ABSTRACT
Zinc (Zn) participates of numerous metabolic processes in plants. However, it can become toxic to plants in excessive concentrations in the soil. Pfaffia glomerata is a Brazilian medicinal species that has stood out because of its numerous chemical and functional properties, mainly by the triterpene saponins and ecdysteroids accumulated in its roots. This study aimed to evaluate the effects of zinc excess on many root morphological parameters of Pfaffia glomerata. A 4 x 3 factorial design was employed in a completely randomized scheme with 3 replicates. The treatments consisted of four concentrations of Zn (2, 100, 200, and 300 µM) and three accessions of P. glomerata (BRA, GD, and JB) grown in a hydroponic system for 7 and 14 days. Differences in root morphology and dry mass production were observed among the three accessions in response to excessive Zn. Some growth parameters of GD accession increased with the addition of Zn, ranging from 36 to 79 µM. However, the GD and JB accessions presented reduction in dry mass production, root area, length, and volume with increasing Zn levels. The BRA accession, which had the lowest growth among accessions, presented chlorotic leaves. The shoot/root dry mass ratio and root diameter increased linearly for BRA and GD accessions at 7 days. Based on the evaluated parameters, we observed the following order of Zn excess tolerance in P. glomerata accessions: GD> JB> BRA.
Keywords
growth; heavy metal; Brazilian ginseng; root length; root volume
RESUMO
O zinco (Zn) participa de numerosos processos metabólicos nas plantas. No entanto, em concentrações excessivas no solo pode tornar-se tóxico para os vegetais. Pfaffia glomerata é uma espécie medicinal brasileira que tem se destacado devido as suas inúmeras propriedades químicas e funcionais, devido principalmente às saponinas triterpênicas e ecdisteróides acumuladas em suas raízes. O objetivo do presente trabalho foi avaliar os efeitos do excesso de Zn sobre vários parâmetros morfológicos radiculares de Pfaffia glomerata. O delineamento experimental utilizado foi o inteiramente casualizado com três repetições, dispostos em um arranjo fatorial (4 x 3). Os tratamentos consistiram em quatro níveis de Zn (2, 100, 200 e 300µM) e três acessos (BRA, GD e JB) de P. glomerata cultivados em sistema hidropônico em casa de vegetação por 7 e 14 dias. Diferenças na morfologia radicular e na produção de material seca foram observadas entre os três acessos em resposta ao excesso de Zn. Alguns parâmetros de crescimento do acesso GD aumentaram sob a adição de Zn variando entre 36 e 79 µM. No entanto, os acessos GD e JB apresentaram redução na matéria seca, bem como na área de superfície, comprimento e volume radicular com o aumento dos níveis de Zn. O acesso BRA, que teve o menor crescimento entre os acessos, apresentou folhas cloróticas. A razão entre matéria seca da parte aérea e raízes e o diâmetro radicular aumentou linearmente para os acessos BRA e GD aos 7 dias de cultivo. Baseando-se nos parâmetros avaliados foi observada a seguinte ordem de tolerância ao excesso de Zn: GD> JB> BRA.
Palavras-chave
crescimento; metal pesado; ginseng brasileiro; comprimento radicular; volume radicular
INTRODUCTION
The contamination by heavy metals and other toxic elements in agricultural areas has received concerns in the last few decades, due to exposure risks to humans and the environment (Wuana & Okieimen, 2011WUANA, R.A.; OKIEIMEN, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. International Scholarly Research Network Ecology, v.2011, p.1-20, 2011.). Many studies describe the negative effects of non-essential and toxic elements like cadmium (Cd), lead (Pb) and arsenic (As) on anatomy, morphology and metabolic reactions for many plant species (Zhao et al., 2009ZHAO, F.J. et al. Arsenic uptake and metabolism in plants. New Phytologist, v.181, n.4, p.777-794, 2009.; Lux et al., 2011LUX, A. et al. Root responses to cadmium in the rhizosphere: a review. Journal Experimental Botany, v.62, n.1, p.21-37, 2011.; Pourrut et al., 2011POURRUT, B. et al. Lead uptake, toxicity, and detoxification in plants. Journal environmental contamination and toxicology, v.213, p.113-136, 2011.). In addition, plant micronutrients such as zinc (Zn), copper (Cu) and manganese (Mn) are being added to agricultural systems, therefore increasing the risk of toxicity to plants (Reichman, 2002REICHMAN, S.M. The responses of plants to metal toxicity: A review focusing on copper, manganese and zinc. Melbourne: Australian Minerals & Energy Environment Foundation. Occasional Paper, n.14, p.1-54, 2002.).
