Abstract
The purpose of this study was to evaluate the effects of long-term storage on the microtensile bond strength (µTBS) to dentin of two self-adhesive and three multi-step resin cements. Two self-adhesive cements RelyX U100 (U100) and seT PP (SET), and 3 multi-step resin cements, one using 2-step etch-and-rinse adhesive AllCem (ALC), and two conventional resin cements with self-etching primer Panavia F (PAN) and Multilink (MULT) were used. Human molars were restored (n=5), sectioned and subjected to the μTBS test after 24 h and 6 months. Fractured specimens were examined by stereomicroscope and SEM. The µTBS were analyzed by one-way ANOVA and Tukey test (α=0.05). ANOVA revealed a difference between groups (p<0.0001). All multi-step resin cements ALC, MULT and PAN showed statistically similar bond strength values that were higher than those of the U100 and SET groups. The bond strength value of ALC, MULT and PAN decreases significantly after 6 months. The majority of the failures were adhesive for all the groups. The µTBS produced by the self-adhesive cements were significantly lower than those observed for multi-step luting agents. Regardless of the numbers of steps of resin cements, the storage time reduces µTBS values to dentin only to multi-step luting agents.
Keywords:
Dentistry; Dental Materials; Resin Cements; Dentin; Dental Bonding
1 INTRODUCTION
Clinicians frequently select resin-bonded fixed partial dentures as a conservative treatment for replacing missing teeth. The clinical success of these restorations depends on the long-term bond stability between resin luting cements and dental tissue. There are two main categories of resin luting cements: the multi-step, which have no inherent adhesion to tooth structure and require an adhesive system, and self-adhesive resin cements, which do not require a separate treatment for bonding to the dental substrate.11 Chen C, He F, Burrow MF, Xie H, Zhu Y, Zhang F. Bond strengths of two self-adhesive resin cements to dentin with different treatments. Journal of Medical and Biological Engineering. 2011; 31(1):73-77. http://dx.doi.org/10.5405/jmbe.681
https://doi.org/10.5405/jmbe.681...
Until a few years ago, the application of resin cements consisted of numerous clinical steps. For preparation of the dental substrate the adhesives used were available for application in 3 steps, which were later combined into 2 steps (etch-and-rinse or self-etching) and a single self-etching application step.22 Viotti RG, Kasaz A, Pena CE, Alexandre RS, Arrais CA, Reis AF. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. The Journal of Prosthetic Dentistry. 2009;102(5):306-312. http://dx.doi.org/ 10.1016/S0022-3913(09)60180-3
https://doi.org/10.1016/S0022-3913(09)60...
However, there was a significant problem with many of the above systems, namely technique sensitivity, which is mainly caused by the mistakes application procedures.
However, professionals prefer materials that are user-friendly, and it has been considered that ease of use facilitates improved performance. A resin luting material that does not require the etching and bonding steps could be considered distinctly advantageous to the clinician when compared with traditional resin luting materials in terms of ease of use and potential savings in time and chairside costs.33 Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements - chemistry, properties and clinical considerations. Journal of Oral Rehabilitation. 2011;38(4):295-314. http://dx.doi.org/10.1111/j.1365-2842.2010.02148.x
https://doi.org/10.1111/j.1365-2842.2010...
,44 Radovic I, Monticelli F, Goracci C, Vulicevic ZR, Ferrari M. Self-adhesive resin cements: a literature review. The Journal of Adhesive Dentistry. 2008;10(4):251-258. Thus, self-adhesive cements that do not require acid treatment were introduced on the market. The bond to tooth structure is promoted by specific functional monomers, such as methacryloxyethyl trimellitate anhydride (4-META), 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) and dipentaerythritol pentaacrylate monophosphate (Penta-P),33 Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements - chemistry, properties and clinical considerations. Journal of Oral Rehabilitation. 2011;38(4):295-314. http://dx.doi.org/10.1111/j.1365-2842.2010.02148.x
https://doi.org/10.1111/j.1365-2842.2010...
which differ among different commercial products. According to the manufacturers, functional monomers are capable of bonding chemically to calcium hydroxyapatite, which is one of the mechanisms responsible for the retention of the restoration.22 Viotti RG, Kasaz A, Pena CE, Alexandre RS, Arrais CA, Reis AF. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. The Journal of Prosthetic Dentistry. 2009;102(5):306-312. http://dx.doi.org/ 10.1016/S0022-3913(09)60180-3
https://doi.org/10.1016/S0022-3913(09)60...
,33 Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements - chemistry, properties and clinical considerations. Journal of Oral Rehabilitation. 2011;38(4):295-314. http://dx.doi.org/10.1111/j.1365-2842.2010.02148.x
https://doi.org/10.1111/j.1365-2842.2010...
