Abstract
The production of a material with rigid, multifunctional three-dimensional porous structure at a low cost is still challenging to date. In this work, a light and rigid carbon foam was prepared using rice husk as the basic element through a simple fermentation process followed by carbonization. For the fermentation process, the amount of biological yeast (7.5 g for the carbon foam CA-1P and 5 g for the carbon foam CA-2P) was used to evaluate its influence on the morphology of the foams. In order to prove that the heat treatment made in the foam alters the hydrophilic character of the rice husk foam, a chemical treatment with steam deposition was carried out. The foams were characterized by the following analyzes: apparent density, micrograph, thermogravimetry, contact angle, water sorption capacity and thermal conductivity. Visually, the CA-1P foams presented a structure with larger pores due to the greater amount of yeast used in its formulation. The heat treatment of rice potato foams proved to be as efficient as the chemical treatment for water contact angle above 90º, proving the ability of the foams to repel water/moisture. The thermal conductivity of the foams (0.029 and 0.026 W m-1 K-1 for CA-1P and CA-2P, respectively) was close to the conductivity of polyurethane foams (0.032 W m-1 K-1). Thus, the method used in the production of the carbon foams produced from the rice husk proved to be effective. In addition, the foams produced have the potential to be used for thermal insulation.
Keywords:
Carbon foams; rice husk; thermal insulation
1. Introduction
The use of agricultural by-products as a source of raw material for the development of added-value products and as an alternative energy source reduces dependence on fossil sources. In addition it is a low cost and sustainable solution11 Quispe I, Navia R, Kahhat R. Energy potential from rice husk through direct combustion and fast pyrolysis: A review. Waste Management. 2016;59:200-210.,22 Lazzari E, Schena T, Caetano M, Marcelo A, Tatiane C, Nunes A, et al. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Industrial Crops and Products. 2018;111:856-64.. Biomass is a qualified raw material for the preparation of carbonaceous materials, since it is available in abundance, besides being a renewable resource. Different materials based on biomass are being studied for applications in different areas, such as: composite rice husks and expanded cork granules and recycled tire rubber for civil construction33 António J, Tadeu A, Marques B, Almeida JAS, Pinto V. Application of rice husk in the development of new composite boards. Construction and Building Materials. 2018;176:432-439.; activated carbon produced from bamboo44 Zhang G, Chen Y, Chen Y, Guo H. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Materials Research Bulletin. 2018;102:391-398.; carbon aerogels produced from starch as electrode material for supercapacitors55 Bakierska M, Molenda M, Majda D, Dziembaj R. Functional starch based carbon aerogels for energy applications. Procedia Engineering. 2014;98:14-19.; barley straw66 Tijani MM, Aqsha A, Mahinpey N. Development of oil-spill sorbent from straw biomass waste : Experiments and modeling studies. Journal of Environmental Management. 2016;171:166-176. e cellulose77 Feng J, Nguyen ST, Fan Z, Duong HM. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chemical Engineering Journal. 2015;270:168-175. as adsorbents for the removal of oils, among others.
Recently, biomass-based carbon foams, such as wheat flour88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861. e tannins99 Jana P, Fierro V, Pizzi A, Celzard A. Biomass-derived, thermally conducting, carbon foams for seasonal thermal storage. Biomass and Bioenergy. 2014;67:312-318., were successfully manufactured by different methods, such as: lyophilization, hydrothermal treatment and direct carbonization. Pyrolysis is a promising way to convert this biomass into high added value products. The pyrolysis process consists of the thermal decomposition of the organic matter contained in the biomass, under a certain temperature and in the total or partial absence of oxygen in: a gaseous, solid (biochar) and liquid fraction (bio-oils)88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861.,1010 Basu P. Biomass Gasification and Pyrolysis: Pratical Design and Theory. Pittsburgh: Eselvier Inc.; 2010..
Among the positives aspects of the carbon foams, are their thermal stability, low density and thermal expansion, good resistance to thermal stress and shock, and a relatively low cost. An important property obtained after the pyrolysis process of the foams is hydrophobicity. This is a result of the loss of oxygen and hydrogen by the dehydration and decarboxylation reactions during the pyrolysis process. They can be applied in thermal insulation for high temperatures, filters, porous electrodes, thermal management, among others99 Jana P, Fierro V, Pizzi A, Celzard A. Biomass-derived, thermally conducting, carbon foams for seasonal thermal storage. Biomass and Bioenergy. 2014;67:312-318.,1111 Gallego NC, Klett JW. Carbon foams for thermal management. Carbon. 2003;41(7):1461-466.,1212 Adhikari S, Nam H, Chakraborty JP. Conversion of Solid Wastes to Fuels and Chemicals Through Pyrolysis. In: Bhaskar T, Pandey A, Lee DJ, Khanal SK, editors. Waste Biorefinery: Potential and Perspectives. Amsterdam: Eselvier BV; 2018. p. 239-263..