Zn has important functions as a cofactor in more than 300 proteins (Palmgren et al., 2008PALMGREN, M.G. et al. Zinc biofortification of cereals: problems and solutions. Trends in Plant Science, v.13, n.9, p.464-473, 2008.). However, it may become toxic to plants under supra-optimal concentrations (Lin et al., 2005LIN, C. et al. Zinc induces mitogen-activated protein kinase activation mediated by reactive oxygen species in rice roots. Plant Physiology and Biochemistry, v.43, n.10-11, p.963-968, 2005.; Broadley et al., 2007BROADLEY, M.R. et al. Zinc in plants. New Phytologist, v.173, n.4, p.677-702, 2007.; Ricachenevsky et al., 2015RICACHENEVSKY, F.K. et al. Molecular control of Zn accumulation and biotechnological applications. Plant Science, v.236, p.1-17, 2015.). For most soils, Zn is found at adequate concentrations (around 60 mg kg-1) for plant growth, but anthropogenic activities can increase its concentration by industrial processes such as mining and smelting or by agricultural techniques such as addition of biosolid fertilizes, mineral fertilization and pesticides (Reichman, 2002REICHMAN, S.M. The responses of plants to metal toxicity: A review focusing on copper, manganese and zinc. Melbourne: Australian Minerals & Energy Environment Foundation. Occasional Paper, n.14, p.1-54, 2002.; Wuana & Okieimen, 2011WUANA, R.A.; OKIEIMEN, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. International Scholarly Research Network Ecology, v.2011, p.1-20, 2011.). When at high concentrations in plant tissues (100 to 300µg Zn g-1 leaf dry weight), Zn can promote severe physiological and morphological changes, including the inhibition of root elongation (Broadley et al., 2007BROADLEY, M.R. et al. Zinc in plants. New Phytologist, v.173, n.4, p.677-702, 2007.; Disante et al., 2010DISANTE, K.B. et al. Sensitivity to zinc of Mediterranean woody species important for restoration. Science of The Total Environment, v.408, n.10, p.2216-2225, 2010.).
Plants have shown different strategies to cope with high (toxic) levels of Zn in their growth environment (Ricachenevsky et al., 2015RICACHENEVSKY, F.K. et al. Molecular control of Zn accumulation and biotechnological applications. Plant Science, v.236, p.1-17, 2015.). These tolerance strategies mainly include detoxification processes such as complexation by organic chelators and sequestering of Zn in vacuoles (Ricachenevsky et al., 2015RICACHENEVSKY, F.K. et al. Molecular control of Zn accumulation and biotechnological applications. Plant Science, v.236, p.1-17, 2015.). The current knowledge on Zn homeostasis of land plants is largely based on the model plant Arabidopsis thaliana, for which our molecular understanding is most developed at present (Sofo et al., 2013SOFO, A. et al. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiologia Plantarum, v.149, n.4, p.287-298, 2013.; Ricachenevsky et al., 2015RICACHENEVSKY, F.K. et al. Molecular control of Zn accumulation and biotechnological applications. Plant Science, v.236, p.1-17, 2015.). Sofo et al. (2013)SOFO, A. et al. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiologia Plantarum, v.149, n.4, p.287-298, 2013. observed that the Cd/Cu/Zn-induced changes in root morphology of Arabidopsis were caused by a hormonal imbalance, mainly controlled by the auxin/cytokinin ratio. It has been indicated that Zn toxicity depends on plant species and growth stage, and that growth inhibition and biomass reduction are common responses of plants to excess Zn (Li et al., 2009LI, T. et al. Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. Journal of hazardous materials, v.169, n.1-3, p.734-741, 2009.; Li et al., 2012LI, X. et al. Zinc induced phytotoxicity mechanism involved in root growth of Triticum aestivum L. Ecotoxicology and Environmental Safety, v.86, p.198-203, 2012.; Marques & Nascimento, 2014MARQUES, M.C.; NASCIMENTO, C.W.A. Tolerância de mamona a zinco avaliada por fluorescência de clorofila e nutrição das plantas. Revista Brasileira de Ciência do Solo, v.38, n.3, p.850-857, 2014.; Alonso-Blázquez et al., 2015ALONSO-BLÁZQUEZ, N. et al. Influence of Zn-contaminated soils in the antioxidative defence system of wheat (Triticum aestivum) and maize (Zea mays) at different exposure times: potential use as biomarkers. Ecotoxicology, v.24, n.2, p.279-291, 2015.).
