Abstracts
Among warm-water marine fishes, cobia is one of the best aquaculture candidate species in the world. Currently there are commercial culture operations in several Asian countries and the industry has started developing elsewhere, including the Western Central Atlantic region. Significant research has been conducted at the University of Miami's Aquaculture Program / University of Miami Experimental Hatchery (UMEH) during the last eight years, involving research to develop and optimize advanced technology to demonstrate the viability of raising hatchery-reared cobia in collaboration with the private sector. This paper reviews some of this recent advances for the development of Hatchery-to-Market Aquaculture Technology for commercial production of cobia.
cobia; commercial aquaculture; research; sea cages
Dentre os peixes marinhos de águas quentes, o bijupirá é um dos grandes candidatos para a aquacultura no mundo. Atualmente, existem operações comerciais em vários países Asiáticos e a indústria iniciou suas operações em outros locais, incluindo a região do Atlântico Central. Pesquisas têm sido realizadas no "University of Miami's Aquaculture Program / University of Miami Experimental Hatchery (UMEH)" durante os últimos oito anos envolvendo o desenvolvimento e otimização de tecnologia avançada para demonstrar a viabilidade da criação de bijupirá com colaboração com o setor privado. Este artigo revisa alguns destes avanços recentes para o desenvolvimento da tecnologia da larvicultura para o mercado para a produção comercial de bijupirá.
aquaculture commercial; bijupirá; gaiolas marinhas; pesquisa
AQUACULTURE
Daniel Benetti; Bruno Sardenberg; Ron Hoenig; Aaron Welch; John Stieglitz; Sasa Miralao; Daniel Farkas; Patrick Brown; Darryl Jory
University of Miami - Rosenstiel School of Marine and Atmospheric Science Aquaculture Program, 4600 Rickenbacker Causeway, Miami, FL 33149 USA
ABSTRACT
Among warm-water marine fishes, cobia is one of the best aquaculture candidate species in the world. Currently there are commercial culture operations in several Asian countries and the industry has started developing elsewhere, including the Western Central Atlantic region. Significant research has been conducted at the University of Miami's Aquaculture Program / University of Miami Experimental Hatchery (UMEH) during the last eight years, involving research to develop and optimize advanced technology to demonstrate the viability of raising hatchery-reared cobia in collaboration with the private sector. This paper reviews some of this recent advances for the development of Hatchery-to-Market Aquaculture Technology for commercial production of cobia.
Key Words: cobia, commercial aquaculture, research, sea cages
RESUMO
Dentre os peixes marinhos de águas quentes, o bijupirá é um dos grandes candidatos para a aquacultura no mundo. Atualmente, existem operações comerciais em vários países Asiáticos e a indústria iniciou suas operações em outros locais, incluindo a região do Atlântico Central. Pesquisas têm sido realizadas no "University of Miami's Aquaculture Program / University of Miami Experimental Hatchery (UMEH)" durante os últimos oito anos envolvendo o desenvolvimento e otimização de tecnologia avançada para demonstrar a viabilidade da criação de bijupirá com colaboração com o setor privado. Este artigo revisa alguns destes avanços recentes para o desenvolvimento da tecnologia da larvicultura para o mercado para a produção comercial de bijupirá.
Palavras-chave: aquaculture commercial, bijupirá, gaiolas marinhas, pesquisa
Introduction
Cobia,Rachycentron canadum, is considered one of the most promising candidates for warm-water marine fish aquaculture in the world (Kaiser & Holt, 2004; Liao et al., 2004; Benetti et al., 2007, 2008, in press ). Cobia, is the only member of the family Rachycentridae; also known as lemonfish or ling, it is widely distributed in the warm-temperate to tropical waters of the West and East Atlantic, throughout the Caribbean and in the Indo-Pacific off India, Australia and Japan (Briggs, 1960; Hassler & Rainville, 1975; Shaffer & Nakamura, 1989; Ditty & Shaw, 1992). In the Eastern Pacific its occurrence has been reported as marginal (Briggs, 1960; Collette, 1999). Cobia are euryhaline and eurythermale, and can tolerate temperature and salinity ranges of 16.8 - 32.2 C and 5 - 44.5 ppt, respectively (Shaffer & Nakamura, 1989; Resley et al., 2006).