Carbon foams obtained from the pyrolysis of foams produced from commercial Mimosa peel tannin extract and exfoliated graphite were studied by Jana et al.99 Jana P, Fierro V, Pizzi A, Celzard A. Biomass-derived, thermally conducting, carbon foams for seasonal thermal storage. Biomass and Bioenergy. 2014;67:312-318.. The authors showed that the porosity and thermal conductivity of the foams were influenced by the density of the foams, which varies according to the amount of exfoliated graphite used. That is, the higher the density, the lower is the porosity and the higher is the thermal conductivity.
A simple and low cost methodology for the production of carbon foams was presented by Yuan et al.88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861.. The authors produced carbon foams from the pyrolysis of foams made from wheat flour. The pyrolysis process chosen by the authors is inexpensive and provides the foam with a structure that is both appropriate mechanically and thermally stable. The porous structure of the material, a determinant factor for the properties of the foam, was controlled based on the amount of water and yeast used. According to the authors, the specific mass and compressive strength are dependent on the amount of yeast used. The higher the amount of yeast used, the greater are the pores formed by decreasing the density of the foams. In addition, the carbon foams exhibited thermal conductivity between 0.09 and 0.17 W.m-1.K-1. The authors concluded that this method can be widely applied to other materials with structures that require thermal conductivity and low density.
The rice husk represents about 20% of the bulk volume of rice. It is a woody bark composed of cellulose, hemicellulose, lignin and inorganic materials. Of this inorganic fraction, about 90-95% is of hydrated silica, contemplating of 13 to 29% of the total of the bark. Due to its characteristics such as abrasiveness and hardness, the rice husk has no commercial value and is discarded in rural areas. However, it presents as a potential precursor in the production of activated carbon due to its physicochemical and biochemical properties88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861.,1313 Almeida SR. Pirólise rápida de casca de arroz : estudo de parâmetros e caracterização de produtos [Dissertação]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2010..
The scientific contribution of this study is the development of carbon foams from the pyrolysis of wood foam produced with the rice husk based on the methodology used by Yuan et al.88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861.. Pyrolysis was used as a method of hydrophobizing the foam surface. The comparison with the traditional silane vapor deposition method was performed to verify the efficiency of the method proposed in the present work. Thermal, chemical and morphological properties of these foams were studied in order to evaluate their use as thermal insulation.