Pfaffia glomerata (Spreng.) Pedersen has been used as a model plant for studies of the effect of heavy metals, in which it was found that young plants showed moderate tolerance to Hg, As and Cd (Calgaroto et al., 2011CALGAROTO, N.S. et al. Zinc alleviates mercury-induced oxidative stress in Pfaffia glomerata (Spreng.) Pedersen. Biometals, v.24, n.5, p.959-971, 2011.; Gupta et al., 2013GUPTA, D.K. et al. Effect of Hg, As and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata. Ecotoxicology, v.22, n.9, p.1403-1412, 2013.). Kamada et al. (2009a)KAMADA, T. et al. Diversidade genética de populações naturais de Pfaffia glomerata (Spreng.) Pedersen estimada por marcadores RAPD. Acta Scientiarum Agronomy. v.31, n.3, p.403-409, 2009a. have reported that this species presented great genetic diversity among its natural populations, which can result in different levels of tolerance to heavy metals. Therefore, the use of different accessions of this species can help to understand how plants cope with high levels of Zn in the environment. Based on this, the aim of the work was to evaluate the influence of Zn levels on many root morphological parameters that can be used to distinguish accessions of P. glomerata according to their tolerance level to excess Zn.
MATERIALS AND METHODS
Three accessions (BRA, JB and GD) of Pfaffia glomerata (Spreng.) Pedersen were used in this study. The accession BRA belongs to Germplasm Bank of Embrapa Recursos Genéticos e Biotecnologia (Cernagem), the JB belongs to collection of the Botanical Garden of Universidade Federal de Santa Maria (UFSM) and the GD belongs to collection of medicinal plants of the Universidade Federal da Grande Dourados (UFGD).
Plants used in the experiment were obtained by in vitro culture from nodal segments (1.0 cm long and without leaves) of the middle portion of micropropagated plantlets cultivated on MS (Murashige & Skoog, 1962MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassays with tabacco tissue cultures. Physiologia Plantarum, v.5, p.473-97, 1962.) medium, which was supplemented with 6g L-1 agar, 30g L-1 sucrose, 0.1g L-1 myo-inositol, and in the absence of growth regulators.
At 25 days of in vitro growth, plants were transferred to plastic containers with a capacity of 17 liters of nutrient solution with the following composition (in µM): 6090.5 N; 974.3 Mg; 4986.76 Cl; 2679.2 K; 2436.2 Ca; 359.9 S; 243.592 P; 0.47 Cu; 2.00 Mn; 1.99 Zn; 0.17 Ni; 24.97 B; 0.52 Mo; 47.99 Fe (FeSO4/Na EDTA). After seven days of acclimatization, the treatments of Zn levels were added as a solution of ZnSO4.7H2O. The treatments were arranged in a completely randomized design with 4 x 3 factorial scheme, being four levels of Zn (2, 100, 200 and 300 µM) and three accessions (BRA, JB and GD) of P. glomerata, with three replicates. The nutrient solution pH was adjusted daily to 5.5±0.2 using HCl or NaOH solution of 0.1mol L-1. The nutrient solution was changed every 7 days of cultivation.
Seven and 14-day-old plants were collected for evaluation of dry matter and parameters of root morphology. For dry matter evaluation, shoot and root from five plants per replicate were collected, gently washed twice with distilled water and dried at 65°C, until a constant dry weight was obtained. For root morphology, roots of five plants per replicate were collected, gently washed twice with distilled water, and frozen in plastic bags for 7 days. Roots were thawed at room temperature for 30 minutes and digitalized with the aid of a scanner Epson 11000 XL. Root morphology analysis was performed with the aid of WinRhizo Pro Software, which determines the total length, surface area, total volume and average diameter of roots.
