Abstract
Tissue engineering has evolved from the use of biomaterials for bone substitution that fulfill the clinical demands of biocompatibility, biodegradability, non-immunogeneity, structural strength and porosity. Porous scaffolds have been developed in many forms and materials, but few reached the need of adequate physical, biological and mechanical properties. In the present paper we report the preparation of hybrid porous polyvinyl alcohol (PVA)/bioactive glass through the sol-gel route, using partially and fully hydrolyzed polyvinyl alcohol, and perform structural characterization. Hybrids containing PVA and bioactive glass with composition 58SiO2-33CaO-9P2O5 were synthesized by foaming a mixture of polymer solution and bioactive glass sol-gel precursor solution. Sol-gel solution was prepared from mixing tetraethoxysilane (TEOS), triethylphosphate (TEP), and calcium chloride as chemical precursors. The hybrid composites obtained after aging and drying at low temperature were chemically and morphologically characterized through infrared spectroscopy and scanning electron microscopy. The degree of hydrolysis of PVA, concentration of PVA solution and different PVA-bioglass composition ratios affect the synthesis procedure. Synthesis parameters must be very well combined in order to allow foaming and gelation. The hybrid scaffolds obtained exhibited macroporous structure with pore size varying from 50 to 600 µm.
biomaterial; bioceramics; polymer; tissue engineering; hybrids; sol-gel; bioglass
Preparation of hybrid biomaterials for bone tissue engineering
Vilma Conceição Costa; Hermes Souza Costa; Wander Luiz Vasconcelos; Marivalda de Magalhães Pereira; Rodrigo Lambert Oréfice; Herman Sander Mansur* * e-mail: hmansur@demet.ufmg.br
Departamento de Engenharia Metalúrgica e Materiais, Universidade Federal de Minas Gerais UFMG, Rua Espírito Santo, 35, s/206, 30160-030 Belo Horizonte - MG, Brazil
ABSTRACT
Tissue engineering has evolved from the use of biomaterials for bone substitution that fulfill the clinical demands of biocompatibility, biodegradability, non-immunogeneity, structural strength and porosity. Porous scaffolds have been developed in many forms and materials, but few reached the need of adequate physical, biological and mechanical properties. In the present paper we report the preparation of hybrid porous polyvinyl alcohol (PVA)/bioactive glass through the sol-gel route, using partially and fully hydrolyzed polyvinyl alcohol, and perform structural characterization. Hybrids containing PVA and bioactive glass with composition 58SiO2-33CaO-9P2O5 were synthesized by foaming a mixture of polymer solution and bioactive glass sol-gel precursor solution. Sol-gel solution was prepared from mixing tetraethoxysilane (TEOS), triethylphosphate (TEP), and calcium chloride as chemical precursors. The hybrid composites obtained after aging and drying at low temperature were chemically and morphologically characterized through infrared spectroscopy and scanning electron microscopy. The degree of hydrolysis of PVA, concentration of PVA solution and different PVA-bioglass composition ratios affect the synthesis procedure. Synthesis parameters must be very well combined in order to allow foaming and gelation. The hybrid scaffolds obtained exhibited macroporous structure with pore size varying from 50 to 600 µm.
Keywords: biomaterial, bioceramics, polymer, tissue engineering, hybrids, sol-gel, bioglass
1. Introduction
Tissue engineering represents a new field that aims to grow complex, three-dimensional tissues or organs to replace damaged tissues1. For this purpose, investigation of novel biomaterials for bone engineering represents an essential area for planning tissue engineering approaches. Next generation biomaterials should combine bioactive and bioresorbable properties to activate in vivo mechanisms of tissue regeneration, stimulating the body to heal itself and to facilitate replacement of the scaffold by the regenerating tissue. A variety of biomaterials, including synthetic polymers2-4, ceramics5 and natural polymers6 are being used to fabricate synthetic scaffold that acts as guide and stimulus for the three-dimensional tissue growth7,8. Controlled porous architecture, high porosity, adequate pore size and interconnectivity, of these scaffolds is necessary to facilitate cell seeding and diffusion throughout the whole structure of both cells and nutrients. The minimum pore diameter required for bone ingrowth and angiogenesis into a scaffold is considered to be 100 µm9. Previous in vitro work has suggested that the ideal pore diameter for bone ingrowth is between 300-400 µm10. The material must exhibit good biocompatibility meaning that the material must not demonstrate severe immunogenicity or cytotoxicity. The ability to control scaffold degradation and mechanical integrity is also a requirement to be fulfilled in the development of optimal scaffolds. Ideally, scaffolds should closely match the properties of the tissue it is to replace.
