Abstract
Mercuric iodide crystals in their platelet habit were grown by the polymer controlled vapor transport method. Mercuric iodide 99% in purity was sublimated at temperatures about 122 - 126 °C and vacuum conditions (10-5 mmHg), after selecting an appropriate polymer. Temperature profiles and experimental heat transfer models were determined for two growth furnaces using different insulator configurations for the cold extreme (air, ceramic wool, grilon, copper and ceramic wool). Growth conditions for few and separate nucleation points and large crystals were determined. Representative samples were characterized by optical microscopy and by measuring the current density and apparent resistivity of the material. Future optimization and comparisons with others mercuric iodide crystal growth methods are included.
mercuric iodide; HgI2; X-ray detectors
Optimization of Mercuric Iodide Platelets Growth by the Polymer Controlled Vapor Transport Method
L. Fornaro*, L. Mussio, M. Köncke, L. Luchini, E. Saucedo,
A. Rivoir, E. Quagliata
Facultad de Química, Cátedra de Radioquímica,
General Flores 2124, Montevideo, Uruguay
*e-mail: lfornaro@bilbo.edu.uy
Received: February 27, 1998; Revised: December 7, 1998
Mercuric iodide crystals in their platelet habit were grown by the polymer controlled vapor transport method. Mercuric iodide 99% in purity was sublimated at temperatures about 122 - 126 °C and vacuum conditions (10-5 mmHg), after selecting an appropriate polymer. Temperature profiles and experimental heat transfer models were determined for two growth furnaces using different insulator configurations for the cold extreme (air, ceramic wool, grilon, copper and ceramic wool). Growth conditions for few and separate nucleation points and large crystals were determined. Representative samples were characterized by optical microscopy and by measuring the current density and apparent resistivity of the material. Future optimization and comparisons with others mercuric iodide crystal growth methods are included.
Keywords: mercuric iodide, HgI2, X-ray detectors
1. Introduction
Due to its appropriate physical properties, mercuric iodide is one of the more suitable semiconductor materials for X-ray and Gamma ray detectors operating at room temperature and it is specially useful for low energy X-ray spectrometry1-9. As X-rays penetrate only about several microns in mercuric iodide crystals10, thin crystals of about hundreds of micrometers in thickness are needed for X-ray detection. Furthermore, the surface effects and treatment are extremely important for the fabrication of good mercuric iodide detector.
The most used method to produce mercuric iodide crystals for X-ray detection is growing large crystals by the physical vapor transport method2,11-17, and then cutting the crystals along the c axis and cleaving perpendicularly to the c axis mechanically, wire-sawn or by hand18. This process results in a mechanical damage of the crystal that usually can not be completely repaired by further treatments (polishing, etching).
Another way to obtain thin mercuric iodide crystals for X-ray detectors is growing them in their platelet habit and then no cleavage or surface polishing is needed to fabricate the detector. Attempts to grow mercuric iodide platelets have and are been made from solution19,20, but, until now, the crystals have exhibited worse quality for X-ray detectors than the vapor grown ones21,22,23. But mercuric iodide detector grade platelets have been grown too by the polymer controlled growth method, introduced by Faile et al.24 and that basically grows mercuric iodide platelets by sublimation of mercuric iodide powder at 230 °C in an evacuated quartz ampoule using polyethylene or styrene 1 wt% as additive. The growth of platelets without polymer addition (not detector grade material) and the decomposition, transport and role of the polymer were studied by Burger et al.25,26. Improvements to the method were done by Barton et al.27, repeating the vapor transport at 230 °C and varying the HgI2-to-polymer ratio and by Przyluski et al.28 by preheating the material at 275 °C, sublimating at 150-220 °C and studying the composition of the gas phase. Finally, Phillips et al.29 studied the possibility of using another additive, concluding in ultra-high purity hydrogen as appropriate and a growth temperature interval of @ 75 °C, although no data about the temperature of mercuric iodide source is mentioned.
