Abstract
A composite of natural rubber (NR) with polyaniline (PANI) was obtained by mixing an aqueous dispersion of dodecylbenzenesulfonic acid (DBSA)-doped PANI with NR latex in different concentrations. Films were obtained by the casting method and characterized by ultraviolet visible near-infrared (UV-Vis-NIR) spectroscopy, thermogravimetry/differential thermogravimetry (TG/DTG), stress-strain testing, differential scanning calorimetry (DSC), and DC electrical conductivity measurements. The UV-vis-NIR spectrum showed that PANI remained doped in the composite, and this improved the mechanical and electrical proprieties of NR films and afforded them good thermal stability up to ~200ºC. The percolation threshold did not follow the universal critical exponent, and in this case, conduction preferentially occurs by hopping and tunneling.
natural rubber; polyaniline; electrical conductivity; percolation threshold
Electrical, mechanical, and thermal analysis of natural rubber/polyaniline-Dbsa composite
Michael Jonas da Silva; Aléx Otávio Sanches; Luiz Francisco Malmonge; José Antonio Malmonge* * e-mail: mal@dfq.feis.unesp.br
Departamento de Física e Química, Faculdade de Engenharia, Univ Estadual Paulista - UNESP, Ilha Solteira, SP, Brazil
ABSTRACT
A composite of natural rubber (NR) with polyaniline (PANI) was obtained by mixing an aqueous dispersion of dodecylbenzenesulfonic acid (DBSA)-doped PANI with NR latex in different concentrations. Films were obtained by the casting method and characterized by ultraviolet visible near-infrared (UV-Vis-NIR) spectroscopy, thermogravimetry/differential thermogravimetry (TG/DTG), stress-strain testing, differential scanning calorimetry (DSC), and DC electrical conductivity measurements. The UV-vis-NIR spectrum showed that PANI remained doped in the composite, and this improved the mechanical and electrical proprieties of NR films and afforded them good thermal stability up to ~200ºC. The percolation threshold did not follow the universal critical exponent, and in this case, conduction preferentially occurs by hopping and tunneling.
Keywords: natural rubber, polyaniline, electrical conductivity, percolation threshold
1. Introduction
Among currently available intrinsically conducting polymers (ICP), polyaniline (PANI) has emerged as one of the most promising ones for technological applications owing to its good electrical properties, environmental stability, low production cost, and ease of synthesis1,2. As a result, PANI is considered a strong candidate for applications such as sensors, electromagnetic shielding (EMI), corrosion protection, and actuators1-3. However, PANI's applications are limited by its poor infusibility, low solubility in organic solvents, and poor mechanical properties2. PANI's solubility can be improved by using organic acids such as dodecylbenzenesulfonic acid (DBSA) and p-toluenesulfonic acid (PTSA) that not only improve the compatibility of PANI with the host matrix but also acts as dopants, thus increasing the electrical conductivity of the material4. PANI's mechanical properties can be improved by mixing it with a polymeric host matrix to form composites or blends3-25. Many polymeric materials can be used as supports for PANI, such as cellulose nanofiber5, epoxy resin7, polyvinyl chloride (PVC)4, polyurethane8, poly(methyl methacrylate) (PMMA)9 , and rubbers10-25 . Among polymeric matrixes, natural rubber (NR) has been increasingly used for forming composites owing to its unique mechanical properties. NR is extensively used in various products that require superior properties such as elasticity, flexibility, and resilience.
NR/PANI blends and composites have already been obtained using different methods such as mill mixing14 , solution/dispersion mixing11,12,16,22 , and electrochemical17 and chemical polymerization10 of aniline in the presence of a host matrix. However, few studies have focused on NR/PANI composites obtained by a mixture of PANI dissolved in an organic solvent with NR latex10-12.