,55 Hikita K, Van Meerbeek B, De Munck J, Ikeda T, Van Landuyt K, Maida T, et al. Bonding effectiveness of adhesive luting agents to enamel and dentin. Dental Materials. 2007;23(1):71-80. http://dx.doi.org/10.1016/j.dental.2005.12.002
https://doi.org/10.1016/j.dental.2005.12...
6 Manso AP, Silva NR, Bonfante EA, Pergoraro TA, Dias RA, Carvalho RM. Cements and adhesives for all-ceramic restorations. Dental Clinics of North America. 2011;55(2):311-332. http://dx.doi.org/10.1016/j.cden.2011.01.011
https://doi.org/10.1016/j.cden.2011.01.0...
7 Vaz RR, Hipólito VD, D’Alpino PH, Goes MF. Bond strenght and interfacial micromorphology of etch-and-rinse and self-adhesive resin cements to dentin. Journal of Prosthodontics. 2012;21(2):101-111. http://dx.doi.org/10.1111/j.1532-849X.2011.00794.x
https://doi.org/10.1111/j.1532-849X.2011...
8 Abo T, Uno S, Yoshiyama M, Yamada T, Hanada N. Microtensile bond strength of self-adhesive luting cements to ceramics. International Journal of Dentistry. 2012;2012:278623. http://dx.doi.org/10.1155/2012/278623.
https://doi.org/10.1155/2012/278623...
-99 Di Hipólito V, Rodrigues FP, Piveta FB, Azevedo LC, Bruschi Alonso RC, Silikas N, et al. Effectiveness of self-adhesive luting cements in bonding to chlorhexidine-treated dentin. Dental Materials. 2012;28(5):495-501. http://dx.doi.org/10.1016/j.dental.2011.11.027
https://doi.org/10.1016/j.dental.2011.11...
The acidic monomers bond to the hydroxyapatite (calcium) of the smear layer, dentin or enamel, creating a link between the ionized phosphoric acid and the network material.1010 Al-Assaf K, Chakmakchi M, Palaghias G, Karanika-Kouma A, Eliades G. Interfacial characteristics of adhesive luting resins and composites with dentine. Dental Materials. 2007; 23(7):829-839. http://dx.doi.org/10.1016/j.dental.2006.06.023
https://doi.org/10.1016/j.dental.2006.06...
On the other hand, self-adhesive cements were unable to demineralize/dissolve the smear layer completely; no decalcification/infiltration of dentin and no hybrid layer and/or resin tag formation was detectable at the bonded interfaces.1111 Monticelli F, Osorio R, Mazzitelli C, Ferrari M, Toledano M. Limited decalcification/diffusion of self-adhesive cements into dentin. Journal of Dental Research. 2008;87(10):974-979. http://dx.doi.org/10.1177/154405910808701012
https://doi.org/10.1177/1544059108087010...
Many studies have evaluated the performance of immediate dentin bond strength resin cements.22 Viotti RG, Kasaz A, Pena CE, Alexandre RS, Arrais CA, Reis AF. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. The Journal of Prosthetic Dentistry. 2009;102(5):306-312. http://dx.doi.org/ 10.1016/S0022-3913(09)60180-3
https://doi.org/10.1016/S0022-3913(09)60...
,88 Abo T, Uno S, Yoshiyama M, Yamada T, Hanada N. Microtensile bond strength of self-adhesive luting cements to ceramics. International Journal of Dentistry. 2012;2012:278623. http://dx.doi.org/10.1155/2012/278623.
https://doi.org/10.1155/2012/278623...
,99 Di Hipólito V, Rodrigues FP, Piveta FB, Azevedo LC, Bruschi Alonso RC, Silikas N, et al. Effectiveness of self-adhesive luting cements in bonding to chlorhexidine-treated dentin. Dental Materials. 2012;28(5):495-501. http://dx.doi.org/10.1016/j.dental.2011.11.027
https://doi.org/10.1016/j.dental.2011.11...
,1212 Orsi IA, Varoli FK, Pieroni CH, Ferreira, MC, Borie E. In vitro tensile strength of luting cements on metallic substrate. Brazilian Dental Journal. 2014;25(2):136-140. http://dx.doi.org/10.1590/0103-6440201302290
https://doi.org/10.1590/0103-64402013022...
Immediate bond effectiveness is adequate to evaluate adhesive ability, whereas long-term clinical trials are the ideal method of assessing the durability of adhesive materials. However, little is known about the behavior of single step (self-adhesive) cement after 6 months storage.
Thus, the aim of this study was to investigate the effects of in vitro long-term degradation on the µTBS to dentin of two single step cements (self-adhesive) and compare them with three multi-step luting resin cements. The null hypotheses to be tested were: (i) no significant difference of µTBS between of resin cements evaluated; and (ii) storage does not influence the µTBS of resin cements.