2. Materials and Methods
2.1 Materials
The raw materials used in the production of the foams were: rice husk, wheat flour, soybean oil, water and dry yeast (these are common for bread making). Triethoxyvinylsilane (VTES) was purchased from Sigma-Aldrich.
2.2 Wood development
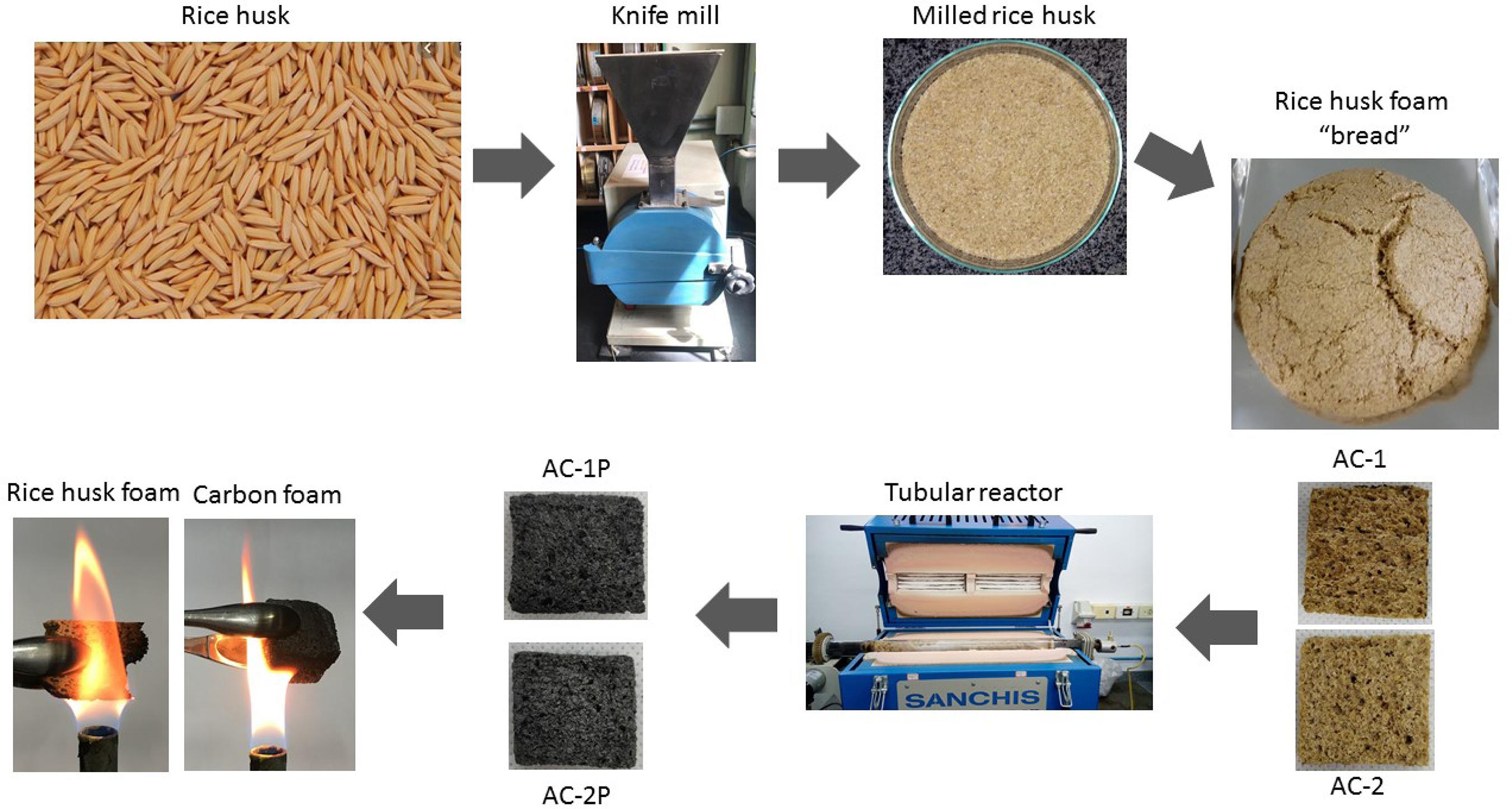
The process used for the production of rice husk foams was based on the fermentation process presented by Yuan et al.88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861., in which the authors associated this process with the process of bread production. Figure 1 shows the flowchart of the production process of the rice husk foams and carbon foams. The rice husk was used as raw material in the production of the foams in order to use an agricultural residue generated in abundance during the production of rice.
Flowchart of the production of rice husk foams and carbon foams. Caption: CA-1 (7.5 g yeast foam), CA-1P (CA-1 foam after pyrolysis), CA-2 (5.0 g yeast foam) and CA-2P (CA-2 foam after pyrolysis).
Initially, the rice husk (CA) was dried at 105ºC for 24 h and was comminuted in a knife mill using a 1 mm sieve. The organic yeast (7.5 g for CA-1 foam and 5 g for CA-2 foam) was then dissolved in 130 g of deionized water at 40 °C under magnetic stirring. After completely dissolved, the mixture was poured into 150 g of wheat flour, 100 g of rice husk powder and approximately 4 g of soybean oil (used to “bind” the dough). The components were mixed for 10 minutes in a planetary mixer until a homogeneous mass was formed, which was the fermented for about 60 min at 35 °C for the formation of porous structures. The foam was baked in an oven at 180 °C for 40 min and then dried for 24 h at 80 °C. The pyrolysis of the foams was carried out in a cylindrical quartz reactor that operates in a batch system, this reactor is described in detail by Perondi et al.1414 Perondi D, Polleto P, Restelatto D, Manera C, Silva JP, Junges J, et al. Steam gasification of poultry litter biochar for bio-syngas production. Process Safety and Environmental Protection. 2017;109:478-488.. The reactor was initially fed with approximately 5.5 g of wood foam. During the pyrolysis reaction, nitrogen gas (N2) was used, with a flow rate of 0.15 L min-1. The heating rate used was 5 ºC min-1 until the final temperature was reached (800 ºC). An isotherm time of 60 min at this temperature (800 °C) was used. Finally, the system was cooled under N2 flow at room temperature. Carbon foams were named CA-1P (CA-1 foam after pyrolysis) and CA-2P (CA-2 foam after pyrolysis).