Either biodegradable polymer or ceramic scaffolds, commonly being considered for bone tissue engineering, lack adequate mechanical properties. Synthetic bioresorbable polymers are easily fabricated into complex structures, yet they are too weak to meet the demands of surgery and the in vivo physiological environment. Conversely, ceramic scaffolds have low toughness and strength. Efforts have been made in developing composite of polymers and ceramics with the aim to increase the mechanical stability and improve tissue interaction11. The material composition might give favorable mechanical properties, strength via ceramic phase, toughness and plasticity via polymer phase. Thus, hybrid materials derived from the integration of biodegradable polymers with bioactive inorganic material might be promising on scaffolds development. The bioactive ceramic phase may be a calcium phosphate ceramic, a glass-ceramic or a bioactive glass. Bioactive glasses are able to form a bond to living bone, which has been attributed to the formation of hydroxyl carbonate apatite layer under physiological conditions12. However bioactive glass is a stiff and brittle material, difficult to form into complex shapes and prone to catastrophic fracture under loads.
The sol-gel method has been employed for the synthesis of hybrid organic-inorganic composite materials13,14. Sol-gel derived bioactive glass foams were also successfully obtained15,16. In vitro cell studies in the presence of these foams have shown an increase in osteoblast proliferation and collagen production17 as well as the stimulation of the formation and mineralisation of bone nodules18 reinforcing their potential. However, their compressive strength is in the range of 0.3 to 2.5 MPa, depending on sintering temperature and final pore structure19, a value lower than even trabecular bone. Nevertheless, it is in the range obtained for several scaffolds for tissue engineering reported in the literature20. However the toughness and tensile strength of the foams are lower than those of bone. It is expected that the preparation of polymer bioactive glass hybrid foams may lead to scaffolds with better mechanical behavior compared to pure bioactive glass foams. It should be emphasized however that these materials are expected to be used in sites free of dynamic load, or with controlled load during tissue regeneration.
Polyvinyl alcohol, PVA, is a water soluble synthetic resin which is obtained through polymerization of vinyl acetate monomer. By hydrolysis, the acetate groups are converted in hydroxyl groups. The degree of hydrolysis in a polyvinyl alcohol reagent is controlled by this process. The polar nature of poly (vinyl alcohol) facilitates the formation of hydrogen bonds and eventual condensation with silanol groups (from developing polysilicate network) formed by hydrolysis of the silicon alkoxides21. Moreover PVA has been proposed for controlled release systems and is employed in a variety of biomedical applications, generally being considered to be biocompatible22,23. Although not a biodegradable polymer itself, when used with molecular weight less than around 10.000 g/mol and associated with a biodegradable sol-gel derived bioactive glass, PVA molecules are expected to be eliminated by the body.
Synthetic bioactive and bioresorbable composite materials are becoming increasingly important as scaffolds for tissue engineering. Bioactive glass reacts with physiological fluids to form tenacious bonds to hard (and in some cases soft) tissue. Composites of tailored physical, biological and mechanical properties as well as predictable degradation behavior can be produced combining bioresorbable polymers and bioactive inorganic phases. Both PVA, as well as bioactive glasses, are being currently used as biomaterials, candidates for tissue engineering applications24,25. In previous work of our group25,26 the synthesis of PVA/silica hybrids obtained via sol-gel method was reported. The hybrids were prepared with low polymer contents (20-40 wt. (%)).