2 MATERIAL AND METHODS
Twenty-five human molars were used after obtaining approval from the Research Ethics Committee of the Local University (Protocol 23115-006642/2011-88/2009-00).
2.1 Preparation of Teeth
Flat surfaces in dentin were created after removing the occlusal portion of the crown by means of a transverse cut performed with a metallographic cutter (ISOMET 1000, Buehler Ltd., Lake Bluff, USA) fitted with a diamond-coated disc, under constant cooling. The surfaces were inspected under a light microscope (Kozo Optical and Electronical Instrumental, Nanjing, China) at 40X magnification to guarantee absence of enamel remainders.
In order to standardize the smear layer, the flat dentin surfaces were manually worn for 30 seconds with nº 600 carbide abrasive paper (Norton Abrasivos, São Paulo, Brazil). After this, the teeth were washed in an ultrasonic tub (Bio Free 2L, Gnatus, Ribeirão Preto, Brazil) for 2 minutes, and prophylaxis was performed with pumice stone for 10 seconds and washing for 20 seconds. The teeth were divided into five groups according to the type of resin cement used (n=5).
2.2 Materials used
Five resin cements were used: Allcem (FGM, Joinville, Brazil), Multilink (Ivoclar Vivadent AG, Shaan, Liechtenstein), Panavia F (Kuraray Medical Inc, Tokyo, Japan), RelyX U-100 (3M/ESPE, St. Paul, USA) and seT PP (SDI, Bayswater, Australia). The application mode and composition of the materials are described in Table 1.
2.3 Cementation
Resin composite blocks were fabricated (Lis, FGM, Joinville, Brazil) measuring 6 mm high, light activated at every 2 mm with a Halogen lamp appliance (Optilux 501, SDS Kerr, Orange, USA) for 40s at intensity of 600mW/cm2 (Radiometer, SDS Kerr, Orange, USA). One side of the composite block was abraded with 600-grit SiC paper under water-cooling to create a flat surface with standardized roughness. The composite blocks were ultrasonically cleaned in distilled water for 10 minutes, rinsed with running water, completely air dried, treated with a prehydrolyzed silane solution (Prosil, FGM, Joinville, Brazil), and blow dried before bonding.
The dentin surfaces of the specimens were treated with the resin cements described, manipulated in accordance with the manufacturers recommendations, as shown in Table 1. After cementation, force of 5N was placed on the resin composite block for 5 minutes. The vestibular, lingual and proximal surfaces were light activated for 20s on each side at an intensity of 600mW/cm2.
2.4 Microtensile Tests
The specimens were fixed with sticky wax to a device on the cutting machine (ISOMET 1000) with the bond interface perpendicular to the cutting disc. Two sequences of longitudinal and perpendicular cuts were performed between them to obtain the test specimens with a cross-sectional surface area of approximately 0.9 mm2 for microtensile bond testing.
The number of specimens prematurely failed during preparation was recorded. For each resin cement, the survived specimens were allocated to two groups, in which one group was subjected to microtensile test immediately. Another one was tested after storage in distilled water at 37 °C for 6 months. Specimens failed before testing after 6 months storage were also noted.
Each specimen was fixed with cyanoacrylate adhesive gel (Pegamil, Anaeróbicos SRL, Buenos Aires, Argentina) to a Geraldelli’s device (Odeme, Joaçaba, Brazil), afterwards coupled to the universal test machine (3342, Instron, Canton, USA) at a speed of 1.0 mm/min. To calculate the rupture stress of each specimen in megapascal (MPa), the cross-sectional area of the specimens was measured with a digital caliper (Absolute Digimatic, Mitutoyo, Tokyo, Japan).
2.5 Fracture Pattern Analysis
The fractured test specimens were analyzed under a stereomicroscope (40x) and classified into the following patterns: 1) Adhesive-Mixed at the interface (A-M); 2) Cohesive in dentin (CD) or 3) cohesive in resin composite (CR). Specimens of each group were randomly selected for evaluation of the interface by scanning electron microscopy (Pro X, Phenom-World, Eindhoven, The Netherlands).
2.6 Statistical Analysis
Statistical analysis was performed using the SigmaPlot 12 software (SigmaPlot v. 12.3, Systat Software Inc., San Jose, USA). Normal distribution of microtensile bond strength data were assumed after Kolmogorov–Smirnov test. The obtained data was analyzed using a one-way ANOVA and Tukey’s post hoc test at α=0.05. Specimens with fracture cohesively in dentine or composite as well as samples that failed before actual test were excluded from the statistical analysis for bond strength test, but were counted individually as percentage distribution of respective failure mode and pre-testing failure (PTF), respectively.