2.3 Surface treatment of foams
In order to verify the efficiency of the pyrolysis process in the hydrophobicization of the foam surface, a silane vapor deposition was carried out. This method is effective for this application1515 Lazzari LK, Zampieri VB, Zanini M, Zattera AJ, Baldasso C. Sorption capacity of hydrophobic cellulose cryogels silanized by two different methods. Cellulose. 2017;24(8):3421-3431., as a way of comparing methods.
Triethoxyvinylsilane (VTES) was deposited onto the rice husk foams as a surface treatment in order to render the surface hydrophobic and thus moisture-absorption free. The samples were suspended, by a screen, in a beaker containing 4.0 mL of MTMS and isolated inside another beaker for 48 h in an oven at 70ºC. Carbon foams were named CA-1H (CA-1 foam after surface treatment) and CA-2H (CA-2 foam after surface treatment).
The water contact angle was measured on the foams to evaluate their degree of hydrophobicity. In the assay, a drop of distilled water was added at three random points on the surface of the foam. The image was recorded with a digital still camera and the images obtained were analysed by using Surftens.
In order to verify the effectiveness of surface treatment with VTES, a water absorption capacity test was performed according to ASTM F726-12 (2012). The foams were previously weighed and then placed in contact with the distillate water at 25ºC ± 2. After 15 minutes they were suspended for 30 s to remove excess liquid and then re-weighed. The sorption capacity (g g-1) of water was calculated by the ratio of the mass (g) of the foam before and after the test. The test was realized in triplicate.
2.4 Caracterization
The percentage of cellulose, hemicellulose and lignin present in rice husk cellulose were quantified according to the TAPPI T222 om-02 (lignin) methodology and by the modified Van Soest method (cellulose and hemicellulose).
The apparent density of the foams was calculated by the mass (g) to volume (cm3) ratio, for five specimens.
The morphology was evaluated by using light microscopy in Meterk (China) optical microscope.
The infrared spectra of the foams were obtained on a Nicolet IS10 Thermo Scientific (USA) spectrometer using a universal attenuated total reflection (ATR) sampling accessory. The absorbance was measured in the spectral range of 700 and 4000 cm-1, with 32 scans and a resolution of 4 cm-1.
The thermal properties of the foams were evaluated by thermogravimetry (TG), by using a Shimadzu model TGA-50, with a heating rate of 10 °C min-1 and a heating flux of 30 to 800 °C under a nitrogen atmosphere (N2) at the rate of 50 mL min-1.
The thermal conductivity of the foams was determined according to the standard NBR 15220-5 (2003). Samples with 50 x 50 x 20 mm width, length and thickness, respectively, were used in a total of 2 samples per analysis. The test was performed in a plate system where the temperature difference between the hot and cold plates is controlled. The test was conducted according to the adaptation of standard NBR 15220-5 (2003). The heat flux applied in the system was determined by measurements with Thermax® rock wool (density 0.032 g cm-3), which has a known thermal conductivity (0.045 W m-1.K-1). The thermal conductivity was determined by the Fourier law.
3. Results and Discussion
3.1 Caracterization of rice husk
Rice husk is a fabric composed of three polymers: cellulose, hemicellulose and lignin. The composition of the rice husk showed a fraction of 41.16 ± 0.52% w/w cellulose, 14.0 ± 1.1% w/w hemicellulose and 19.02 ± 0.14% w/w lignin. Second McKendry1616 McKendry P. Energy production from biomass (part 1): overview of biomass. Bioresource Technology. 2002;83(1):37-46., cellulose and hemicellulose are present in a larger amount than lignin, unlike forest residues. Still, there may be a wide variation for each component. For instance, cellulose ranges from 28.6% to 41.5%, hemicellulose from 14.0% to 28.6% and lignin from 20.4% to 33.7%, the variation depend on climatic conditions, agronomic management, soil type, among other parameters11 Quispe I, Navia R, Kahhat R. Energy potential from rice husk through direct combustion and fast pyrolysis: A review. Waste Management. 2016;59:200-210..
In the pyrolysis process of this biomass the lignin is the main constituent of the biochar formed, becoming responsible for the adsorption process when the biochar is used for this application. Cellulose and hemicellulose are related to the low carbon content in the biochar, as they are responsible for the volatile fractions which enable the loss of a significant fraction of carbon during the pyrolysis process1717 Menya E, Olupot PW, Storz H, Lubwama M, Kiros Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chemical Engineering Research and Design. 2017;129:271-96..