In this work, we present results concerning the sol-gel synthesis of polyvinyl alcohol-bioactive glass hybrid foams, with polymer contents from 60 to 80 wt. (%), and different types of PVA. The purpose of this study was to investigate the effects of some important synthesis parameters such as degree of hydrolysis of PVA, concentration of PVA solution and higher PVA content on the final properties of composites. As far as we know, this is the first research in this technology field to address the influence of PVA chemical properties such as degree of hydrolysis on the fabrication of bioactive glass-polymer (PVA) hybrid tri-dimensional scaffolds.
2. Experimental
2.1. Hybrids preparation
Sol-gel derived bioactive glass/polyvinyl alcohol hybrid foams were prepared using a procedure similar to the one used by Pereira et al.25,26.
2.1.1. Preparation of PVA solution
The Polyvinyl alcohol (PVA) selected for use were: from Celanese Chemicals, Celvol 103, degree of hydrolysis 98.0-98.8%, molecular weight range: 13,000-23,000 g/mol and from Aldrich-Sigma, degree of hydrolysis 80%, molecular weight: 9,000-10,000 g/mol. PVA aqueous solutions were prepared at 23, 28 and 35 wt. (%) by dissolving the PVA powder in a water bath at 80 °C, under constant stirring, for 2 hours. The pH of the solution was adjusted to 2.0 by hydrochloric acid solution 2 N.
2.1.2. Preparation of the starting bioglass solution
The starting sol solution with composition 58 wt. (%) SiO2- 33 wt. (%) CaO- 9 wt. (%) P2O5 was synthesized from mixing tetraethoxysilane (TEOS), D.I. water, triethylphosphate (TEP), and calcium chloride in presence of hydrochloric acid solution 2 N. The H2O/TEOS molar ratio used was 12.
2.1.3. Preparation of the PVA-Bioactive glass hybrid foams
The hybrid compositions prepared were 20, 30, 40 wt. (%) glass and 80, 70, 60 wt. (%) polymer. The hybrids were obtained using a procedure similar to the one described by in our previous work25,26. An appropriate amount of the starting sol was added to the PVA solution. To this resulting solution were added the surfactant, sodium laureate sulfate (LESS), and HF 10%v/v solution. The mixture was foamed by vigorous agitation. Hydrofluoric acid was used to catalyze the gelation. The foams were cast just before gelation in plastic containers and sealed. The samples were, basically, aged at 40 °C for 3 days and then dried at 40 °C for 7 days.
2.2. Hybrids characterization
FTIR was used to characterize the presence of chemical groups. The FTIR absorption spectra were obtained within the range between 4000 and 400 cm-1 (Perkin Elmer, Paragon 1000), using the KBr pellet method. For the PVA sample was prepared a thin film and used the transmission spectroscopy. Porous morphologies of the hybrids were examined with a scanning electron microscope (JEOL, JSM-6360L) after carbon coating. The examination was carried out at accelerating voltage of 15 kV. To expose the internal architecture, the samples were cut with a razor blade.
3. Results and Discussion
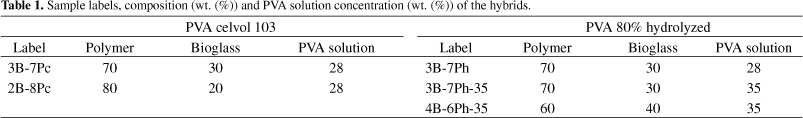
Table 1 presents the labels, composition (wt. (%)) of the hybrids and PVA solution concentration (wt %) used to prepare the hybrids.