From the previous references it can be deduced that all the experiences were performed at above 150 °C, over the aHgI2 - bHgI2 transition temperature. The presence of bHgI2 platelets in the ampoules has been mentioned several times24,27,28 and was confirmed by attempts made at our Laboratory, when a high yield of b material was observed sublimating at 230 °C. These yellow platelets (orthorhombic bHgI2) transform in red tetragonal aHgI2 platelets but the transition creates huge crystal defects.
Taken the above into account, this report studies the growth of aHgI2 platelets controlled by polymer but maintaining the source temperature bellow the phase a-b transition temperature to assure, theoretically, no transition occurs during the process. Within this framework, different temperature profiles and heat transport models for the furnaces of growing crystals were studied, looking for the optimum growth conditions. Finally, further developing and a comparison with the alternative methods for growing mercuric iodide platelets are considered.
2. Experimental
The growth experiments were performed in a pyrex glass ampoule, cleaned with acqua regia (25% HNO3, 75% HCl) during 12 h, rinsed 10 times with fresh distilled water and dried in a furnace for 10 days. The ampoules were 3.5 cm ID and 27 cm in length. Before filling the ampoule, it was outgased two hours at 10 -5 mmHg and 240 °C. Then, 25 g of mercuric iodide from Aldrich, 99% in purity, dried at 60 °C until constant mass and 1 wt% of polymer (small pellets) were charged inside the ampoule by a long stem funnel to avoid the powder spread that would produce nucleation points on the wall of the ampoule. Then the filled ampoule was evacuated at 10-2 mmHg, washed two times with high purity grade Ar (2 ppm O2, 2 ppm N2), evacuated at 10-5 mmHg while cooling with liquid nitrogen to prevent the mercuric iodide sublimation and finally sealed at this vacuum. The vacuum was measured by a Pirani TPR 010 and a cold cathode IKR 020 controlled by the module TPG 300 (Balzers). Special care was taken for selecting the polymer. Several studies were made looking for an appropriate one. The cerit poly CP-10 was finally selected, with low molecular weight (average: 6000) and a melting point 98 - 105 °C. The ampoule was inserted in a preheated horizontal and tubular furnace, maintaining the end with the material source at a temperature below 128 °C, the lowest temperature at which the a-b transition takes place25 and with variation ±2 °C. Two different furnaces were used: F1, heated by air forced convection (no direct radiation from a resistance) and F2, a conventional tubular furnace heated by a resistance. As in previous runs a crowded and thin growth zone was observed, efforts were made for enlarging this zone. Therefore, the growth was performed in the two furnaces using different insulator configurations: only air without insulator, ceramic wool, grilon (industrial plastic) and a copper tube out of the ampoule covered with ceramic wool. Temperature profiles of this configurations were measured by fixing very thin thermocouples on the outside wall of the ampoule along the insulator. As different sublimation rates were observed according the furnace type, growing times of 12 days for F1 and 6 days for F2 were used. After this time, the ampoule was left inside the furnaces until room temperature was achieved and then was opened first in a small point to avoid a strong air flush and finally cut.
The crystals were studied by optical microscopy using a Zeiss MC 63 A microscope, observing the (0 0 1) faces. In order to investigate the electrical properties of the best crystal obtained, 5% KI solution etching at room temperature, 2 min each face, was performed. Pd was deposited by thermal evaporation right after etching the crystal to minimize surface oxidation. The Pd deposition was performed in a high vacuum evaporator Denton Vacuum DV-502 system. Typical deposition conditions were 10-5 mmHg, deposition time 10 min, maintaining the distance Pd source - crystals at about 10 cm to have a maximum temperature of 82 °C in the crystal position. Immediately after Pd deposition, Pd leads with a diameter of 0.001 inch were attached using graphite suspension (aquadag from Achelson Inc.) A protective coating (Humiseal from Chase Corp.) was then applied to cover the device surfaces. The sample was then mounted on a Teflon holder and inserted in a closed testing box. Room temperature I-V measurements were carried out using a Keithley electrometer (Model 614) and an EG&G Ortec (Model 556) DC high voltage power supply.