All the data were tested by the assumptions of the mathematical model (normality and homogeneity of variance). The analysis of variance of the experimental data was performed using the F-test. The quantitative factor, when significant (P<0.05), was subjected to polynomial regression analysis, by testing the linear and quadratic models. Pearson’s correlations between dry weight and root morphological parameters were performed using SigmaPlot software version 12.3 (P<0.05).
RESULTS AND DISCUSSION
We measured the effect of increasing Zn concentrations on dry mass production. Interestingly, at 7 days, GD accession showed increase in root, shoot and total dry mass when excess Zn is added at moderate concentrations (50, 36 and 40 µM, respectively; Figure 1). However, at 14 days, root dry mass linearly decreased upon addition of Zn, whereas shoot and total dry mass increased at about 79 and 70μM Zn, respectively (Figure 1). The increase in dry mass observed for GD accession is due to the fact that the accession presented higher growth rate (Figure 1) and, consequently greater demand for Zn. On the other hand, the accession JB showed linear decrease to all growth parameters of dry mass with increasing levels of Zn in both 7 and 14 days (Figure 1). The threshold of Zn toxicity sharply varies among plant species, time of exposure and composition of the nutrient solution. Significant decrease in total dry mass of Populus deltoids and Populus nigra developed after additions of 100µM and 1000µM Zn, respectively (Di Baccio et al., 2003DI BACCIO, D.R. et al. Responses of Populus deltoides x Populus nigra (Populus x euramericana) clone I-214 to high zinc concentrations. New Phytologist, v.159, n.2, p.443-452, 2003.).
Effect of zinc levels on dry matter production of roots (A and B), shoot (C and D) and whole plant (E and F), as well as shoot/root dry matter ratio (G and H) of three accessions (BRA, GD and JB) of P. glomerata cultivated in hydroponics for 7 and 14 days.
In general, all P. glomerata accessions showed some reduction in growth based on dry mass with increasing levels of Zn in nutrient solution, but this response was genotype dependent (Figure 1). The accession BRA showed either no alteration in dry mass or a small linear increase in shoot and total plant dry mass with increasing levels of Zn. However, independently of the Zn level tested, this accession showed chlorotic leaves and low growth during the evaluation periods, indicating that it was not adapted to the hydroponic system used, when compared to the other accessions. The observed typical symptoms of inhibition of growth of P. glomerata by excess Zn have also been reported for other species such as Sedum alfredii (Li et al., 2009LI, T. et al. Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. Journal of hazardous materials, v.169, n.1-3, p.734-741, 2009.), Ricinus communis (Marques & Nascimento, 2014MARQUES, M.C.; NASCIMENTO, C.W.A. Tolerância de mamona a zinco avaliada por fluorescência de clorofila e nutrição das plantas. Revista Brasileira de Ciência do Solo, v.38, n.3, p.850-857, 2014.), Triticum aestivum (Li et al., 2012LI, X. et al. Zinc induced phytotoxicity mechanism involved in root growth of Triticum aestivum L. Ecotoxicology and Environmental Safety, v.86, p.198-203, 2012.) and Zea mays (Alonso-Blázquez et al., 2015ALONSO-BLÁZQUEZ, N. et al. Influence of Zn-contaminated soils in the antioxidative defence system of wheat (Triticum aestivum) and maize (Zea mays) at different exposure times: potential use as biomarkers. Ecotoxicology, v.24, n.2, p.279-291, 2015.).
For shoot/root dry matter ratio there were no significant interaction among accessions and Zn levels at 7 days (Figure 1G). In this parameter the BRA and GD accessions showed linear increased with increasing Zn levels in solution. At 14 days the accessions GD and JB showed a cubical response (Figure 1H). Differences between root and shoot growth under Zn excess were also observed for the ecotypes of Holcus lanatus by Rengel (2000)RENGEL, Z. Ecotypes of Holcus lanatus tolerant to zinc toxicity also tolerate zinc deficiency. Annals of Botany, v.86, n.6, p.1119-1126, 2000. and in Phyllostachys pubescens by Liu et al. (2014)LIU, D. et al. Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environmental Science & Pollution Research, v.21, n.23, p.13615-24, 2014.. Disante et al. (2010)DISANTE, K.B. et al. Sensitivity to zinc of Mediterranean woody species important for restoration. Science of The Total Environment, v.408, n.10, p.2216-2225, 2010. reported reduction in leaf biomass of several woody species to excess Zn, as well as increase in root biomass production under relatively low levels of Zn.