3 RESULTS
Mean and standard deviation values for μTBS to dentin (MPa), the number of specimens tested (n), failure distribution (in percentages), and premature failures (PF) according to resin cements and storage time are shown in Table 2. The ANOVA revealed a significant difference between groups (p < 0.001).
– Mean and standard deviation of microtensile bond strength values (MPa) obtained for the different cements. Total number of beams (N tested stick/pre-load failure) and percentage distribution of failure mode after microtensile bond strength testing [Failure % Adhesive-Mixed / Cohesive Dentin / Cohesive Resin].
The AllCem, Multilink and Panavia F cements presented similar bond strengths among them, and statistically higher values than the U-100 and seT PP cements. The U-100 and seT PP cements presented no statistically significant difference between them (p < 0.001). The ANOVA revealed that significant differences were found among immediate bond strength and 6 months aging in water storage for AllCem, Multilink and Panavia F cements (p < 0.001). Although no significant difference was found to U-100 and seT PP cements when immediate and 6-mont results are compared, the values after 6-month are statistically significant and lower than AllCem, Multilink and Panavia F cements (p < 0.001). The majority of the failures were adhesive/mixed for all the groups tested.
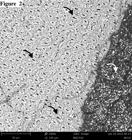
Representative SEM images of all cements are shown in Figures 1 to 5. The images of AllCem, Multilink and Panavia F specimens present different demineralization patterns (Figures 1, 2 and 3). On the other hand, U-100 and seT PP images shows a presence of smear layer on dentin and large number of dentin tubules with smear plug (Figures 4 and 5).
– (Magnification: 3900x – Scale bar: 20µm) - Representative SEM image of fractured specimen from AllCem group (dentin side). Note open tubules with resin tag of Ambar adhesive. Black arrow: dentin tubule open.
– (Magnification: 2000x – Scale bar: 30µm) - Representative SEM image of fractured specimen from Multilink group (dentin side). Note the difference between the aspect of dentin surface (black arrows), poor sign of demineralization could be detected and the layer of resin cement (white arrow).
– (Magnification: 1650x – Scale bar: 50µm) - Representative SEM image of fractured specimen from Panavia F group (dentin side). Is possible to observe some empty dentin tubules and others with resin cement remnants was detected (black arrows). White arrows: layer of resin cement.
– (Magnification: 2000x – Scale bar: 30µm) - Representative SEM image of fractured specimen from RelyX U-100 group (dentin side). White arrow: dentin tubule with smear plug. Black arrows: dentin tubules open.
– (Magnification: 3900x – Scale bar: 20µm) - Representative SEM image of fractured specimen from seT PP group (dentin side). Black arrows: dentin tubules with smear plug.
4 DISCUSSION
The values of microtensile bond strength of resin cements evaluated in this study were statistically different. The multi-step resin cements showed better performance when compared to self-adhesives cements. Based on the results obtained in this study, the first null hypothesis must be rejected.
In order to speed up the dental substrate pre-treatment steps, self-adhesive resin cements were developed.1313 Hitz T, Stawarczyk B, Fischer J, Hämmerle CH, Sailer I. Are self-adhesive resin cements a valid alternative to conventional resin cements? A laboratory study of the long-term bond strength. Dental Materials. 2012;28(11):1183-1190. http://dx.doi.org/10.1016/j.dental.2012.09.006
https://doi.org/10.1016/j.dental.2012.09...
Ferracane et al.33 Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements - chemistry, properties and clinical considerations. Journal of Oral Rehabilitation. 2011;38(4):295-314. http://dx.doi.org/10.1111/j.1365-2842.2010.02148.x
https://doi.org/10.1111/j.1365-2842.2010...
affirmed that the acidity of the cement arising from the functional acid group of the monomer is controlled, so that it would be sufficiently strong to promote hybridization with the tooth structure and sufficiently low to prevent hydrophilicity, which could compromise the mechanical properties of the material.1414 Jongsma LA, Kleveriaan CJ, Pallav P, Feilzer AJ. Influence of polymerization mode and C-fator on cohesive strength of dual-cured resin cements. Dental Materials. 2012;28(7):722-728. http://dx.doi.org/10.1016/j.dental.2012.03.002
https://doi.org/10.1016/j.dental.2012.03...
When one beings to make the mixture, the cement becomes rather hydrophilic, facilitating its flow and adaptation to the surface. Over time, the material becomes more hydrophobic, as the acid group is consumed through the acid-base reaction with the minerals of dentin.33 Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements - chemistry, properties and clinical considerations. Journal of Oral Rehabilitation. 2011;38(4):295-314. http://dx.doi.org/10.1111/j.1365-2842.2010.02148.x
https://doi.org/10.1111/j.1365-2842.2010...