3. Results and Discussion
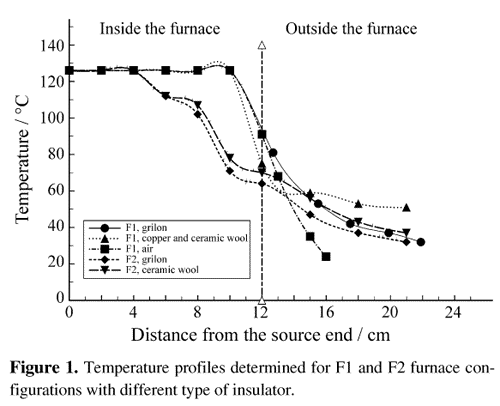
Figure 1 shows the temperature profiles determined for F1 and F2 furnace configurations with different type of insulator. As can be observed the profiles outside the furnace are very similar for the same insulator whatever the furnace and are very similar too for the several insulators used. Heat transfer models were developed for the configurations showing that the radiation from the furnace is the main mechanism of heat transfer outside for all the insulators.
From about twenty runs performed at 122 - 126 °C with F1 and F2 configurations, several results are coincident to all of them. The length of the zone where the crystals grew depends slightly on the insulator and therefore on the temperature profile, but the zone itself is always close to the furnace, in the hottest place. This result agrees with previous ones28 where the largest platelets grew in the vicinity of the bHgI2 platelets zone. This b zone doesn't exist in the present experiences; only a small quantity of b material appears as nucleation points (although the source material and all the ampoule temperature is always below the lowest phase transition temperature).
Therefore, no important difference on the effect of these three insulators can be appointed from the growth experiences. Nevertheless, as remarkably the nucleation points are more separated for the temperature profile with the lowest slope, for further developments a possible better insulation by using the copper tube covering the total ampoule, with insulator outside the furnace is suggested. This configuration would determine a temperature profile where the heat transfer model implies radiation from the furnace and conduction along the copper tube, with a very small slope in the crystal growth zone.
In the whole series of experiments the platelets grew perpendicular to the ampoule wall, in the radial direction. This fact disagrees with previous works24 where this result was obtained only for quartz ampoules and no for the glass ones, but agrees with another reference28. Related to purity and stoichemistry of the crystals, no special studies were carried out. No black residue was observed in the remaining source material and small quantities of Hg2I2 were detected by X-ray powder diffraction in the colder zone of nucleation points and in the coldest extreme of the ampoule, although no iodine vapor was observed. This result disagrees with previous ones25 where the Hg2I2 was only observed in experiences without polymer (polyethylene). To conclude about the process that determines the Hg2I2 presence more studies are needed, for example DSC (Differential Scanning Calorimetry) of starting material, a-platelets and no stoichemistry material.
Unfortunately, one of the main problems observed for the present method was the lack of reproducibility, because some results are not obtained systematically. Basically, the morphology of the crystals could be different although they were grown with identical experimental conditions. Nevertheless, there are some repetitive results: the time needed for sublimating a given quantity of starting material and the type of crystals obtained are different for the two furnace configurations. F1 needs double of time for equal mass sublimation and the crystals obtained are smaller, usually with rectangular (0 0 1) faces and with tendency to have polycrystal growth on the face opposite to the direction of HgI2 vapor flow. Instead of this, F2 gives some thin polycrystalline flakes and also platelets, sometimes with irregular shape and constant thickness and in other cases with perfect right angles but irregular thickness. Figure 2 shows these two last possibilities for platelets obtained in two independent runs with source material sublimating at 126 °C, 6 days of growing time in the F2 furnace configuration and with ceramic wool as insulator.
One possible explanation for the different results obtained for the platelet growth in the two types of furnace sublimating the source material at the same temperature can be tried. Due to the different heating mechanism of the furnaces, the heat transfer to the ampoule is also different. This fact determines different sublimation rates and possible different vapor transport inside the ampoule, important factors that determine the crystal growth mechanisms30. From the results obtained can be concluded that the F2 configuration is more appropriate for growing better mercuric iodide platelet by the method studied.