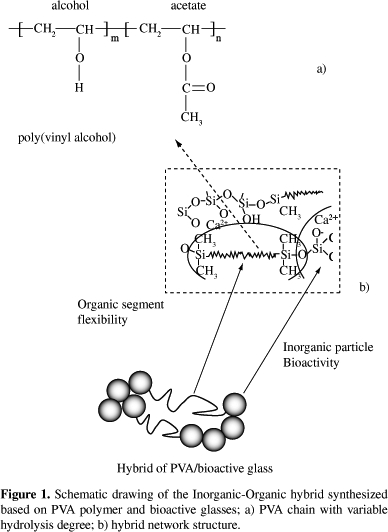
Hybrid PVA/bioglass foams were obtained for polymer contents of 60, 70 and 80 wt. (%). The use of two degree of hydrolysis of PVA changed the solution behavior. For PVA, fully hydrolyzed (Celvol 103), the concentrations of the starting PVA solution that proved to be adequate to allow the production and gelation of the foam were 23 and 28 wt. (%). Using 23 wt. (%), the resulting final foams have less residual water and the drying procedure is more efficient, compared to lower concentration PVA solutions used in previous work. Comparing with the 28 wt. (%) concentration, the amount of hydrofluoric acid necessary to gel the foam was higher. Reaching gelation can be better controlled when working with PVA fully hydrolyzed, Celvol 103, since its solution has higher viscosity and the higher predominance of hydroxyl groups may contribute to crosslink with the polysiloxane network, through hydrogen bonds and condensation with silanol groups. The chemical crosslinking between the organic and inorganic network is illustrated in Figure 1, which shows a schematic representation of the hybrid network produced based on polyvinyl alcohol.
For PVA, 80% hydrolyzed, the starting PVA concentration of 23 wt. (%) did not work, even for higher HF concentration used foams could not be stabilized, since the gel-sol transition could not be reached using the procedure adopted. At 28 wt. (%), depending of the hybrid composition, it was possible obtain the hybrid foams. For hybrid composition of PVA higher than 70 wt. (%), the control of the foam casting could not be done, since the gel-sol transition is a very slow process in the conditions used. PVA, 80% hydrolyzed, presents higher amount of acetate groups left in the backbone, providing less reaction sites for reaction with silanol groups formed during hydrolysis of silicon precursors (Figure 1). The presence of acetate groups in partially hydrolyzed polymers weakens the hydrogen bonding. This suggests that the mechanism involving interaction between the hydroxyl groups of PVA and hydroxyl groups of silanol, from hydrolyzed silica, is delayed. Chemical crosslinking, as illustrates Figure 1, is a versatile method to modify and improve properties in materials. In this way, there are several advantages on using organic-inorganic hybrid, because one may be able to tailor physical, biological and mechanical properties as well as predictable degradation behavior where novel materials can be produced combining bioresorbable polymers and bioactive inorganic phases.
3.1. FTIR Characterization
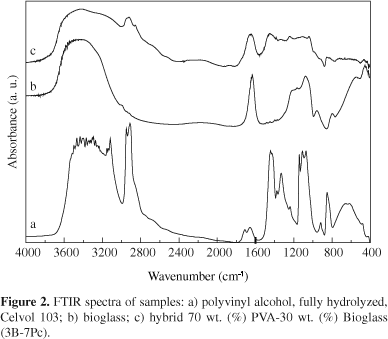
Representative IR spectra of the pure PVA films, the bioglass and the hybrid foams are shown in Figure 2, for the sample group of PVA fully hydrolyzed, and in Figure 3 for the PVA 80% hydrolyzed sample group. Although not shown in the figures, the spectra of the other hybrids obtained in this work are very similar to the spectra exhibited in Figures 2c and 3c. No relevant differences were found by using the two PVA precursors neither for varying the PVA solution and composition of the hybrids.
In Figures 2a and 3a, FTIR spectra of pure PVA are shown. For both PVA spectra, the broad band observed from 3100 to 3600 cm-1 may be assigned to O-H stretching due the strong hydrogen bond of intramolecular and intermolecular type27. The presence of a higher hydroxyl content in the fully hydrolyzed PVA compared to the PVA 80% hydrolyzed can be seen from the broader band around 3300 cm 1. The C-H alkyl stretching band21 can be observed at 2850-2950 cm-1. The absorption peaks at approximately 1710 cm-1 (for fully hydrolyzed PVA) or 1740 cm-1 (for 80% hydrolyzed) and at 1090-1150 cm-1 may be attributed to the stretching vibration of C = O and C-O of the remaining vinyl acetate non-hydrolyzed group of PVA polymer21. The absorption band around 1700 cm-1 arises due to the carbonyl band (C=O) of the acetate group found in partially hydrolyzed PVA polymer and has higher relative intensity for the PVA 80% hydrolyzed as expected from the higher concentration of remaining acetate groups.