But, some studies have demonstrated the limited capacity to demineralize and dissolve the smear layer in order to attain the adjacent dentin.11 Chen C, He F, Burrow MF, Xie H, Zhu Y, Zhang F. Bond strengths of two self-adhesive resin cements to dentin with different treatments. Journal of Medical and Biological Engineering. 2011; 31(1):73-77. http://dx.doi.org/10.5405/jmbe.681
https://doi.org/10.5405/jmbe.681...
,1515 Kambara K, Nakajima M, Hosaka K, Takahashi M, Thanatvarakorn O, Ichinose S, Foxton RM, Tagami J. Effect of smear layer treatment on dentin bond of self-adhesive cements. Dental Materials Journal. 2012;31(6):980-987. http://dx.doi.org/10.4012/dmj.2012-031
https://doi.org/10.4012/dmj.2012-031...
The explanation for the low microtensile bond strength obtained with the self-adhesive cements are: (1) this limitation is attributed to the high viscosity of the cement, which makes it difficult for it to penetrate into the dentin tubules11 Chen C, He F, Burrow MF, Xie H, Zhu Y, Zhang F. Bond strengths of two self-adhesive resin cements to dentin with different treatments. Journal of Medical and Biological Engineering. 2011; 31(1):73-77. http://dx.doi.org/10.5405/jmbe.681
https://doi.org/10.5405/jmbe.681...
,99 Di Hipólito V, Rodrigues FP, Piveta FB, Azevedo LC, Bruschi Alonso RC, Silikas N, et al. Effectiveness of self-adhesive luting cements in bonding to chlorhexidine-treated dentin. Dental Materials. 2012;28(5):495-501. http://dx.doi.org/10.1016/j.dental.2011.11.027
https://doi.org/10.1016/j.dental.2011.11...
(Figures 4 and 5) and (2) the low penetration potential of acidic monomers of the self-adhesive cements into the smear layer may interfere in hybrid layer formation, thereby compromising the bond of self-adhesive cements. The result of this is observed in the large number of premature failures and percentage of adhesive failure of this type of cement found for the groups of self-adhesive cements in the study (Table 2).
In multi-steps cements the bond to dentin is obtained by pretreatment of the surface with acid, followed by the application of an adhesive system that contains hydrophilic and hydrophobic components. These steps remove the smear layer and demineralize the dentin surface, thereby exposing the collagen fibrils to enable infiltration of the adhesive system, consequently forming the hybrid layer (Figures 1, 2 and 3). Hikita et al.55 Hikita K, Van Meerbeek B, De Munck J, Ikeda T, Van Landuyt K, Maida T, et al. Bonding effectiveness of adhesive luting agents to enamel and dentin. Dental Materials. 2007;23(1):71-80. http://dx.doi.org/10.1016/j.dental.2005.12.002
https://doi.org/10.1016/j.dental.2005.12...
showed statistically superior performance in microtensile tests for multi-steps that used an etch-and-rinse or self-etching adhesive system before cementation, compared with the self-adhesive cements.
The All Cem group, in which the conventional adhesive system Ambar and resin cement All Cem were used, exhibited the highest bond strength mean with statistical difference in comparison with the self-adhesive cement. The previous application of 37% phosphoric acid and the application of the adhesive system is required, this adhesive system, classified as a two-step/wet-bonding technique, exhibits a mechanism of action that promotes greater micromechanical retention, because it results in deeper demineralization of the substrate by the action of the phosphoric acid, which is afterwards replaced by resin monomers.1616 Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004; 83(3):216-221. http://dx.doi.org/10.1177/154405910408300306
https://doi.org/10.1177/1544059104083003...
For resin cement Panavia F, the rationale of higher bond strength values could lie in its chemical composition. It has been demonstrated that the phosphate group of the functional monomer 10-MDP, present in Panavia F, bonds to hydroxyapatite and forms a nano-layered structure on dentin.1717 Fukegawa D, Hayakawa S, Yoshida Y, Suzuki K, Osaka A, Van Meerbeek B. Chemical interaction of phosphoric acid ester with hydroxyapatite. Journal of Dental Research. 2006; 85(10):941-944 . http://dx.doi.org/10.1177/154405910608501014
https://doi.org/10.1177/1544059106085010...
,1818 Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, et al. Comparative study on adhesive performance of functional monomers. Journal of Dental Research. 2004;83(6):454-458. http://dx.doi.org/10.1177/154405910408300604
https://doi.org/10.1177/1544059104083006...