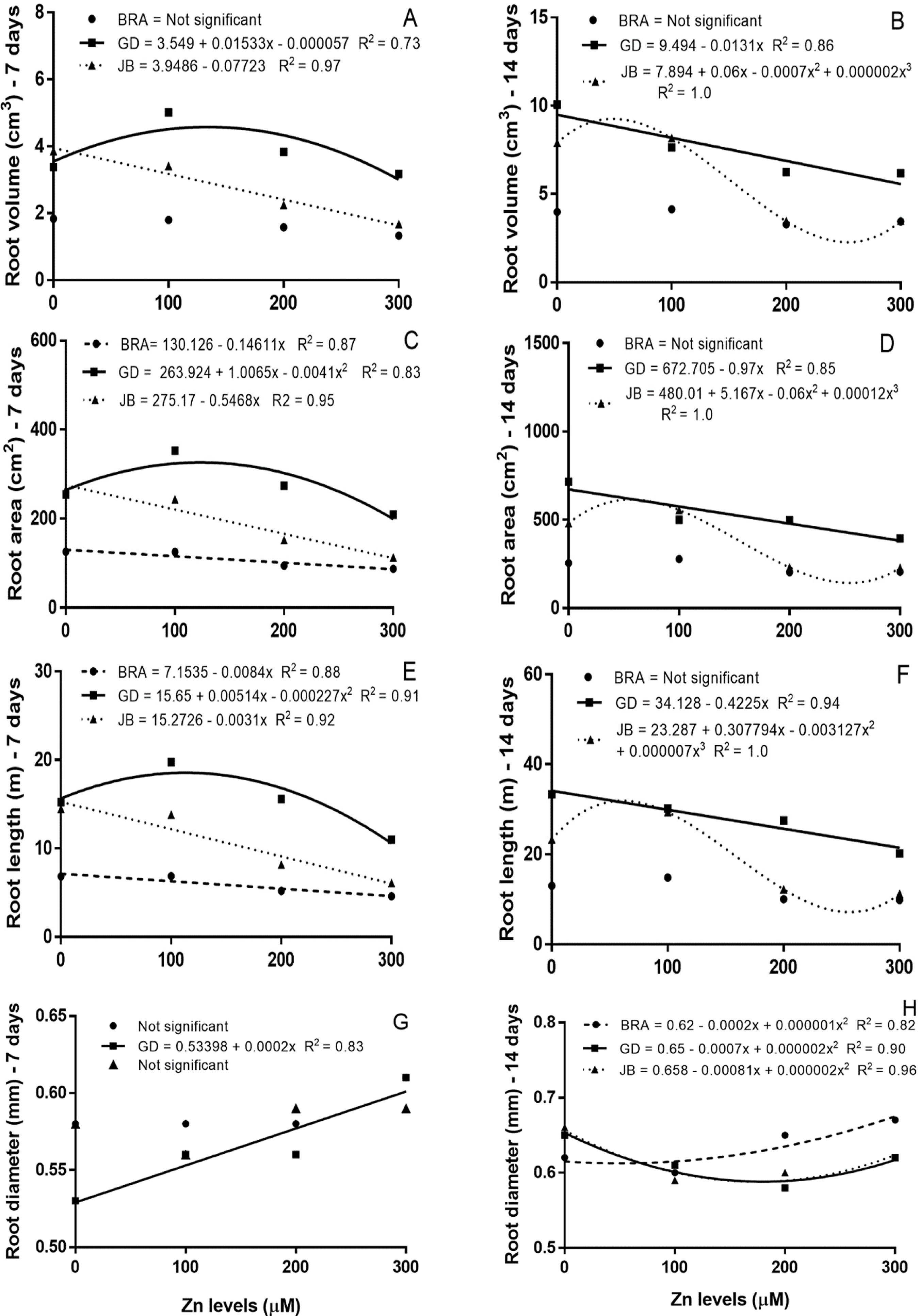
In general, the volume (Figure 2A and 2B), surface area (Figure 2C and 2D) and length (Figure 2E and 2F) of roots to all accessions of P. glomerata decreased with increasing Zn levels in nutrient solution. The length, surface area, diameter and volume of roots may be valuable parameters to compare the response of different genotypes under excess heavy metals (Li et al., 2009LI, T. et al. Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. Journal of hazardous materials, v.169, n.1-3, p.734-741, 2009.; Disante et al., 2010DISANTE, K.B. et al. Sensitivity to zinc of Mediterranean woody species important for restoration. Science of The Total Environment, v.408, n.10, p.2216-2225, 2010.). Li et al. (2012)LI, X. et al. Zinc induced phytotoxicity mechanism involved in root growth of Triticum aestivum L. Ecotoxicology and Environmental Safety, v.86, p.198-203, 2012. observed that the loss of cell viability and the significant increases of lignification in response to excess Zn may be associated with the remarkable reduction of root growth in Triticum aestivum seedlings.