In the FTIR spectra of the bioglass presented in both figures, the vibrational modes due to Si-O-Si asymmetric stretching are observed at approximately 1070 cm-1 and 1200 cm-1. Additional peaks are seen at 790 cm-1 (for symmetric Si-O-Si stretching vibration, and at 450 cm1 (for Si-O-Si bending modes)28. A typical absorption band observed in silica gel is located at 1640 cm-1 and is attributed to the deformation mode of adsorbed molecular water in the pores. The band at 950 cm-1 has been ascribed to the stretching vibration of silanol groups in pure silica (vibrational modes of Si-O- stretching)28.
FTIR spectra of hybrid in the composition 70 wt. (%) PVA30 wt. (%) bioglass are presented in Figures 2c and 3c, for Celvol and 80% hydrolyzed, respectively. The bioglass component is identified by major vibration bands, (Si-O-Si, at 1080 and 450 cm1). For both hybrids, the peak at 950 cm-1 associated with the Si-OH vibrational mode remains as a shoulder. In the frequency range of 3000-3650 cm1, a broader band was noted, especially for hybrid prepared from PVA fully hydrolyzed (Figure 2c). The band located at 1640 cm-1 remains in the spectra of the hybrids since the low heating temperature is not enough to remove the molecular water of the pores. The terminal vinyl groups of the PVA which appear at 1710 cm-1 (for fully hydrolyzed PVA) or 1740 cm-1 (for 80% hydrolyzed) are not shown in the hybrids, which suggests that these bonds might be involved in crosslinking with silica. In the range 1500-900 cm-1 there is a superposition of the bands derived from the bioglass and the PVA components.
3.2. Scanning electron microscopy
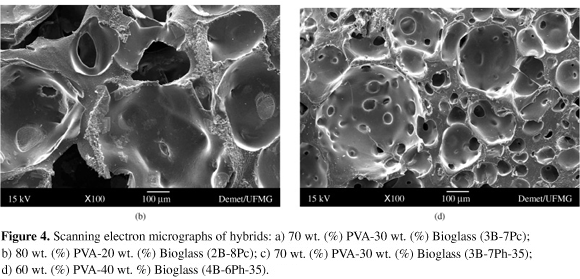
The pore morphology and distribution of the hybrid foams can be seen by the SEM micrographs presented in Figure 4a-d. Figure 4a shows SEM micrograph of hybrid 70 wt. (%) PVA-30 wt. (%) Bioglass (3B-7Pc), Figure 4b shows SEM micrograph of hybrid 80 wt. (%) PVA-20 wt. (%) Bioglass (2B-8Pc), Figure 4c shows SEM micrograph of hybrid 70 wt. (%) PVA-30 wt. (%) Bioglass (3B-7Ph 35), and Figure 4d shows the micrograph of 60 wt. (%) PVA-40 wt. (%) Bioglass (4B-6Ph-35). Very distinct features can be observed in the different hybrids produced. The observed morphologies of the foams vary considering the processing variables. The porosity and pore sizes are strongly dependent on the hybrid composition, concentration of the polymer solution used and gelation time. The control of the gelation time is very important to establish an adequate morphology of the hybrids produced by this method. For composition of 70 wt. (%) PVA, for both PVA polymers used (Figure 4a, c), the gelation time was found to be approximately 13 minutes and this is a proper time to gel the three-dimensional hybrids structure and stabilize the bubbles formed during the process. These hybrids exhibited macroporous structure with interconnected open pores and pore size varied from 50 to 350 mm. For the composition of 60 wt. (%) PVA (Figure 4d), the gelation time was shorter and the time at which the foam was cast, the gelation point almost reached, affecting the final porous structure.
For this hybrid, the distribution of pores is more homogeneous and pore size ranged from 50 to 90 mm and reaching 450 mm for the bubble-like pores. For the composition of 80 wt. (%) PVA, Figure 4b, for both PVA polymers used, the gelation time was delayed and the pore morphology was semi-spherical. Hybrid showed in Figure 4b has the largest pores (pore size from about 160 to 500 mm) and hybrid in showed Figure 4d has the smallest.