In addition to this chemical interaction, Panavia F is also capable of providing micromechanical retention, since its monomer is capable of infiltrating into the dentinal substrate.1919 Reis AF, Giannini M, Pereira PN. Long-term TEM analysis of the nanoleakage patterns in resin-dentin interfaces produed by different bonding strategies. Dental Materials. 2007; 23(9):1164-1172. http://dx.doi.org/10.1016/j.dental.2006.10.006
https://doi.org/10.1016/j.dental.2006.10...
The bond strength values for multi-step cement (Panavia F) were close to those found in the literature.22 Viotti RG, Kasaz A, Pena CE, Alexandre RS, Arrais CA, Reis AF. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. The Journal of Prosthetic Dentistry. 2009;102(5):306-312. http://dx.doi.org/ 10.1016/S0022-3913(09)60180-3
https://doi.org/10.1016/S0022-3913(09)60...
,2020 Kasaz AC, Pena CE, de Alexandre RS, Viotti RG, Santana VB, Arrais CA, et al. Effects of a peripheral enamel margin on the long-term bond strength and nanoleakage of composite/dentin interfaces produced by self-adhesive and conventional resin cements. The Journal of Adhesive Dentistry. 2012;14(3):251-263. http://dx.doi.org/10.3290/j.jad.a22517
https://doi.org/10.3290/j.jad.a22517...
The high bond strength results obtained by the resin cement Multilink are in agreement with another studies.2121 Salz U, Zimmermann J, Salzer T. Self-curing, self-etching adhesive cement systems. The Journal of Adhesive Dentistry. 2005;7(1):7-17.,2222 Holderegger C, Sailer I, Schuhmacher C, Schläpfer R, Hämmerle C, Fischer J. Shear bond strength of resin cements to human dentin. Dental Materials. 2008;24(7):944-950. http://dx.doi.org/10.1016/j.dental.2007.11.021
https://doi.org/10.1016/j.dental.2007.11...
One reason that may explain the high bond strength results of the cement Multilink is the prior application of a self-etching primer, which was vigorously applied, as used in Panavia F. Another reason is that the self-etching system (Primer A + Primer B) used has an acidic monomer supplied in a separate bottle of water, to prevent premature hydrolysis of the methacrylate.2121 Salz U, Zimmermann J, Salzer T. Self-curing, self-etching adhesive cement systems. The Journal of Adhesive Dentistry. 2005;7(1):7-17.
All self-adhesive luting agents evaluated in the present study provided low bond strength values to dentin surfaces with high prevalence of pre-failure specimens. Despite the shorter working time, luting of a non-retentive preparation with use of self-adhesive cements should be avoided with these systems.22 Viotti RG, Kasaz A, Pena CE, Alexandre RS, Arrais CA, Reis AF. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. The Journal of Prosthetic Dentistry. 2009;102(5):306-312. http://dx.doi.org/ 10.1016/S0022-3913(09)60180-3
https://doi.org/10.1016/S0022-3913(09)60...
The only advantage of this material is gained with some sacrifice in bonding performance.
Regarding the storage factor, statistical analysis revealed that the µTBS mean values after 24h of storage were statistically higher compared to mean values after 6 months only for multi-step resin cements. The second null hypothesis therefore has partially rejected. These findings can be explained by the adhesive system degradation that probably occurs because of adhesive displacement by water into the adhesive-dentin interface, as a result of hydrolysis.2323 Hashimoto M. A review-micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. Journal of Biomedical Materials Research B. 2010; 92(1):268-280. http://dx.doi.org/10.1002/jbm.b.31535
https://doi.org/10.1002/jbm.b.31535...
Although the degradation of naked collagen fibril by MMPs occurs into adhesive-dentin interface,1616 Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004; 83(3):216-221. http://dx.doi.org/10.1177/154405910408300306
https://doi.org/10.1177/1544059104083003...
in the case of conventional resin cements with self-etching primer, the hydrolysis of adhesive resin may be more damaging to long-term bonding effectiveness due to their bond structure having less demineralized dentin.2323 Hashimoto M. A review-micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. Journal of Biomedical Materials Research B. 2010; 92(1):268-280. http://dx.doi.org/10.1002/jbm.b.31535
https://doi.org/10.1002/jbm.b.31535...
Additionally, physical changes such as plasticization, softening, and chemical changes such as oxidation alter permanently the mechanical properties of the polymer network in the adhesive-dentin interface. The increased water sorption will result in a polymer plasticization, thereby reducing interchange interactions, such as entanglements and secondary bonds. Thus, this sponge effect results in reduction of the polymer mechanical properties (hardness and strength).2424 Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22(3):211-222. http://dx.doi.org/10.1016/j.dental.2005.05.005
https://doi.org/10.1016/j.dental.2005.05...