In this study, NR/PANI-DBSA composites were obtained by mixing NR latex and an aqueous dispersion of DBSA-doped PANI in different concentrations. These composites were characterized by ultraviolet visible near-infrared (UV-Vis-NIR) spectroscopy, thermogravimetric/differential thermogravimetric (TG/DTG) analysis, differential scanning calorimetry (DSC), stress-strain measurements, and DC electrical conductivity measurements. It was found that PANI-DBSA improved the mechanical proprieties of NR and that the composite showed low electrical percolation threshold.
2. Material and Methods
2.1. Material
Analytical grade aniline was purchased from Sigma-Aldrich, distilled under vacuum, and stored in a refrigerator before being polymerized. Ammonium peroxydisulfate (APS) and DBSA (70 wt% in 2-propanol) were purchased from Sigma-Aldrich and used as received. NR latex was collected from Hevea brasiliensis trees (Clone RRIM 600) planted in the Experimental Farm of the University of São Paulo State (UNESP), campus of Ilha Solteira, Brazil, and stabilized in a commercial solution of ammonium hydroxide to avoid coagulation. The dry rubber content was determined by standard methods13 .
2.2. Synthesis of PANI-DBSA
An aqueous dispersion of PANI-DBSA complex was prepared by the oxidative polymerization of aniline in the presence of DBSA in aqueous media. In a typical procedure, 1.0 mL of aniline and 7.6 mL of DBSA were mixed in 500 mL of deionized water under constant stirring. After 1 h, 10 mL of an aqueous solution containing 0.61 g of APS was added to the mixture. The medium was kept at 5ºC under magnetic stirring and after 12 h of reaction, the PANI-DBSA complex was separated from the medium by centrifugation. PANI-DBSA was re-dispersed in water and centrifuged again. This procedure was repeated three times, and the final content was either re-dispersed in water in the desirable concentration (e.g., for characterization) or kept at high concentration.
2.3. Preparation of NR/PANI-DBSA composite
The NR/PANI-DBSA composite was obtained by the mixture of PANI-DBSA aqueous solution (5.2 w/v) in NR latex (pH = 7.0, 41.0 w/v of NR) at concentrations of 3-10 wt%. The mixture was kept under constant stirring for 2 h at ambient temperature, following which it was cast on a glass substrate and dried in a conventional oven at 60ºC for 12 h to obtain ~200-µm-thick films.
2.4. Methods
UV-Vis-NIR absorption spectra of NR/PANI-DBSA films were obtained using a Cary 50 spectrophotometer (Varian). Spectra were recorded from 300-1000 nm. Thermogravimetric analysis was carried out in the temperature range of 25-600ºC at a heating rate of 10ºC/min in nitrogen atmosphere with a flow rate of 60 mL/min using a Q500 (TA Instruments). Approximately 10 mg were used for each sample. The glass transition temperature (Tg) of the samples (10.0 mg) was measured using a MDSC 292 (TA Instruments) with a scan rate of 10ºC/min within the temperature range of -100 to 150ºC under nitrogen atmosphere.
Mechanical tests were conducted in accordance with ASTM D882 using an Instron tensometer at a crosshead speed of 500 mm/min and a 100-N load cell. Electrical conductivity measurements of the samples were carried out using a two-probe method. Gold electrodes were evaporated onto both faces of the film for electrical contact. A power source that provides a constant voltage and measures the current (Model 247, Keithley Instruments) was used to measure the current through the sample. The electrical conductivity σ (S/cm) was calculated according to Equation 1:
where d (cm) is the thickness of the film; I (A), the current driven through the sample; Ae (cm2), the electrode area; and V (V), the applied voltage.
3. Results and Discussion
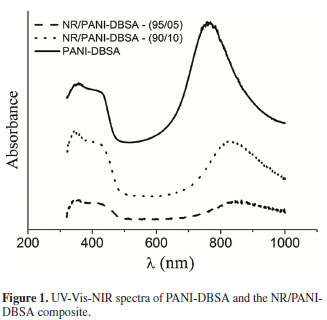
UV-Vis-NIR absorption spectra of DBSA-doped PANI and the NR/PANI-DBSA composite are shown in Figure 1. Three bands are observed in the PANI-DBSA spectrum, indicating that the polymer is in its emeraldine salt form. The band at 340 nm is assigned to the π-π* transition of benzene rings and those at ~800 nm and ~420 nm, to polaron bands related to the doping process and conductivity of PANI5,10,19. The same bands are observed in the UV-VIS-NIR spectra of NR/PANI-DBSA composites, indicating that the high pH of natural latex did not lead to the dedoping of PANI.