The number of possible combinations of bioactive glasses with biocompatible polymers such as PVA is almost countless. That means each and every proportion of inorganic phase CaO, SiO2 and P2O5, not to mention the synthesis parameters themselves, will result on a different hybrid system with some specific properties and bioactive behavior. Therefore, synthetic bioactive and bioresorbable composite materials are becoming increasingly important as scaffolds for tissue engineering. Next generation biomaterials must combine bioactive and bioresorbable properties to activate in vivo mechanisms of tissue regeneration, stimulating the body to heal itself and to facilitate replacement of the scaffold by the regenerating tissue. Bioactive glass reacts with physiological fluids to form tenacious bonds to hard (and in some cases soft) tissue. However bioactive glass is a stiff and brittle material, difficult to form into complex shapes and prone to catastrophic fracture under loads. Conversely, synthetic bioresorbable polymers are easily fabricated into complex structures, yet they are too weak to meet the demands of surgery and the in vivo physiological environment. Composites of tailored physical, biological and mechanical properties as well as predictable degradation behavior can be produced combining bioresorbable polymers and bioactive inorganic phases.
4. Conclusions
Hybrid Polyvinyl alcohol/bioglass foams have been prepared by the sol-gel method, with a high content of polymer (60 to 80%). The synthesis parameters need to be very well combined in order to allow the control of foaming and gelation. Pore characteristics of the obtained scaffolds have been related to hybrid composition, concentration of the polymer solution used, and gelation time. The macropore diameter ranged from 50 to 600 mm.
The results suggest that there is scope for designing materials with controlled compositions and morphologies. Such system has a potential to be used in bone tissue engineering, but further studies must be conducted.
Acknowledgments
The authors acknowledge CNPq/FAPEMIG/CAPES for financial support on this project. We are also grateful to Prof. Dr. Dagoberto B. Santos for the facilities of Electron Microscopy Laboratory.
Received: August 16, 2006; Revised: December 19, 2006
Article presented at the IV Congresso Latino Americano de Órgãos Artificiais e Biomateriais (COLAOB 2006), August 8 and 11, 2006, Caxambu, MG, Brazil
- 1. Stock UA, Vacanti JP. Tissue Engineering: Current State and Prospects. Annu. Rev. Med. 2001; 52:443-451.
- 2. Ignjatovic N, Tomic S, Dakic M, Miljkovic M, Plavsic M, Uskokovic D. Synthesis and properties of hydroxyapatite/poly-L-lactide composite biomaterials. Biomaterials 1999; 20(9):809-816.
- 3. Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Fabrication of Biodegradable Polymer Scaffolds to Engineer Trabecular Bone. Journal Biomater. Science Polym Ed. 1995; 7(1):23-28.
- 4. Wang K, Thomas CH, Healy KE, Nuber, G. A Novel Method to Fabricate Bioabsorbable Scaffolds. Polymer 1995; 36(4):837-842.
- 5. Herath HMTU, DiSilvio L, Evans JR. G. Porous Hydroxyapatite Ceramics for Tissue Engineering. Journal of Applied Biomaterials & Biomechanics 2005; 3(3):192-198.
- 6. Liu LS, Thompson AY, Heidaran MA, Poser JW, Spiro RC. An Osteoconductive collagen/Hyaluronate Matrix for Bone Regeneration. Biomaterials 1999; 20(12):1097-1108.
- 7. Hutmacher DW. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000; 21(24):2529-2543.
- 8. Hench LL. Bioactive Materials: The potential for Tissue Regeneration. Journal of Biomedical Materials Research 1998; 41(4):511-518.
- 9. Karageorgiou V, Kaplan D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005; 26(27):5474-5491
- 10. Tsuruga E, Tahita H, Itoh H, Wakisaka Y, Kuboki Y. Pore Size of Porous Hydroxyapatite as the Cell-Substratum Controls BMP-Induced Osteogenesis. J. Biochem 1997; 121(2):317-324.
- 11. Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Indirect Solid Free Form Fabrication of Local and Global Porous, Biomimetic and Composite 3D Polymer-Ceramic Scaffolds. Biomaterials 2003; 24(1):181-194.
- 12. Hench LL, LaTorre GP. The Reaction Kinetics of Bioactive Ceramics: Part IV. Effect of Glass and Solution Composition. Bioceramics 1992; 5:67-74.