Cobia aquaculture in cages began in the early 1990's in Taiwan (Yeh, 2000; Liao et al., 2004), and by 1997 the development of technology for mass production of juveniles supported the expansion of the cage culture industry in Taiwan (Yeh et al., 1998). By 1999, four commercial hatcheries were operating in Taiwan, producing around 3 million cobia juveniles annually (Yeh, 2000). Since then, cobia aquaculture production has been steadily expanding in Asia, primarily in Taiwan, Vietnam and China, and in other Southeast and Indo-Pacific Asian countries including the Philippines, Indonesia, Iran and Reunion Island. During the last decade the industry has started a fast development trend in tropical and subtropical regions throughout the world. Most recently, Australia and the Marshall Islands have begun developing cobia aquaculture and it is also expanding in the Americas and the Caribbean. Current commercial project locations include the United States, Puerto Rico, the Bahamas, Belize, the Dominican Republic, Mexico, Panama, and Brazil (Benetti et al., 2007; Holt et al., 2007). Floating cages continue to be the preferred method of growout worldwide. Although production is expanding rapidly, combined production of Asian countries is still rather low, on the order of tens of thousands of metric tons per year (Liao et al., 2004).
In the Western Hemisphere, cobia aquaculture is a promising industry. Early attempts to culture cobia in the US began in the 1970s using eggs collected from the wild (Hassler & Rainville, 1975). Research accelerated in the late 1990s with the first successful spawns obtained from captive broodstock fish (Arnold et al., 2002) and today a large body of knowledge on cobia culture in the United States continues to be produced (Benetti et al., 2008a, b, in press).
During the last eight years, the University of Miami's Aquaculture Program / University of Miami Experimental Hatchery (UMEH) has been involved in basic and applied research to develop advanced technology that has been used to demonstrate the viability of raising hatchery-reared cobia in collaboration with the private sector (Snapperfarm, Inc. and AquaSense LLC) using SeaStation (Net Systems LLC) and Aquapod (Ocean Technologies LLC) submerged cages in exposed sites in Puerto Rico, US (PR) and the Bahamas (BA). Hatchery-reared cobia are also being cultured in traditional gravity cages in Belize, Panama, Brazil, Dominican Republic, Martinique, and Mexico (Benetti et al., 2008a), and in tanks in Bonaire and elsewhere. In this paper we review some recent advances for the development of Hatchery-to-Market Aquaculture Technology for commercial production of cobia.
Selective breeding & Broodstock management
The ability to use artificial control of water temperature to induce year-round volitional spawning of cobia will allow aquaculture production facilities access to viable cobia eggs uninterruptedly, enabling continuous production of this species with no seasonal constrains (Benetti, 2010). The University of Miami Experimental Hatchery (UMEH) has developed a cobia breeding program aimed at year-round control of the spawning cycle. Through implementation of a superior broodstock nutrition program and manipulation of water temperature, on and off-season volitional spawning of cobia were continuously induced from April 2008 - December 2009.
Temperatures in the maturation tank were maintained between 27 - 29ºC for the period between April 2008 and April 2009, mimicking the natural conditions during the cobia spawning season. There were 17 spawning events during the off-season between October 27, 2008 and April 3, 2009. We observed a slight decrease in fertilization was observed for the eggs produced from the off-season spawns, as fertilization rates in the off-season averaged 89.48% (± 12.95%) compared to on-season fertilization rates, which averaged 93.97% (± 6.32%), but this decrease was not significant. To the best of our knowledge, this is the first time these methods have been successfully applied to this species in the Western Hemisphere. Using the same protocol, this study was continued from April 3, to December 16, 2009 and produced an additional 28 natural spawns. Fertilization rates during this period were similar on the on-season, 92.13% (±5.59%), and off-season, 92.07% (± 2.38%). From April 2009 - December 2009 UMEH produced over 60 million fertilized cobia eggs. A total of 108 on and off-season volitional spawns of cobia were obtained in a semi-recirculating conditioning maturation system at UMEH from April 2008 - December 2009 resulting in the production of over 200 million fertilized eggs, which were used for larval rearing trials and shipped to several academic and research institutions as well as the private sector for experimental and production trials (Benetti, 2010).