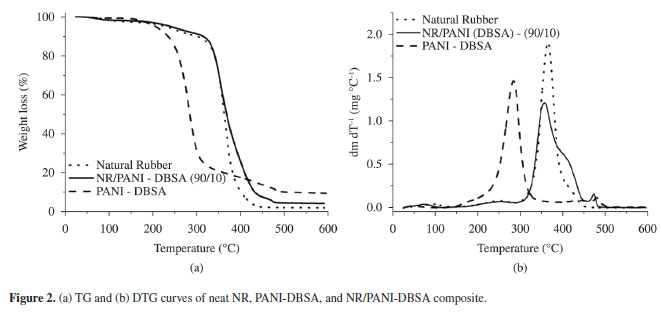
Figure 2 shows typical TG/DTG curves obtained for the neat NR, PANI-DBSA, and NR/PANI-DBSA composite with 10 wt% of PANI-DBSA. The TG curve of DBSA-PANI shows three main weight loss stages. The first weight loss occurred before 100ºC owing to the loss of water and other volatiles; the second, in a temperature range of 200-350ºC owing to the evaporation and degradation of DBSA and the oxidation of the PANI structure20,21; and the third, in a relatively wide temperature range of 400-500ºC owing to the degradation of the bound PANI-DBSA and the decomposition of PANI20,21.
The TG/DTG curves of the neat NR and composite basically show the same decomposition mechanism. The NR TG profile shows remarkable weight loss in the temperature range of 300-450ºC corresponding to the structural decomposition of rubber in nitrogen atmosphere26. The NR/PANI-DBSA composite also showed a peak at ~370ºC (major peak) that was mainly attributed to rubber decomposition and two discrete weight loss steps in the temperature ranges of 60-100ºC and 400-500ºC, corresponding to the loss of water and the degradation of PANI and DBSA bounded in the composite, respectively.
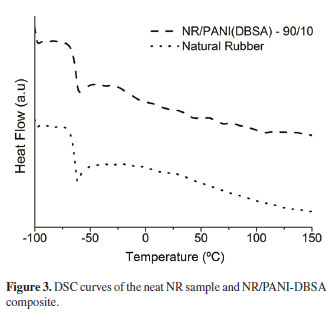
The effect of the addition of PANI-DBSA on the Tg of NR was investigated by DSC, and the results did not show a significant change in the Tg of NR with addition of up to 10 wt% of PANI-DBSA, as shown in Figure 3. In both samples, the Tg was around - 63ºC.
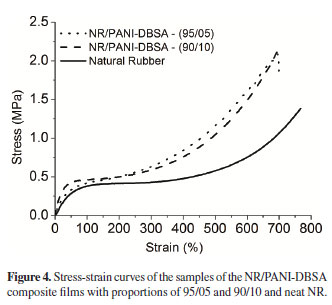
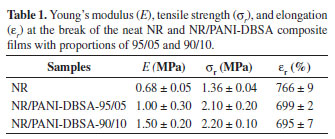
Stress-strain tests were performed under uniaxial extension; Table 1 shows the analytical results of the mechanical properties and Figure 4, the tensile curves. The addition of PANI-DBSA improved the mechanical properties of the composites. Both Young's modulus (determined from the initial slope of the tensile curves) and tensile strength significantly increased with the addition of PANI-DBSA to rubber.
This effect is attributed to the rigidity of PANI. Increasing the PANI-DBSA content in the composite to 10 wt% led to no significant change in the tensile profile compared to a proportion of 5 wt%. This behavior can be related to the amount of water uptake in the composite owing to the hygroscopic characteristics of DBSA-doped PANI27 .