The percentage of ash in the rice husk sample was 15.82 ± 0.08% w/w, whose cost is higher than in other biomasses. These values are close to other studies that also characterized rice husk, 14.8 %w/w for Abu Bakar and Titiloye1818 Bakar MSA, Titiloye JO. Catalytic pyrolysis of rice husk for bio-oil production. Journal of Analytical and Applied Pyrolysis. 2013;103:362-368. and 16.9 %w/w for Fang et al.1919 Fang M, Yang L, Chen G, Shi Z, Luo Z, Cen K. Experimental study on rice husk combustion in a circulating fluidized bed. Fuel Processing Technology. 2004;85(11):1273-1282.. These ashes have silicon dioxide (SiO2) as main component, which can be presented in: crystalline form or amorphous form, very useful for different applications. According to Quispe et al11 Quispe I, Navia R, Kahhat R. Energy potential from rice husk through direct combustion and fast pyrolysis: A review. Waste Management. 2016;59:200-210., the values of silicon dioxide in the ashes of rice husk vary from 87.4% to 91.4%. Amorphous silica is reactive and can be used to improve concrete properties, as well as being used as a cementitious additive for the solidification of hazardous waste. In addition to ash, the sample also presented 9.62 ± 0.57% w/w of moisture and 7.87 ± 0.39% w/w of extractives.
According to Diniz2020 Diniz J. Conversão térmica de casca de arroz à baixa temperatura: produção de bioóleo e resíduo sílico-carbonoso adsorvente [Tese]. Santa Maria: Universidade Federal de Santa Maria; 2005., the solid fraction obtained in the pyrolysis of the rice husk presents a high mineral content in comparison with other biomasses, in which approximately 40% of the coal obtained from the rice husk corresponds to inorganic material, mainly silica.
Figure 2 shows the FTIR spectrum of the rice husk and the attributions of the identified bands.
The band at 3320 cm-1 is attributed to OH stretching vibrations related to the cellulosic material or water, the bands at 1030 cm-1 can be attributed to CO, C = C and CCO related to cellulose, hemicellulose and lignin22 Lazzari E, Schena T, Caetano M, Marcelo A, Tatiane C, Nunes A, et al. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Industrial Crops and Products. 2018;111:856-64.,88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861..
The symmetrical and asymmetric stretching vibration bands seen between 3500 and 3000 cm-1 show the presence of moisture in the biomass structure and that the dehydration reactions occur during pyrolysis. In this region, there is also the presence of -OH groups, these stretch mainly due to the presence of silanols (Si-OH) that are adsorbed on the surface of the bark1717 Menya E, Olupot PW, Storz H, Lubwama M, Kiros Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chemical Engineering Research and Design. 2017;129:271-96.,1818 Bakar MSA, Titiloye JO. Catalytic pyrolysis of rice husk for bio-oil production. Journal of Analytical and Applied Pyrolysis. 2013;103:362-368..
Figure 3 shows the results for thermal analysis for the rice husk sample and the CA-1 (produced with 7.5 g yeast) and CA-2 (produced with 5.0 g yeast) samples.
Thermogravimetry and thermogravimetry derived rice husk. Caption: CA-1 (7.5 g yeast foam) and CA-2 (5.0 g yeast foam).
Analyzing Figure 3 it is possible to notice that mass loss occurs below 100 ºC, at which point the evaporation of water absorbed in the biomass structure takes place (8.4%). The main region of mass loss can be observed between 200 and 370 ºC, with a mass loss of 43.8%. For Ozsin & Putun2121 Özsin G. Insights into pyrolysis and co-pyrolysis of biomass and polystyrene : Thermochemical behaviors, kinetics and evolved gas analysis. Energy Conversion and Management. 2017;149:675-85. this region is called active pyrolysis with the release of organic and non-condensable gases. At 370 °C the mass loss is about 25.9%. This phase is also defined by the authors as passive pyrolysis, when a secondary decomposition of solid wastes occurs. In this region it is possible to observe two stages of mass loss, the first, from 200 to 300 ºC, corresponds to the degradation of the hemicellulose and part of the cellulose. In the second stage, from 300 °C onwards, lignin decomposes, showing a greater stability in relation to cellulose and hemicellulose.
According to Almeida1313 Almeida SR. Pirólise rápida de casca de arroz : estudo de parâmetros e caracterização de produtos [Dissertação]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2010., due to the shape of the thermogravimetric curve and the temperatures involved in the thermal degradation of the rice husk, it can be considered richer in cellulose than lignin. These pyrolysis characteristics can be explained by the degradation patterns of cellulose, hemicellulose and lignin and their overlapping zones. It is known that the decomposition of hemicellulose and cellulose occurs in the temperature ranges of 200-350 °C and 200-500 °C, respectively. On the other hand, the decomposition of lignin occurs at a rather slow rate in relation to hemicellulose and cellulose, which covers a broader temperature range of 150-900 °C1010 Basu P. Biomass Gasification and Pyrolysis: Pratical Design and Theory. Pittsburgh: Eselvier Inc.; 2010.,1818 Bakar MSA, Titiloye JO. Catalytic pyrolysis of rice husk for bio-oil production. Journal of Analytical and Applied Pyrolysis. 2013;103:362-368..