The crystal showed in Fig. 2a is one of the best we have obtained by this method. These kind of platelet exhibits worse morphology and surface properties although higher transparency that the ones grown from solution using the same starting mercuric iodide22.
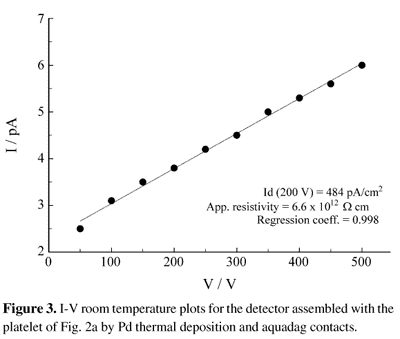
The platelet of Fig. 2a was used to measure the electrical properties of the material. Figure 3 shows the dark current curves for the detector assembled with this platelet. The detector reaches the dark current saturation value in few minutes, exhibits current densities of 764 pA/cm2 and 484 pA/cm2 at 500 V and 200 V respectively and an apparent resistivity of 6.6 x 1012W. cm in the 100 - 500 V range. References about the correspondent values for another platelets grown by this method could not been found, but the comparison of the results with the correspondent for other growth methods are summarized in Table 1. The current density and the apparent resistivity of the obtained platelet are worse than the correspondent to a crystal grown by vapor transport from a purest starting material as could be predicted, but are better than the correspondent to platelets grown from solution with the same starting material. Although no surface studies were performed, the linearity of the I-V plot indicates a very good contact attachment, which is an indirect measure of appropriate surface quality.
4. Conclusions
Several conclusions can be achieved from the results obtained. Mercuric iodide platelets can be grown by the polymer controlled vapor transport method sublimating at temperature bellow the aHgI2-bHgI2 phase transition temperature. Besides, a conventional furnace with an external insulator like ceramic wool determines an appropriate sublimation rate for the platelet growth. Although this method exhibits a considerable lack of reproducibility, the detector assembled with the obtained platelet has better electrical parameters (although worse morphology and surface properties) than the ones obtained by the alternative method of growing mercuric iodide platelets from solution with the same starting material. By now, the electrical parameters don't reach the correspondent values of bulk crystals grown by the vapor transport method starting from a high purity material, cut and cleaved.
Therefore, efforts must be made looking for increasing the reproducibility of the method and for improving the growth conditions using the F2 configuration and a purer starting material. Besides, carrier parameters studies and X-ray spectrometry tests would be appropriate for further comparisons.
Acknowledgments
The authors acknowledge the support of PEDECIBA (Project URU-84/002) and CONICYT (Project CE N° 1106) and Dr. Alba S. Leon for her fruitful suggestions and useful discussions.
- 1. Schieber, M. Nucl. Instr. and Meth., n. 144, p. 469, 1977.
- 2. Schieber, M.; Roth, M.; Schnepple, W.F. J. Cryst. Growth, n. 65, p. 353, 1983.
- 3. Merz, J.L.; Wu, Z.L. Nucl. Instr. and Meth., n. 213, p. 51, 1983.
- 4. Nicolau, Y.F. Nucl. Instr. and Meth., n. 213, p. 13, 1983.
- 5. Beyerle, A.; Hull, K.; Markakis, J.; Lopez, B. Nucl. Instr. and Meth., n. 213, p. 107, 1983.
- 6. Burger, A.; Nason, D. J. Appl. Phys., n. 71, p. 2717, 1992.
- 7. Iwanczyk, J.S. Nucl. Instr. and Meth. A, n. 322, p. 543, 1992.
- 8. Iwanczyk, J.S.; Patt, B.E.; Wang, Y.J.; Khusainov, A. Kh. Nucl. Instr. and Meth. A, n. 380, p. 186, 1996.