Effect of zinc levels on the volume (A and B), surface area (C and D), length (E and F) and average diameter (G and H) of roots of three accessions (BRA, GD and JB) of P. glomerata grown in hydroponics at 7 and 14 days.
Nevertheless, at 7 days of exposure to Zn, GD accession showed higher volume, surface area and length of roots than the other accessions (Figure 2). In addition, this response was quadratic, where the dose of maximum yields observed to volume (Figure 2A), surface area (Figure 2C) and length of roots (Figure 2E) were 134.5, 122.7 and 11.3μM Zn, respectively. On the other hand, the other two accessions showed a negative linear response; except for root volume of BRA that was not altered (Figure 2A, 2C and 2E). Meanwhile, at 14 days, accession GD presented a negative linear response, and the JB accession showed a cubic response, with similar volume (Figure 2B), surface area (Figure 2D) and length (Figure 2F) of roots up to about 100µM Zn for both accessions.
Several environmental factors influence root morphology, such as metal stress (Li et al., 2005LI, T. et al. Root responses and metal accumulation in two contrasting ecotypes of Sedum alfredii Hance under lead and zinc toxic stress. Journal of Environmental Science and Health, v.40, n.5, p.1081-1096, 2005.) and nutrient availability (Zhang et al., 2003ZHANG, Y.J. et al. Ethylene phosphorus availability has interacting yet distinct effects on root hair development. Journal Experimental Botany, v.54, n.391, p.2351-2361, 2003.). The inhibition of root elongation is one of the major responses of plants to excess metals and may occur faster than other physiological responses. Zinc toxicity symptoms are striking in the root system, mainly on root elongation and cell division (Broadley et al., 2007BROADLEY, M.R. et al. Zinc in plants. New Phytologist, v.173, n.4, p.677-702, 2007.). Sharifianpour et al. (2014)SHARIFIANPOUR, G. et al. Effect of different rates of zinc on root morphological traits among different upland rice Landraces in Malaysia. International Journal of Agriculture and Forestry, v.4, n.3, p.255-260, 2014. observed that among seven upland rice varieties, root parameters (length, average diameter, surface area, volume, and number of root tips) showed increased up 20 mg Zn L-1, but they decreased significantly under 30 mg Zn L-1 after four weeks. Liu et al. (2014)LIU, D. et al. Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environmental Science & Pollution Research, v.21, n.23, p.13615-24, 2014. observed that root morphological parameters in Moso bamboo were initially increased by low levels of Zn, but on the other hand under Zn stress of 400μM, the length, surface area and volume of roots decreased 51%, 24% and 57%, respectively.
Among the three accessions, only the GD showed a positive correlation between root, shoot and total dry mass with root length; however, this correlation was weak (ρ above 0.3) (Table 1). This result indicates that the production of biomass is partially dependent of the root length, since higher root length promotes higher uptake of water and nutrients. Li et al. (2005)LI, T. et al. Root responses and metal accumulation in two contrasting ecotypes of Sedum alfredii Hance under lead and zinc toxic stress. Journal of Environmental Science and Health, v.40, n.5, p.1081-1096, 2005. reported that length, surface area and volume of roots of the hyperaccumulator ecotype of Sedum alfredii increased with high levels of Zn, whereas in the non-hyperaccumulator these parameters were significantly decreased. It has been reported that under Zn excess, the DNA synthesis and mitotic activity of root tips may be inhibited, which subsequently results in inhibition of root growth (Jain et al., 2010JAIN, R. et al. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiologiae Plantarum, v.32, n.5, p.979-986, 2010.).