Several authors1212 Adhikari S, Nam H, Chakraborty JP. Conversion of Solid Wastes to Fuels and Chemicals Through Pyrolysis. In: Bhaskar T, Pandey A, Lee DJ, Khanal SK, editors. Waste Biorefinery: Potential and Perspectives. Amsterdam: Eselvier BV; 2018. p. 239-263.,1717 Menya E, Olupot PW, Storz H, Lubwama M, Kiros Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chemical Engineering Research and Design. 2017;129:271-96. also found this thermal degradation behavior for samples of rice husk. Since, in general, the total degradation of the biomass can be affected at temperatures between 600 and 800 °C. The residual mass (ashes) was approximately 22%, also in agreement with the other authors. According to Dunnigan et al.2222 Dunnigan L, Ashman PJ, Zhang X, Kwong CW. Production of biochar from rice husk : Particulate emissions from the combustion of raw pyrolysis volatiles. Journal of Cleaner Production. 2018;172:1639-1645., the high ash content in the rice husk (21.5%), causes a reduction in the carbon content present in the biochar (± 40%).
3.2 Rice husk and carbon foams
Figure 4 shows the images and optical micrographs of the foams produced from the rice husk.
Photographs: (a) CA-1, (c) CA-1P, (e) CA-2 and (g) CA-2P. Optical micrographs: (b) CA-1, (d) CA-1P, (e) CA-2 and (h) CA-2P. Caption: CA-1 (7.5 g yeast foam), CA-1P (CA-1 foam after pyrolysis), CA-2 (5.0 g yeast foam) and CA-1P (CA-2 foam after pyrolysis).
It is possible to visually verify that the formulations of the foams visually had an influence on the three-dimensional stiffness of the foams. With increasing amount of yeast in the CA-1 (Figure 4 (a)) and CA-1P foams (Figure 4 (c)) a foam with a larger pore structure was obtained in relation to the CA-2 foams (Figure 4 (e)) and CA-2P (Figure 4 (g)). Yuan et al.88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861. studied the influence of yeast concentration on carbon foams derived from wheat flour, and also observed the same structural behavior of the foams. According to the authors, yeast and water play a vital role during the formation of the pore structure. After adding water, the starches can be gelatinized and the proteins in the flour fully absorb the water to form a gluten network structure. Meanwhile, the yeast produces and releases carbon dioxide gases to form pores as it disperses in the paste.
After pyrolysis under an inert atmosphere (N2), the rice husk was transformed into carbon foam. The size of the foams reduces which was also observed by Yuan et al.88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861.. According to the authors, the original shape of the pore structure remains, but its size reduces by about 50% in volume, which can be observed by comparing Figures 4 (a) and (c) for the foams with 7.5 g of yeast and Figures 4 (e) and (g) for the foams with 5.0 g of yeast. This process comprises two reactions, involving dehydration and carbonization, in which condensable gases are released.
During the vapor deposition process of VTES, a silane film is formed on the surface of the foam, which does not alter its morphology and thermal and physical properties. After treatment, the hydrogen bonds formed at the interface act as a separation system, preventing the fiber from interacting with the water present in the medium2323 Salon MCB, Abdelmouleh M, Boufi S, Belgacem MN, Gandini A. Silane adsorption onto cellulose fibers : Hydrolysis and condensation reactions. Journal of Colloids and Interface Science. 2005;289(1):249-261..
In Figure 5, the apparent density results of the foams are presented. It is possible to verify that the variation in the amount of yeast used does interfere in the specific mass, that is to say, the lower the amount of yeast, the higher is the specific mass. This happen, because there is a smaller formation of pores in the structure.
Apparent density of foams. Caption: CA-1 (7.5 g yeast foam), CA-1P (CA-1 foam after pyrolysis), CA-2 (5.0 g yeast foam) and CA-1P (CA-2 foam after pyrolysis).