Figure 5 shows the electrical conductivity of the composite films as a function of the PANI-DBSA content. The conductivity increased with an increase in the PANI-DBSA content in the NR matrix, reaching a value of 10-6 S/cm for 10 wt% of PANI-DBSA. The percolation threshold was found to be ~3.1 wt%.
By percolation theory, when a conducting continuous network is formed in the composite through connections between adjacent conducting particles, the electrical conductivity behavior can be calculated using a typical power-law28,29:
where pc is the critical concentration or percolation threshold; p, the concentration of the conductive phase; k, a constant; and t, the conductivity critical exponent. The data from Figure 5 were fitted to a plot of log (σ) versus log (p-pc) according to Equation 2, as illustrated in Figure 6, to estimate the values of the critical exponent (t) and constant (k). The values t and k were estimated by fitting the data shown in Figure 6, in which the values were t = 3.3 and k = 3.7 × 10-9.
In polymeric composites filled with a low proportion of conducting particles, the mean distance between particles or clusters is sufficiently large and the conductivity is restricted by the presence of the polymeric matrix. However, by increasing the conducting phase content at the percolation threshold, a physical path is formed. The t value obtained is larger than that obtained by the universal percolation theory. The behavior of the nonuniversal critical exponent of conductivity in polymer composites has been reported in literature30,31; it is attributed to the formation of an electrical percolation network in which the particles are not in direct physical contact32 . In this case, the conduction process in the composite preferentially occurs via hopping and tunneling of charge carriers between neighboring particles or particles clusters32 .
Above the percolation threshold, the electrical conductivity of the composite increased by seven orders of magnitude compared to that of neat NR (10-14 S/cm).
4. Conclusion
An NR/PANI-DBSA composite was obtained by incorporating an aqueous dispersion of DBSA-doped PANI into NR latex, and films of the composite were obtained by casting methods. The mechanical and electrical proprieties of the NR films were improved by the incorporation of PANI-DBSA with good thermal stability up to ~200ºC. The percolation threshold (pc) and critical exponent values were pc = 3.1 and t = 3.3, respectively. The behavior of the nonuniversal critical exponent was attributed to electrical percolation. For a composite with 10% of PANI-DBSA content, an electrical conductivity of ~10-6 S/cm was attained, which is seven orders of magnitude higher than that of neat NR.
Acknowledgments
The authors acknowledge the CNPQ (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for financial support.
Received: June 20, 2013
Revised: March 18, 2014
- 1. Chen CH. Thermal studies of polyaniline doped with dodecyl benzene sulfonic acid directly prepared via aqueous dispersions. Journal of Polymer Research 2002;9(3):195-200. http://dx.doi.org/10.1023/A:1021395726060
- 2. Bhadra S, Khastgir D, Singha NK and Lee JH. Progress in preparation, processing and applications of polyaniline. Progress in Polymer Science 2009;34(8):783-810. http://dx.doi.org/10.1016/j.progpolymsci.2009.04.003
- 3. Mattoso LHC, Medeiros ES, Baker DA, Avloni J, Wood DF and Orts WJ. Electrically conductive nanocomposites made from cellulose nanofibrils and polyaniline. Journal of Nanoscience and Nanotechnology 2008;8(5):2917-2922.