Larval rearing and fingerling production
Reliable fingerling production is the main bottleneck for commercial aquaculture of most high value marine species. The production levels for cobia seedstock we have achieved are adequate to support the development of commercial grow-out operations and can be improved if demand increases, indicating that cobia aquaculture can be viable in the Americas and the Caribbean in the next few years. In 2009, approximately 70% of the cobia fingerlings produced at UMEH were shipped to commercial companies like Snapperfarm and other private companies in the Caribbean and Latin America for grow-out in cages. The remaining 30% were retained for shipping and salinity trails. During the past years, we shipped eggs, larvae, fingerlings, juveniles and broodstock to numerous universities, institutions and private companies, supporting research and the development of the aquaculture industry in the US and abroad.
We have produced several hundred thousand cobia fingerlings at UMEH through innovative research on early developmental stages and based on a proactive health management strategy including the use of probiotics, prophylaxis and improved nutrition of live feeds.
Some of our current research on larval rearing and nursery stages (until 35 days post hatch - DPH) uses ultra-violet filtered flow-through seawater filtered down to 10 µm prior to entering the tanks, and ranging in temperature from 26 to 33ºC. We have increased daily water turnover rates from 100-600%, to 300 - 1,500%, and rotifers (Brachionus plicatilis) and Artemia sp concentrations from 5/ml and 0.1-1.0/ml to 5-10/ml and 0.5 - 2/ml, respectively.
We have developed standard protocols for microalgae (Isochrysis galbana and Nannochloropsis oculata) concentrations (@ 10,000 cells/ml) and live feeds feeding frequency. Cobia larvae are fed "ad libitum" using a "pulse feeding" technique, with feeds being added 3-5 times a day as needed to maintain desired concentrations. Microalgae and rotifers are added between 2 and 10 days post-hatch (DPH). We typically wean out 100% of the post-larvae onto starting diets (Otohime and Gemma) at 23 DPH, but Artemia is continually offered through an extended period ranging from 7 - 28 DPH instead of 7 - 22 DPH. During this stage, survival rates as high as 90% are achieved. Survival rates ranging from 15.11 - 38.63% were achieved from egg to shipping size fingerlings (0.8-2.5 grams) in 2009.
In addition to careful monitoring, cleaning routine, adequate nutrition (enriched rotifers and Artemia), prophylaxis (50-75 ppm formalin bath for one hour as needed) and probiotics (Ecomicrobials @ 100 ppm in the live feeds prior to feeding the larvae), the higher concentrations of live feeds and water exchange ratios, combined with an extended Artemia period, were critical for the higher survival rates and excellent health of the 204,073 fingerlings produced during 2009 (Table 1; Benetti, 2010).
We have been working to improve methods for reliable fingerling production of cobia since 2004 (Benetti et al., 2007) and recently published these results Benetti et al. (2008b) discussed methods and results of two cobia larval rearing trials designed to test the efûcacy of protocols developed over several years of research in cobia larviculture at the UMEH. The protocols incorporate the use of probiotics and prophylaxis, minimize microalgae use, and include various commercially available ingredients for live feed enrichment. In these trials, fertilized eggs were stocked at 400/L and incubated in 1000-L cylinder-conical tanks with flow-through seawater at 500% daily exchange rate. Moderate aeration and pure oxygen were used to maintain dissolved oxygen concentrations above saturation (6.5 mg/L at 26°C). Hatching occurred at 22-24 h post fertilization. Two day-post-hatch (dph) yolk-sac larvae were stocked in four 12,000-L cylinder-conical tanks at 5 and 10 larvae/L. Beginning on 3 dph, larvae were fed microalgae (Isochyrsis galbana C-strain) at low concentrations (5-10,000 cell/ml) and enriched rotifers (Brachionus plicatilis) at 3-5/mL through 9 dph. Beginning on 7 dph, enriched Artemia (Artemia franciscana GSL Strain) nauplii were fed to larvae at rates of 0.1-1/mL.