For the two-step etch-and-rinse adhesive systems, the presence of water in their composition produces a semipermeable membrane due its high concentration of hydrophilic monomers and solvents.2525 Koshiro K, Inoue S, Tanaka T, Koase K, Fujita M, Hashimoto M, et al. In vivo degradation of resin-dentin bonds produced by a self-etch vs. a total-etch adhesive system. European Journal of Oral Sciences. 2004;112(4):368-375. http://dx.doi.org/10.1111/j.1600-0722.2004.00141.x
https://doi.org/10.1111/j.1600-0722.2004...
Although no degradation was found in the self-adhesive resin cements, the results of these materials after 6-month are lower than multi-step resin cements after 6-month. More long-term results need to be done to prove the hypothesis of no degradation of self-adhesive resin cements.
5 CONCLUSION
The self-adhesive luting agents evaluated in the current study yielded lower bond strength values than the multi-step systems All Cem, Panavia F and Multilink. The storage in water for 6 months decreases the microtensile bond strength of all resin cements tested to dentin.
ACKNOWLEDGEMENTS
This study was supported by the Foundation for the Support of Scientific and Technological Research of Maranhão (FAPEMA - BEPP-03730/13). The authors thank 3M/ESPE, SDI and FGM for providing restorative materials.
REFERENCES
-
1Chen C, He F, Burrow MF, Xie H, Zhu Y, Zhang F. Bond strengths of two self-adhesive resin cements to dentin with different treatments. Journal of Medical and Biological Engineering. 2011; 31(1):73-77. http://dx.doi.org/10.5405/jmbe.681
» https://doi.org/10.5405/jmbe.681 -
2Viotti RG, Kasaz A, Pena CE, Alexandre RS, Arrais CA, Reis AF. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. The Journal of Prosthetic Dentistry. 2009;102(5):306-312. http://dx.doi.org/ 10.1016/S0022-3913(09)60180-3
» https://doi.org/10.1016/S0022-3913(09)60180-3 -
3Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements - chemistry, properties and clinical considerations. Journal of Oral Rehabilitation. 2011;38(4):295-314. http://dx.doi.org/10.1111/j.1365-2842.2010.02148.x
» https://doi.org/10.1111/j.1365-2842.2010.02148.x -
4Radovic I, Monticelli F, Goracci C, Vulicevic ZR, Ferrari M. Self-adhesive resin cements: a literature review. The Journal of Adhesive Dentistry. 2008;10(4):251-258.
-
5Hikita K, Van Meerbeek B, De Munck J, Ikeda T, Van Landuyt K, Maida T, et al. Bonding effectiveness of adhesive luting agents to enamel and dentin. Dental Materials. 2007;23(1):71-80. http://dx.doi.org/10.1016/j.dental.2005.12.002
» https://doi.org/10.1016/j.dental.2005.12.002 -
6Manso AP, Silva NR, Bonfante EA, Pergoraro TA, Dias RA, Carvalho RM. Cements and adhesives for all-ceramic restorations. Dental Clinics of North America. 2011;55(2):311-332. http://dx.doi.org/10.1016/j.cden.2011.01.011
» https://doi.org/10.1016/j.cden.2011.01.011 -
7Vaz RR, Hipólito VD, D’Alpino PH, Goes MF. Bond strenght and interfacial micromorphology of etch-and-rinse and self-adhesive resin cements to dentin. Journal of Prosthodontics. 2012;21(2):101-111. http://dx.doi.org/10.1111/j.1532-849X.2011.00794.x
» https://doi.org/10.1111/j.1532-849X.2011.00794.x -
8Abo T, Uno S, Yoshiyama M, Yamada T, Hanada N. Microtensile bond strength of self-adhesive luting cements to ceramics. International Journal of Dentistry. 2012;2012:278623. http://dx.doi.org/10.1155/2012/278623.