It regards to the flame test conducted with the foams (before and after pyrolysis), which was presented visually through Figure 1, it is possible to relate it to the thermal analysis presented and discussed in Figure 3. The carbon foams present only one mass loss event below 100 °C due to the moisture present in the sample. After that, the mass loss ratio becomes constant, which shows that the organic matter (cellulose, hemicellulose and lignin) present in the rice husk was degraded during the pyrolysis process. The residual mass of the carbon foams was about 90%, while the residual mass of the rice husk foam is approximately 20%. This result shows that carbon foams have a higher thermal stability than rice husk foams, which is proved when the foams are exposed to direct flames. In the flame test (Figure 1) it was possible to visually perceive that the carbon foam does not burn when in contact with the flame. This is due to the pyrolysis carried out on rice husk foam, in which much of the organic matter present in the foam is carbonized, serving as an anti-flame treatment. The rice husk foam, on the other hand, when exposed to direct flame, burns completely.
The result found for the water contact angle test of the foams is presented in Table 1.
Table 1 shows that the CA-1 foam absorbs the water drop momentarily which prevents the contact angle from being measured. On the other hand, for the CA-2 foam, it was possible to measure the contact angle of water, because the structure had smaller pores, the water droplet remained under the surface for a few minutes and was then absorbed by the foam. This results shows that the two foams are hydrophilic, that is, have an affinity with water, thus justifying the need to perform chemical (vapor deposition) or thermal (pyrolysis) treatment for the modification of this characteristic.
By evaluating the treated surface foams, CA-1H and CA-2H were more efficient than CA-1P and CA-2P, especially in foams with the lowest amount of yeast used (CA-2H). However, both treatments were efficient for the hydrophobization of the foams because in all cases the contact angle was higher than 90º, thus classifying the foams as hydrophobic2424 Aegerter MA, Leventis N, Koebel MM. Aerogels Handbook. New York: Springer-Verlag; 2011..
The hydrophobicity demonstrated by the contact angle is corroborated by the water sorption capacity test of the foams, shown in Figure 6. The CA-1 and CA-2 foams have a higher water sorption capacity (2.14 and 2.57 g g-1, respectively) than the others, precisely because they do not have a hydrophobic surface, thus having more affinity with water.
The water sorption capacity of the surface treated foams did not show any difference between the chemical and thermal treatments. Even with the highest water contact angle, the CA-2H foam presented sorption capacity close to the other treated foams, about 0.5 g of water sorbed per gram of foam. This test shows that if exposed to a humid or rainy environment, heat treated or chemically treated foams would draw a lower amount of water than the wood foams, and thus delay degradation.
Figure 7 shows the thermal conductivity results of the rice husk and carbon foams. Formulation 1 foams (CA-1 and CA-1P) presented lower thermal conductivity than CA-2 and CA-2P foams, because CA-1 and CA-1P foams have larger pores (Figure 4) due to the higher amount of yeast used in this formulation. In comparing the rice husk and carbon foams, the rice husk foams presented lower thermal conductivity than the carbon foams, because after the pyrolysis process the amount of carbon present in the foams is higher, which causes the thermal conductivity.
Thermal conductivity of foams. Caption: CA-1 (7.5 g yeast foam), CA-1P (CA-1 foam after pyrolysis), CA-2 (5.0 g yeast foam) and CA-1P (CA-2 foam after pyrolysis).
The foams produced by Yuan et al.88 Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces. 2016;8(26):16852-16861., with a specific mass of 0.09 - 0.23 g cm-3, presented thermal conductivity varying from 0.08 to 0.17 W m-1 K-1. According to the authors, after pyrolysis, the structure of the foams has less impurities. At the same time the carbon structure is aligned. Therefore, fewer defects and edges in the sample contribute to a higher heat transfer.
4. Conclusion
The carbon foams produced from the rice husk showed similar thermal conductivity to materials used commercially for thermal insulation. The methodology adopted in this study uses a combination of simple (agricultural residue and wheat flour) and environmentally friendly elements. In addition, it is, an easy process that does not require the use of reagents toxic to the environment. Considering that they have been resistant to direct flame, which makes them a potential anti-flame material preventing the spread of fire. In the comparison between the two methods of hydrophobizing rice husk foams (pyrolysis and vapor deposition of VTES), both treatments were positive regarding the modification of the hydrophilic to hydrophobic characteristic of the rice husk foams surface. contact greater than 90°. Besides that, carbon foams have the same ability to repel water/moisture as chemically treated foams. Thus, with the results found for carbon foams there are strong reasons to believe that the alternative product analyzed in this study is promising from a thermal insulation perspective.
5. Acknowledgments
The authors would like to express their gratitude to the Federal University of Rio Grande do Sul (UFRGS), the University of Caxias do Sul (UCS), the National Council for Scientific and Technological Development (CNPq) and the Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS ).
6. References
-
1Quispe I, Navia R, Kahhat R. Energy potential from rice husk through direct combustion and fast pyrolysis: A review. Waste Management 2016;59:200-210.