Coefficient of Pearson correlation (ρ) between the dry matter (DW) production of roots, shoot and whole plant, as well as the length, average diameter of roots and numbers of root tips of three accessions (JB, GD and BRA) of P glomerata grown in hydroponics with increasing levels of Zn.
The accession GD showed a moderate negative correlation for between root length and root diameter (ρ = -0.60) (Table 1). In addition, the BRA accession presented moderate positive correlation between shoot and total dry mass with root diameter (ρ above 0.6) (Table 1). There were no significant interaction among accessions and Zn levels for root diameter at 7 days (Figure 2G), for this parameter only the GD accession showed linear increase with increasing Zn levels. However, at 14 days, the accessions GD and JB showed a quadratic response, where the dose of minimum yield observed to root diameter was about 200µM Zn (Figure 2H). The increase in root diameter probably was a response related to decrease in cellular division in the apical portion of roots (Jain et al. 2010JAIN, R. et al. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiologiae Plantarum, v.32, n.5, p.979-986, 2010.). Sofo et al. (2013)SOFO, A. et al. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiologia Plantarum, v.149, n.4, p.287-298, 2013. observed that the Zn-induced changes in root morphology of Arabidopsis were caused by a hormonal unbalance, mainly governed by the auxin/cytokinin ratio. Therefore, it will be very interesting to check whether the differences in root morphology and partition of root and shoot biomass among P. glomerata accessions is linked to an auxin/cytokinin unbalance ratio.
It is desirable that plant species used for phytoremediation purposes show a higher rate of growth (Disante et al., 2010DISANTE, K.B. et al. Sensitivity to zinc of Mediterranean woody species important for restoration. Science of The Total Environment, v.408, n.10, p.2216-2225, 2010.). In the present study, although the accession GD of P. glomerata showed symptoms of Zn toxicity, this accession may be used in environments with high levels of Zn, because its growth rate is much higher when compared to both JB and BRA accessions. Moreover, according to the Zn responses, GD accession is the best candidate for field trials to test the value of herbaceous species to restore contaminated areas.
Kamada et al. (2009b)KAMADA, T. et al. Variação de caracteres morfológicos e fisiológicos de populações naturais de Pfaffia glomerata (Spreng.) Pedersen e correlação com a produção de β-ecdisona. Revista Brasileira de Plantas Medicinais, v.11, n.3, p.247-256, 2009b. observed higher divergence among individuals of P. glomerata from different populations and lower divergence among those from the same population in relation to β-ecdysone tissue-specific content. Soil abiotic factors have not been studied in relation to root morphological parameters in P. glomerata. The present work is the first to demonstrate the effect of increasing levels of Zn on the root morphological parameters of P. glomerata, which confirm that this species has high genetic diversity in natural populations (Kamada et al., 2009aKAMADA, T. et al. Diversidade genética de populações naturais de Pfaffia glomerata (Spreng.) Pedersen estimada por marcadores RAPD. Acta Scientiarum Agronomy. v.31, n.3, p.403-409, 2009a.), resulting in significant variability, which can promote different levels of tolerance to heavy metals.
CONCLUSIONS
There were differences in root morphology and dry matter production among the three accessions of P. glomerata plants grown under Zn excess. The accession GD showed increase in dry matter production, root volume, root surface area and root length at Zn levels below 70µM. Based on the evaluated parameters the following order of Zn tolerance in P. glomerata accessions was observed: GD> JB> BRA.
REFERENCES
- ALONSO-BLÁZQUEZ, N. et al. Influence of Zn-contaminated soils in the antioxidative defence system of wheat (Triticum aestivum) and maize (Zea mays) at different exposure times: potential use as biomarkers. Ecotoxicology, v.24, n.2, p.279-291, 2015.
- BROADLEY, M.R. et al. Zinc in plants. New Phytologist, v.173, n.4, p.677-702, 2007.
- CALGAROTO, N.S. et al. Zinc alleviates mercury-induced oxidative stress in Pfaffia glomerata (Spreng.) Pedersen. Biometals, v.24, n.5, p.959-971, 2011.