- 4. Afzal AB, Akhtar MJ and Ahmad M. Morphological studies of DBSA-doped polyaniline/PVC blends. Journal of Electron Microscopy 2010;59(5):339-344. PMid:20601353. http://dx.doi.org/10.1093/jmicro/dfq050
- 5. Silva MJ, Sanches AO, Malmonge LF, Medeiros ES, Rosa MF, McMahan CM et al. Conductive nanocomposites based on cellulose nanofibrils coated with polyaniline-DBSA via In Situ polymerization. Macromolecular Symposia 2012;319(1):196-202. http://dx.doi.org/10.1002/masy.201100156
- 6. Zilberman M, Titelman GI, Siegmann A, Haba Y, Narkis M and Alperstein A. Conductive blends of thermally dodecylbenzene sulfonic acid-doped polyaniline with thermoplastic polymers. Journal of Applied Polymer Science 1997;66(2):243-253. http://dx.doi.org/10.1002/(SICI)1097-4628(19971010)66:2<243::AID-APP5>3.0.CO;2-W
- 7. Soares BG, Celestino ML, Magioli M, Moreira VX and Khastgir D. Synthesis of conductive adhesives based on epoxy resin and polyaniline. DBSA using the in situ polymerization and physical mixing procedures. Synthetic Metals 2010;160:1981-1986. http://dx.doi.org/10.1016/j.synthmet.2010.07.021
- 8. Diniz FB, Andrade GF, Martins CR and Azevedo WM. A comparative study of epoxy and polyurethane based coatings containing polyaniline-DBSA pigments for corrosion protection on mild steel. Progress in Organic Coatings 2013;76:912-916. http://dx.doi.org/10.1016/j.porgcoat.2013.02.010
- 9. Fattouma A, Othman ZB and Arous M. Dc and Ac conductivity of polyaniline/poly(methyl methacrylathe) blends below the percolation threshold. Materials Chemistry and Physics 2012;135(1):117-122. http://dx.doi.org/10.1016/j.matchemphys.2012.04.033
- 10. Galiani PD, Malmonge JA, Santos DP and Malmonge LF. Compósitos de borracha natural com polianilina. Polímeros 2007;17(2):93-97. http://dx.doi.org/10.1590/S0104-14282007000200007
- 11. Camillo EC, Constantino CJL, Teruya MY, Alves N, Mattoso LHC and Job AE. Dependence of the electrical conductivity and elastomeric properties on sample preparation of blends of polyaniline and natural rubber. Journal of Applied Polymer Science 2005;97(4):1498-1503. http://dx.doi.org/10.1002/app.21899
- 12. Sukitpaneenit P, Thanpitcha T, Sirivat A, Weder C and Rujiravanit R. Electrical conductivity and mechanical properties of polyaniline/natural rubber composite fibers. Journal of Applied Polymer Science 2007;106(6):4038-4046. http://dx.doi.org/10.1002/app.27101
- 13. Malmonge JA, Camilo EC, Moreno RMB, Mattoso LHC and McMahan CM. Comparative study on the technological properties of latex and natural rubber from hancornia speciosa gomes and Hevea brasiliensis. Journal of Applied Polymer Science 2009;111(6):2986-2991. http://dx.doi.org/10.1002/app.29316
- 14. Al-Ghamdi AA, Al-Hartomy OA, Al-Solamy F, Al-Hazmi F, Al-Ghamdi AA, El-Mossalamy EH et al. On the prospects of conducting polyaniline/natural rubber composites for electromagnetic shielding effectiveness applications. Journal of Thermoplastic Composite Materials 2012;1-18.