-
2Lazzari E, Schena T, Caetano M, Marcelo A, Tatiane C, Nunes A, et al. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Industrial Crops and Products 2018;111:856-64.
-
3António J, Tadeu A, Marques B, Almeida JAS, Pinto V. Application of rice husk in the development of new composite boards. Construction and Building Materials 2018;176:432-439.
-
4Zhang G, Chen Y, Chen Y, Guo H. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Materials Research Bulletin 2018;102:391-398.
-
5Bakierska M, Molenda M, Majda D, Dziembaj R. Functional starch based carbon aerogels for energy applications. Procedia Engineering 2014;98:14-19.
-
6Tijani MM, Aqsha A, Mahinpey N. Development of oil-spill sorbent from straw biomass waste : Experiments and modeling studies. Journal of Environmental Management 2016;171:166-176.
-
7Feng J, Nguyen ST, Fan Z, Duong HM. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chemical Engineering Journal 2015;270:168-175.
-
8Ye Y, Ding Y, Wang C, Xu F, Lin Z, Qin Y, et al. Multifunctional Stiff Carbon Foam Derived from Bread Multifunctional Stiff Carbon Foam Derived from Bread. ACS Applied Materials and Interfaces 2016;8(26):16852-16861.
-
9Jana P, Fierro V, Pizzi A, Celzard A. Biomass-derived, thermally conducting, carbon foams for seasonal thermal storage. Biomass and Bioenergy 2014;67:312-318.
-
10Basu P. Biomass Gasification and Pyrolysis: Pratical Design and Theory Pittsburgh: Eselvier Inc.; 2010.
-
11Gallego NC, Klett JW. Carbon foams for thermal management. Carbon 2003;41(7):1461-466.
-
12Adhikari S, Nam H, Chakraborty JP. Conversion of Solid Wastes to Fuels and Chemicals Through Pyrolysis. In: Bhaskar T, Pandey A, Lee DJ, Khanal SK, editors. Waste Biorefinery: Potential and Perspectives Amsterdam: Eselvier BV; 2018. p. 239-263.
-
13Almeida SR. Pirólise rápida de casca de arroz : estudo de parâmetros e caracterização de produtos [Dissertação]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2010.
-
14Perondi D, Polleto P, Restelatto D, Manera C, Silva JP, Junges J, et al. Steam gasification of poultry litter biochar for bio-syngas production. Process Safety and Environmental Protection 2017;109:478-488.
-
15Lazzari LK, Zampieri VB, Zanini M, Zattera AJ, Baldasso C. Sorption capacity of hydrophobic cellulose cryogels silanized by two different methods. Cellulose 2017;24(8):3421-3431.
-
16McKendry P. Energy production from biomass (part 1): overview of biomass. Bioresource Technology 2002;83(1):37-46.
-
17Menya E, Olupot PW, Storz H, Lubwama M, Kiros Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chemical Engineering Research and Design 2017;129:271-96.
-
18Bakar MSA, Titiloye JO. Catalytic pyrolysis of rice husk for bio-oil production. Journal of Analytical and Applied Pyrolysis 2013;103:362-368.
-
19Fang M, Yang L, Chen G, Shi Z, Luo Z, Cen K. Experimental study on rice husk combustion in a circulating fluidized bed. Fuel Processing Technology 2004;85(11):1273-1282.
-
20Diniz J. Conversão térmica de casca de arroz à baixa temperatura: produção de bioóleo e resíduo sílico-carbonoso adsorvente [Tese]. Santa Maria: Universidade Federal de Santa Maria; 2005.
-
21Özsin G. Insights into pyrolysis and co-pyrolysis of biomass and polystyrene : Thermochemical behaviors, kinetics and evolved gas analysis. Energy Conversion and Management 2017;149:675-85.
-
22Dunnigan L, Ashman PJ, Zhang X, Kwong CW. Production of biochar from rice husk : Particulate emissions from the combustion of raw pyrolysis volatiles. Journal of Cleaner Production 2018;172:1639-1645.
-
23Salon MCB, Abdelmouleh M, Boufi S, Belgacem MN, Gandini A. Silane adsorption onto cellulose fibers : Hydrolysis and condensation reactions. Journal of Colloids and Interface Science 2005;289(1):249-261.
-
24Aegerter MA, Leventis N, Koebel MM. Aerogels Handbook New York: Springer-Verlag; 2011.
Publication Dates
-
Publication in this collection
11 Nov 2019 -
Date of issue
2019
History
-
Received
12 July 2019 -
Reviewed
19 Sept 2019 -
Accepted
25 Sept 2019