These cobia larvae were reared at water temperatures ranging from 24.3 - 31.8 °C. Water quality parameters were within normal ranges for seawater: salinity 26-34 ppt, pH 7.92-8.16, and NH 3 b 0.18 mg/L. Vigorous aeration and supplemental oxygen were applied continuously during both larval rearing trials to maintain adequate water movement and levels of dissolved oxygen (DO) (7.0-9.0 mg/L). Water was filtered down to 10 μm using standard sand filters filled with broken glass media and bag filters prior to filling the tanks. Daily water exchange rates in tanks from 17 dph onwards ranged from 100% at 3 dph to 500%. Between 20-22 dph, all post-larvae were fully weaned onto dry starting diets. Survival rates of post-larvae measuring 1.5-2.0 cm SL and weighing 0.5 g at 20-22 dph were estimated to be > 50%. Further mortality during the nursery stage to 3-5 cm and 1-3 g fingerlings prior to shipping at 27 dph brought the overall survival rate to an average of 25.7%. Survival rates of fingerlings cultured in tanks initially stocked at lower densities (5 larvae/L) was significantly higher (P =0.0078). From 15 dph, post-larvae and fingerlings were graded by size daily, and large individuals were singled out and stocked into another tank. These trials produced 125,328 fingerlings in four tanks in just two months; this is sufficient production to support a commercial operation and indicate that cobia aquaculture can be viable in the Americas (Benetti et al., 2008).
Health management
Routine water quality testing and husbandry procedures are performed by the hatchery staff at UMEH
We strictly follow strict protocols for health management of fish, including disinfection of eggs, larvae and broodstock fish, feeding schedule, and management and enrichment of rotifers and Artemia with essential nutrients. Prophylactic methods (fresh water and formalin dip with Paracide-F) are used to prevent and control diseases at the hatchery. All fish are given a prophylaxis treatment with (100 ppm/1 hour) every 30-45 days, even if they do not look injured or present any sign of pathogens. Formalin and freshwater dips are effective against skin and gill parasites (ectoparasites). In addition, the fish are disinfected using iodine compound, according to the recommendation of the International Zoosanitary Code.
Routine data acquisition includes quantitative measures of water quality parameters, as dissolved oxygen, temperature, total ammonia, nitrite, nitrate, pH, feeds administered as well as qualitative descriptions of observations made during the servicing period, specially for any signs of pathogens and fish behavior deviation. These procedures are daily performed and the information is recorded on the appropriate data sheets.
Probiotics
We routinely used probiotics in our hatchery protocols, with significant success. Several peer-reviewed and technical articles have been recently published about our research on this topic.
Nodavirus surveillance program
Together with veterinarians at the University of Miami School of Medicine (Department of Veterinary Resources, UM/SM/DVR), we have jointly developed a program to prevent and detect nodavirus (also known as Viral Nervous Necrosis Virus or VNNV) infection in cultured cobia at our production colony. Morbid fish have been routinely tested negative since April 2008 and consistently found to be negative for infection. This protocol outlines a surveillance plan to identify and monitor this closed colony in the event of nodaviral introduction. In the event of fish morbidity, a licensed UM/SM/DVR veterinarian performs a standard diagnostic work-up. If clinical signs are consistent with nodaviral infection, samples from morbid fish also are submitted for viral examination
Health protocols prior to shipment
A licensed UM/SM/DVR veterinarian examines cobia fingerlings, and fish are only shipped if they are found to be in good health, specifically for the following:
The fish have no clinical signs, diseases or lesions, including those included in list of the World Organization for Animal Health (OIE), to which this species may be susceptible.
VNN, KHV, RUS and white spot syndrome are not observed.