- 13. Li H, Du R, Chang, J. Fabrication, Characterization and In Vitro Degradation of Composite Scaffolds Based on PHBV and Bioactive Glass. Journal of Biomaterials Applications 2005; 20(2):137-155.
- 14. Martín AI, Salinas AJ, Vallet-Regí M. Bioactive and Degradable Organic-Inorganic Hybrids. Journal of European Ceramic Society 2005; 25(16):3533-3538.
- 15. Coelho MB, Pereira MM. Sol-Gel Synthesis of Bioactive Glass Scaffolds for Tissue Engineering: Effect of Surfactant Type and Concentration. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2005; 75(2):451-456.
- 16. Sepulveda P, Jones JR, Hench LL. Bioactive Sol-gel Foams for Tissue Repair. J. Biomed. Mater. Res 2002; 59(2):340-348.
- 17. Valerio P, Guimarães MHR., Pereira MM, Leite MF, Goes AM. Primary Osteoblast Cell Response to Sol-Gel Derived Bioactive Glass Foams. J. Mater. Sci: Mater in Med 2005; 16(9):851-856.
- 18. Gough JE, Jones JR, Hench LL. Nodule Formation and Mineralization of Human Primary Osteoblasts Cultured on a Porous Bioactive Glass Scaffold. Biomaterials 2005; 25(11):2039-2046.
- 19. Jones JR, Ehrenfried LM, Hench LL. Optimizing the strength of macroporous bioactive glass scaffolds. Key Eng. Mater 2004; 254256:981-984.
- 20. Ramay HR, Zhang M. Preparation of porous hydroxyapatite scaffolds by combination of the gel-casting and polymer sponge methods. Biomaterials. 2003; 24(19):3293-3302.
- 21. Mansur HS, Oréfice RL, Mansur AAP. Characterization of Poly(vinyl alcohol)/Poly(ethylene glycol) Hydrogels and PVA-Derived Hybrids by Small-Angle Scattering and FTIR Spectroscopy. Polymer 2004; 45(21):7193-7202.
- 22. Soppimath KS, Kulkarni AR, Aminabhavi M. Controlled release of antihypertensive drug from the interpenetrating network poly(vinyl alcohol)guar gum hydrogel microspheres. J. Biomater. Sci. Polym 2000; 11(1):27-43.
- 23. Kelly, CM, DeMerlis, CC, Schoneker, DR, Borzelleca, JF. Subchronic toxicity study in rats and genotoxicity tests with polyvinyl alcohol. Food and Chemical Toxicology 2003; 41(5):719-727.
- 24. Dolui SK, Kotoky T. Synthesis and Characterisation of Polyvinyl alcohol (PVA)/Silica Hybrid Composites Derived Through the Sol-Gel Method in Aqueous Medium: Effect of Acid Content, Silica Content and Viscosity of PVA on the Dispersion Characteristics of Silica and the Physical Properties of the Composite. Journal of Sol-Gel Science and Technology 2004; 29(2):107-117.
- 25. Pereira MM, Jones JR, Orefice RL, Hench, LL. Preparation of Bioactive Glass-Polyvinyl Alcohol Hybrids Foams by the Sol-Gel Method. Journal of Materials Science: Materials in Medicine 2005; 16(11):1045-1050.
- 26. Pereira MM, Jones JR, Hench, LL. Bioactive glass and Hybrid Scaffolds Prepared by Sol-Gel Method for Bone Tissue Engineering. Advances in Applied Ceramics, 2005; 104(1):35-42.
- 27. Peppas N. A. Tear propagation resistance of semicrystalline polymeric networks. Polymer 1977; 18(2):403-407.
- 28. Almeida RM, Pantano CG. Structural Investigation of Silica Gel Films by Infrared Spectroscopy. Journal of Applied Physics 1990; 68(8):4225-4232.
Publication Dates
-
Publication in this collection
03 May 2007 -
Date of issue
Mar 2007
History
-
Accepted
19 Dec 2006 -
Reviewed
19 Dec 2006 -
Received
16 Aug 2006