- DI BACCIO, D.R. et al. Responses of Populus deltoides x Populus nigra (Populus x euramericana) clone I-214 to high zinc concentrations. New Phytologist, v.159, n.2, p.443-452, 2003.
- DISANTE, K.B. et al. Sensitivity to zinc of Mediterranean woody species important for restoration. Science of The Total Environment, v.408, n.10, p.2216-2225, 2010.
- GUPTA, D.K. et al. Effect of Hg, As and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata Ecotoxicology, v.22, n.9, p.1403-1412, 2013.
- JAIN, R. et al. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiologiae Plantarum, v.32, n.5, p.979-986, 2010.
- KAMADA, T. et al. Diversidade genética de populações naturais de Pfaffia glomerata (Spreng.) Pedersen estimada por marcadores RAPD. Acta Scientiarum Agronomy v.31, n.3, p.403-409, 2009a.
- KAMADA, T. et al. Variação de caracteres morfológicos e fisiológicos de populações naturais de Pfaffia glomerata (Spreng.) Pedersen e correlação com a produção de β-ecdisona. Revista Brasileira de Plantas Medicinais, v.11, n.3, p.247-256, 2009b.
- LI, T. et al. Root responses and metal accumulation in two contrasting ecotypes of Sedum alfredii Hance under lead and zinc toxic stress. Journal of Environmental Science and Health, v.40, n.5, p.1081-1096, 2005.
- LI, T. et al. Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. Journal of hazardous materials, v.169, n.1-3, p.734-741, 2009.
- LI, X. et al. Zinc induced phytotoxicity mechanism involved in root growth of Triticum aestivum L. Ecotoxicology and Environmental Safety, v.86, p.198-203, 2012.
- LIN, C. et al. Zinc induces mitogen-activated protein kinase activation mediated by reactive oxygen species in rice roots. Plant Physiology and Biochemistry, v.43, n.10-11, p.963-968, 2005.
- LIU, D. et al. Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environmental Science & Pollution Research, v.21, n.23, p.13615-24, 2014.
- LUX, A. et al. Root responses to cadmium in the rhizosphere: a review. Journal Experimental Botany, v.62, n.1, p.21-37, 2011.
- MARQUES, M.C.; NASCIMENTO, C.W.A. Tolerância de mamona a zinco avaliada por fluorescência de clorofila e nutrição das plantas. Revista Brasileira de Ciência do Solo, v.38, n.3, p.850-857, 2014.
- MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassays with tabacco tissue cultures. Physiologia Plantarum, v.5, p.473-97, 1962.
- PALMGREN, M.G. et al. Zinc biofortification of cereals: problems and solutions. Trends in Plant Science, v.13, n.9, p.464-473, 2008.
- POURRUT, B. et al. Lead uptake, toxicity, and detoxification in plants. Journal environmental contamination and toxicology, v.213, p.113-136, 2011.
- RICACHENEVSKY, F.K. et al. Molecular control of Zn accumulation and biotechnological applications. Plant Science, v.236, p.1-17, 2015.
- REICHMAN, S.M. The responses of plants to metal toxicity: A review focusing on copper, manganese and zinc. Melbourne: Australian Minerals & Energy Environment Foundation Occasional Paper, n.14, p.1-54, 2002.
- RENGEL, Z. Ecotypes of Holcus lanatus tolerant to zinc toxicity also tolerate zinc deficiency. Annals of Botany, v.86, n.6, p.1119-1126, 2000.
- SHARIFIANPOUR, G. et al. Effect of different rates of zinc on root morphological traits among different upland rice Landraces in Malaysia. International Journal of Agriculture and Forestry, v.4, n.3, p.255-260, 2014.
- SOFO, A. et al. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiologia Plantarum, v.149, n.4, p.287-298, 2013.
- WUANA, R.A.; OKIEIMEN, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. International Scholarly Research Network Ecology, v.2011, p.1-20, 2011.
- ZHANG, Y.J. et al. Ethylene phosphorus availability has interacting yet distinct effects on root hair development. Journal Experimental Botany, v.54, n.391, p.2351-2361, 2003.
- ZHAO, F.J. et al. Arsenic uptake and metabolism in plants. New Phytologist, v.181, n.4, p.777-794, 2009.
Publication Dates
-
Publication in this collection
2016
History
-
Received
17 Nov 2015 -
Accepted
22 Aug 2016