- 15. John H, Joseph R and Mathew KT. Dielectric behavior of natural rubber composites in microwave fields. Journal of Applied Polymer Science 2007;103(4):2682-2686. http://dx.doi.org/10.1002/app.25420
- 16. Kalasad MN, Gadyal MA, Hiremath RK, Ikram IM, Mulimani BG, Khazi IM et al. Synthesis and characterization of polyaniline rubber composites. Composites Science and Technology 2008;68(7-8):1787-1793. http://dx.doi.org/10.1016/j.compscitech.2008.02.001
- 17. Anisha MM, Faseena NM and Predeep P. Electrochemical synthesis of conducting natural rubber nanocomposite films. Plastics, Rubber and Composites 2013;42(6):264-267. http://dx.doi.org/10.1179/1743289812Y.0000000050
- 18. Yong KC and Saad CSM. Novel peroxide-vulcanized NBR-PAni.DBSA blends, part 1: preparation and characterization. Journal of Applied Polymer Science 2009,112(6):3199-3208. http://dx.doi.org/10.1002/app.29619
- 19. Han GM, Cho SK, Oh SG and Im SS. Preparation and characterization of polyaniline nanoparticles synthetized from DBSA micellar solution. Synthetic Metal 2002;126(1):53-60. http://dx.doi.org/10.1016/S0379-6779(01)00494-5
- 20. Han D, Chu Y, Yang L, Liu Y and Lv Z. Reversed micelle polymerization: a new route for the synthesis of DBSA-polyaniline nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2005;259(1-3):179-187. http://dx.doi.org/10.1016/j.colsurfa.2005.02.017
- 21. Chen T, Dong C, Li X and Gao J. Thermal degradation mechanism of dodecylbenzene sulfonic acid- hydrochloric acid co-doped polyaniline. Polymer Degradation and Stability 2009;94(10):1788-1794. http://dx.doi.org/10.1016/j.polymdegradstab.2009.06.011
- 22. Faez R and Paoli MA. A conductive rubber based on EPDM and polyaniline I. Doping effect. European Polymer Journal 2001;37(6):1139-1143. http://dx.doi.org/10.1016/S0014-3057(00)00235-4
- 23. Soares BG, Amorim GS, Souza FG, Oliveira MG and Silva JE. The in situ polymerization of aniline in nitrile rubber. Synthetic Metals. 2006;156(2-4):91-98. http://dx.doi.org/10.1016/j.synthmet.2005.09.045
- 24. Soares BG, Amorim GS, Souza FG, Oliveira MG and Silva JE. Conducting elastomer blends based on nitrile rubber and Pani.DBSA. Macromolecular Symposia. 2006;233(1):95-101. http://dx.doi.org/10.1002/masy.200690033
- 25. Job AE, Constantino CJL, Mendes TSG, Teruya MY, Alves N and Mattoso LHC. Effect of natural rubber latex on the conducting state of polyaniline blends determined by Raman spectroscopy. Journal of Raman Spectroscopy 2003;34(10):831-836. http://dx.doi.org/10.1002/jrs.1060
- 26. Oliveira LCS, Arruda EJ, Costa RB, Gonçalves PS and Delben A. Evaluation of latex from five Hevea clones grown in São Paulo State, Brazil. Thermochimica Acta 2003;398(1-2):259-263. http://dx.doi.org/10.1016/S0040-6031(02)00225-3
- 27. Ostwal MM, Sahimi M and Tsotsis TT. Water harvesting using a conducting polymer: a study by molecular dynamics simulation. Physical Review E 2009;79(6):1-16. http://dx.doi.org/10.1103/PhysRevE.79.061801
- 28. Silva MJ, Kanda DHF and Nagashima HN. Mechanism of charge transport in castor oil-based polyurethane/carbon black composite (PU/CB). Journal of Non-Crystalline Solids 2012;358(2):270-275. http://dx.doi.org/10.1016/j.jnoncrysol.2011.09.032
- 29. Kirkpatrick S. Percolation and conduction. Reviews of Modern Physics 1973;45(4):574-588. http://dx.doi.org/10.1103/RevModPhys.45.574
- 30. Heaney MB. Measurement and interpretation of nonuniversal critical exponents in disordered conductor-insulator composites. Physical Review B 1995;52(17):12477-12480. http://dx.doi.org/10.1103/PhysRevB.52.12477
- 31. Ezquerra TA, Connor MT, Roy S, Kulescza M, Fernandes-Nascimento J and Baltá-Calleja FJ. Alternating-current electrical properties of graphite, carbon Black and carbon-fiber polymeric composites. Composites Science and Technology 2001;61(9):903-909. http://dx.doi.org/10.1016/S0266-3538(00)00176-7
- 32. Nakamura S, Saito K and Sawa G. Electrical Field dependence of conductivity and critical exponent of it for carbon black-resin composites. Seventh International conference on Dielectric Materials Measurements & Applications. 1996;430(1):96-99.
Publication Dates
-
Publication in this collection
20 May 2014 -
Date of issue
Aug 2014
History
-
Accepted
18 Mar 2014 -
Received
20 June 2013