouten JP, Meijer CJ, Snijders PJ and Steenbergen RD (2007) Sequential gene promoter methylation during HPV-induced cervical carcinogenesis. Br J Cancer 97:1457-1464.
- Huang RL, Chang CC, Su PH, Chen YC, Liao YP, Wang HC, Yo YT, Chao TK, Huang HC, Lin CY, et al. (2012) Methylomic analysis identifies frequent DNA methylation of zinc finger protein 582 (ZNF582) in cervical neoplasms. PLoS One 7:e41060.
- Inoue H, Rudnick A, German MS, Veile R, Donis-Keller H and Permutt MA (1997) Isolation, characterization, and chromosomal mapping of the human NKX6-1 gene (NKX6A), a new pancreatic islet homeobox gene. Genomics 40:367-370.
- Izumi H, Inoue J, Yokoi S, Hosoda H, Shibata T, Sunamori M, Hirohashi S, Inazawa J and Imoto I (2005) Frequent silencing of DBC1 is by genetic or epigenetic mechanisms in non-small cell lung cancers. Hum Mol Genet 14:997-1007.
- Jacobs DI, Mao Y, Fu A, Kelly WK and Zhu Y (2013) Dysregulated methylation at imprinted genes in prostate tumor tissue detected by Methylation microarray. BMC Urol 13:37.
- Jiang Y, Chu Y, Tang W, Wan Y, Zhang L and Cheng W (2014) Transcription factor WT1 and promoter CpG hypomethylation coactivate HOX-A10 expression in ovarian cancer. Curr Pharm Des 20:167-1654.
- Lai HC, Lin YW, Huang TH, Yan P, Huang RL, Wang HC, Liu J, Chan MW, Chu TY, Sun CA, et al. (2008) Identification of novel DNA methylation markers in cervical cancer. Int J Cancer 123:161-167.
- Leonard SM, Wei W, Collins SI, Pereira M, Diyaf A, Constandinou-Williams C, Young LS, Roberts S and Woodman CB (2012) Oncogenic human papillomavirus imposes an instructive pattern of DNA methylation changes which parallel the natural history of cervical HPV infection in young women. Carcinogenesis 33:1286-1293.
- Lo KW, Wong YF, Chan MK, Li JC, Poon JS, Wang VW, Zhu SN, Zhang TM, He ZG, Wu QL, et a1. (2002) Prevalence of human papillomavirus in cervical cancer: A multicenter study in China. Int J Cancer 100:327-331.
- Moscicki AB, Ma Y, Wibbelsman C, Powers A, Darragh TM, Farhat S, Shaber R and Shiboski S (2008) Risks for cervical intraepithelial neoplasia 3 among adolescents and young women with abnormal cytology. Obstet Gynecol 112:1335-1342.
- Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD and Matchar DB (2000) Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 132:810-819.
- Pan Z, Chen S, Pan X, Wang Z, Han H, Zheng W, Wang X, Li F, Qu S and Shao R (2010) Differential gene expression identified in Uygur women cervical squamous cell carcinoma by suppression subtractive hybridization. Neoplasma 57:123-128.
- Parkin DM, Bray F, Ferlay J and Pisani P (2000) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94:153-156.
- Rankeillor KL, Cairns DA, Loughrey C, Short SC, Chumas P, Ismail A, Chakrabarty A, Lawler SE and Roberts P (2014) Methylation-specific multiplex ligation-dependent probe amplification identifies promoter methylation events associated with survival in glioblastoma. J Neurooncol 117:243-251.
- Rauscher 3rd FJ (1993) The WT1 Wilms tumor gene product: A developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J 7:896-903.
- Ribeiro AA, Costa MC, Alves RR, Villa LL, Saddi VA, Carneiro MA, Zeferino LC and Rabelo-Santos SH (2015) HPV infection and cervical neoplasia: associated risk factors. Infect Agent Cancer 10:16.
- Scharnhorst V, van-der-Eb AJ and Jochemsen AG (2001) WT1 proteins: Functions in growth and differentiation. Gene 273:141-161.
- Schlecht NF, Ben-Dayan M, Anayannis N, Lleras RA, Thomas C, Wang Y, Smith RV, Burk RD, Harris TM, Childs G, et al. (2015) Epigenetic changes in the CDKN2A locus are associated with differential expression of P16INK4A and P14ARF in HPV-positive oropharyngeal squamous cell carcinoma. Cancer Med 4:342-353.
- Shimazu T, Asada K, Charvat H, Kusano C, Otake Y, Kakugawa Y, Watanabe H, Gotoda T, Ushijima T and Tsugane S (2015) Association of gastric cancer risk factors with DNA methylation levels in gastric mucosa of healthy Japanese: A cross-sectional study. Carcinogenesis 36:1291-1298.
- Sohrabi A, Hajia M, Jamali F and Kharazi F (2017) Is incidence of multiple HPV genotypes rising in genital infections? J Infect Public Health 16:S1876-0341(17)30041-2.
- Sova P, Feng Q, Geiss G, Wood T, Strauss R, Rudolf V, Lieber A and Kiviat N (2006) Discovery of novel methylation biomarkers in cervical carcinoma by global demethylation and microarray analysis. Cancer Epidemiol Biomarkers Prev 15:114-123.
- Wright KO, Messing EM and Reeder JE (2004) DBCCR1 mediates death in cultured bladder tumor cells. Oncogene 23:82-90.
- Yang N, Eijsink JJ, Lendvai A, Volders HH, Klip H, Buikema HJ, van Hemel BM, Schuuring E, van der Zee AG and Wisman GB (2009) Methylation markers for CCNA1 and C13ORF18 are strongly associated with high-grade cervical intraepithelial neoplasia and cervical cancer in cervical scrapings. Cancer Epidemiol Biomarkers Prev 18:3000-3007.
- Zhang XR, Chen D and Tian XY (2012) Quantitative test of methylation suppressor gene locus in significance for early diagnosis of cervical cancer. Chin J Clin Physicians 6:8028-8032.
- Zuo Q, Zheng W, Zhang J, Pan Z, Liu Y, Long H, Fan P, Guo C, Li F and Shao R (2014) Methylation in the promoters of HS3ST2 and CCNA1 genes is associated with cervical cancer in Uygur women in Xinjiang. Int J Biol Markers 29:e354-e362.
Internet Resources
- Ferlay J, Soerjomataram I and Ervik M (2013) International Agency for Research on Cancer. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11.http://www.globocan.iarc.fr (accessed December 12, 2012)
» http://www.globocan.iarc.fr
-
Associate Editor: Carlos F. M. Menck
Publication Dates
-
Publication in this collection
Jan-Mar 2018
History
-
Received
26 May 2016 -
Accepted
07 June 2017