» https://doi.org/10.1155/2012/278623 -
9Di Hipólito V, Rodrigues FP, Piveta FB, Azevedo LC, Bruschi Alonso RC, Silikas N, et al. Effectiveness of self-adhesive luting cements in bonding to chlorhexidine-treated dentin. Dental Materials. 2012;28(5):495-501. http://dx.doi.org/10.1016/j.dental.2011.11.027
» https://doi.org/10.1016/j.dental.2011.11.027 -
10Al-Assaf K, Chakmakchi M, Palaghias G, Karanika-Kouma A, Eliades G. Interfacial characteristics of adhesive luting resins and composites with dentine. Dental Materials. 2007; 23(7):829-839. http://dx.doi.org/10.1016/j.dental.2006.06.023
» https://doi.org/10.1016/j.dental.2006.06.023 -
11Monticelli F, Osorio R, Mazzitelli C, Ferrari M, Toledano M. Limited decalcification/diffusion of self-adhesive cements into dentin. Journal of Dental Research. 2008;87(10):974-979. http://dx.doi.org/10.1177/154405910808701012
» https://doi.org/10.1177/154405910808701012 -
12Orsi IA, Varoli FK, Pieroni CH, Ferreira, MC, Borie E. In vitro tensile strength of luting cements on metallic substrate. Brazilian Dental Journal. 2014;25(2):136-140. http://dx.doi.org/10.1590/0103-6440201302290
» https://doi.org/10.1590/0103-6440201302290 -
13Hitz T, Stawarczyk B, Fischer J, Hämmerle CH, Sailer I. Are self-adhesive resin cements a valid alternative to conventional resin cements? A laboratory study of the long-term bond strength. Dental Materials. 2012;28(11):1183-1190. http://dx.doi.org/10.1016/j.dental.2012.09.006
» https://doi.org/10.1016/j.dental.2012.09.006 -
14Jongsma LA, Kleveriaan CJ, Pallav P, Feilzer AJ. Influence of polymerization mode and C-fator on cohesive strength of dual-cured resin cements. Dental Materials. 2012;28(7):722-728. http://dx.doi.org/10.1016/j.dental.2012.03.002
» https://doi.org/10.1016/j.dental.2012.03.002 -
15Kambara K, Nakajima M, Hosaka K, Takahashi M, Thanatvarakorn O, Ichinose S, Foxton RM, Tagami J. Effect of smear layer treatment on dentin bond of self-adhesive cements. Dental Materials Journal. 2012;31(6):980-987. http://dx.doi.org/10.4012/dmj.2012-031
» https://doi.org/10.4012/dmj.2012-031 -
16Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004; 83(3):216-221. http://dx.doi.org/10.1177/154405910408300306
» https://doi.org/10.1177/154405910408300306 -
17Fukegawa D, Hayakawa S, Yoshida Y, Suzuki K, Osaka A, Van Meerbeek B. Chemical interaction of phosphoric acid ester with hydroxyapatite. Journal of Dental Research. 2006; 85(10):941-944 . http://dx.doi.org/10.1177/154405910608501014
» https://doi.org/10.1177/154405910608501014 -
18Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, et al. Comparative study on adhesive performance of functional monomers. Journal of Dental Research. 2004;83(6):454-458. http://dx.doi.org/10.1177/154405910408300604
» https://doi.org/10.1177/154405910408300604 -
19Reis AF, Giannini M, Pereira PN. Long-term TEM analysis of the nanoleakage patterns in resin-dentin interfaces produed by different bonding strategies. Dental Materials. 2007; 23(9):1164-1172. http://dx.doi.org/10.1016/j.dental.2006.10.006
» https://doi.org/10.1016/j.dental.2006.10.006 -
20Kasaz AC, Pena CE, de Alexandre RS, Viotti RG, Santana VB, Arrais CA, et al. Effects of a peripheral enamel margin on the long-term bond strength and nanoleakage of composite/dentin interfaces produced by self-adhesive and conventional resin cements. The Journal of Adhesive Dentistry. 2012;14(3):251-263. http://dx.doi.org/10.3290/j.jad.a22517
» https://doi.org/10.3290/j.jad.a22517 -
21Salz U, Zimmermann J, Salzer T. Self-curing, self-etching adhesive cement systems. The Journal of Adhesive Dentistry. 2005;7(1):7-17.
-
22Holderegger C, Sailer I, Schuhmacher C, Schläpfer R, Hämmerle C, Fischer J. Shear bond strength of resin cements to human dentin. Dental Materials. 2008;24(7):944-950. http://dx.doi.org/10.1016/j.dental.2007.11.021
» https://doi.org/10.1016/j.dental.2007.11.021 -
23Hashimoto M. A review-micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. Journal of Biomedical Materials Research B. 2010; 92(1):268-280. http://dx.doi.org/10.1002/jbm.b.31535
» https://doi.org/10.1002/jbm.b.31535 -
24Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22(3):211-222. http://dx.doi.org/10.1016/j.dental.2005.05.005
» https://doi.org/10.1016/j.dental.2005.05.005 -
25Koshiro K, Inoue S, Tanaka T, Koase K, Fujita M, Hashimoto M, et al. In vivo degradation of resin-dentin bonds produced by a self-etch vs. a total-etch adhesive system. European Journal of Oral Sciences. 2004;112(4):368-375. http://dx.doi.org/10.1111/j.1600-0722.2004.00141.x
» https://doi.org/10.1111/j.1600-0722.2004.00141.x
Publication Dates
-
Publication in this collection
May-Jun 2016
History
-
Received
28 Sept 2015 -
Reviewed
15 Feb 2016 -
Accepted
04 Apr 2016