- 9. Ponpon, J.P.; Sieskind, M. Nucl. Instr. and Meth. A, n. 380, p. 173, 1996.
- 10. Levi, A.; Burger, A.; Nissenbaum, J.; Schieber, M. Nucl. Instr. and Meth., n. 213, p. 35, 1983.
- 11. Schieber, M.; Carlston, R.C.; Lamonds, H.A.; Randtke, P.T.; Shnepple, F.W.; Llacer, J. J. Cryst. Growth, n. 24/25 p. 205, 1974.
- 12. Lamonds, H.A. Nucl. Instr. and Meth., n. 213, p. 5, 1983.
- 13. Berg, L. Van den; Schnepple, W.F. Nucl. Instr. and Meth. A, n. 283, p. 335, 1989.
- 14. Piechotka, M.; Zha, M.; Kaldis, E. J. Cryst. Growth, n. 113, p. 251, 1991.
- 15. Zha, M.; Piechotka, M.; Kaldis, E. J. Cryst. Growth, n. 115, p. 43, 1991.
- 16. Hermon, H.; Roth, M.; Schieber, M. Nucl. Instr. and Meth., n. 322, p. 432, 1992.
- 17. Cadoret, R. J. Cryst. Growth, n. 146, p. 9, 1995.
- 18. Levi, A.; Burger, A.; Schieber, M.; Berg, L. van den; Yelon, W.B.; Alkire, R.W. Nucl. Instr. and Meth., n. 213, p. 31 1983.
- 19. Nicolau, Y.F. J. Cryst. Growth, n. 48, p. 61, 1980.
- 20. Fornaro, L.; Luchini, L.; Köncke, M.; Mussio, L.; Quagliata, E.; Chattopadhyay, K.; Burger, A. Send to Journal of Crystal Growth, Israel, July 1998.
- 21. Burger, A.; Nason, D.; Berg, L. van den; Schieber, M. Semiconductors for Room Temperature Nuclear Detector Applications, Semiconductors and Semimetals, v. 43, Schlesinger, T.E.; James, R.B., eds., Academic Press, Inc., San Diego, CA, USA, p. 98, 1995.
- 22. Fornaro, L.; Chen, H.; Chattopadhyay, K.; Chen, K-T.; Burger, A. Mat. Res. Soc. Symp. Proc., n. 487, p. 339, 1998
- 23. Fornaro, L.; Mussio, L.; Quagliata, E.; Luchini, L.; Köncke, M.; Chen, H.; Chattopadhyay, K.; Burger, A. Send to Nuclear Instuments and Methods in Physics Research
- 24. Faile, P.; Dabrowski, A.J.; Huth, G.C.; Iwanczyk, J.S. J. Cryst. Growth, n. 50, p. 752, 1980.
- 25. Burger, A.; Roth, M.; Schieber, M. J. Cryst. Growth, n. 56, p. 526, 1982.
- 26. Burger, A.; Levi, A.; Nissenbaum, J.; Roth, M.; Schieber, M. J. Cryst. Growth, n. 72, p. 643, 1985.
- 27. Barton, J.B.; Dabrowski, A.J.; Iwanczyk, J.S.; Kusmiss, J.H.; Ricker, G.; Vallerga, J.; Warren, A.; Squillante, M.R.; Lis, S.; Entine, G. Ad. in X-ray Analysis, n. 25, p. 31 1982.
- 28. Przyluski, J.; Laskowski, J. Nucl. Instr. and Meth. A, n. 283, p. 144, 1989.
- 29. Phillips, J.D.; Lund, M.W.; Kevin, J.; Allred, W.P. Nucl. Instr. and Meth. A, n. 380, p. 50, 1996.
- 30. Chernov, A.A.; Kaldis, E.; Piechotka, M.; Zha, M. J. Cryst. Growth, n. 125, p. 627, 1992.
Publication Dates
-
Publication in this collection
21 Jan 2000 -
Date of issue
Apr 1999
History
-
Reviewed
07 Dec 1998 -
Received
27 Feb 1998