These diseases have not been observed during the last twelve months prior the shipment: erythrodermatitis (Aeromonas salmonicida); spring viraemia of carps; yersiniosis; viral hemorrhagic septicaemia; and infectious hematopoietic necrosis.
In addition, the high quality cobia fingerlings shipped are held solely at UMEH since hatching (i.e. in excess of thirty days prior to shipment) and do not come into contact with any newly introduced animals. To prevent parasites infection during the 15 days prior to shipment, fingerlings are given a prophylaxis formalin dip with the FDA-approved Paracide-F (37% formaldehyde). Fingerlings are shipped only if on the day of shipment are in good health, free of signs of infectious and contagious diseases, free of ectoparasites, and show no fresh wound or healing wounds.
Certifications
UMEH is certified with the State of Florida Department of Agriculture and Consumer Services Aquaculture Certificate, the Fish and Wild Life Import and Export License (U.S. Fish and Wildlife Service) and the Institutional Animal Care and Use Committee (IACUC). It also has permits to export fish to Belize, Panama, Bahamas and Colombia. All export permitting process are conducted with local sanitary authorities and follows strict guidelines.
Ongoing research
We are studying the use of hydrogen peroxide as a therapeutic. Initial studies have been conducted to determine the lethal dose (LD50) of hydrogen peroxide. Initial results are promising and indicate that cobia can withstand doses sufficient to have therapeutic value. Once an LD50 dosage is established, we will carry out additional tests to determine the efficacy of hydrogen peroxide against Amyloodinium in cobia.
The U.S. Food and Drug Administration (FDA) recently approved 35% PEROX-AID (Eka Chemicals, Marietta, Georgia), for use in aquaculture as a treatment for external parasites, bacteria, and fungi. PEROX-AID is a hydrogen peroxide product, with 35% active ingredient. Hydrogen peroxide has been reported to be an effective agent against Amyloodinium, a common pathogen in cobia culture systems (Brock et al., 2001). Because hydrogen peroxide breaks down into hydrogen and water over time, it is an environmentally sensitive alternative to the more persistent therapeutics (like copper) often used to deal with Amyloodinium and other diseases.
Shipping of live animals and eggs
We have developed successful shipping methods for different life stages of cobia, with survival rates exceeding 95% for shipments lasting 24-36 h. This ability has allowed us to transfer the products of our cobia research, such as eggs, larvae, and fully-weaned fingerlings, to institutions and private companies throughout the world. We have also developed a bioeconomic model to determine optimal shipping densities based on the input variables of average fish weight (g) and per-box shipping costs. The use of this bioeconomic model to optimize shipping practices at UMEH will improve informed decision-making and economical shipping practices (Benetti, 2010).
Recent research has focused on the optimization of shipping methods for live cobia fingerlings (1-5 g in weight). Experiments investigating the tolerance of juvenile cobia to abrupt transfers of salinity were conducted on multiple batches of juvenile fish, which emanated from successful larval rearing trials. The fish were transferred directly from flow-through holding tanks at an average salinity of 35 ppt to treatment tanks of varying salinities (0 ppt to 55 ppt). During the 24-h long trials, juvenile cobia exhibited tolerance to a wide range of salinities, with 100% survival in salinities from 11 ppt to 45 ppt. Additional salinity preference experiments were conducted using a salinity gradient to determine the salinity preference of cobia acclimated to hyper and hypo-osmotic conditions. Afterwards, simulated shipping trials were conducted to test the effects of shipping fish at varying packing densities (5 kg/m3 to 20 kg/m3) in two different water salinities (35 ppt and 12 ppt) over a period of 24.5 hours. Results show a significant increase in survival in juvenile cobia shipped at 12 ppt (iso-osmotic) water salinity across all packing densities.
Growout
We have comprehensively studied growth rates of hatchery-reared cobia cultured in submersible cages off Puerto Rico and the Bahamas and presented, discussed and compared to those of other teleosts (Table 2). Growth rates of hatchery-reared cobia Rachycentron canadum cultured in submersible cages off Puerto Rico and the Bahamas were comprehensively studied and are presented, discussed and compared to those of other teleosts (Benetti et al., in press).
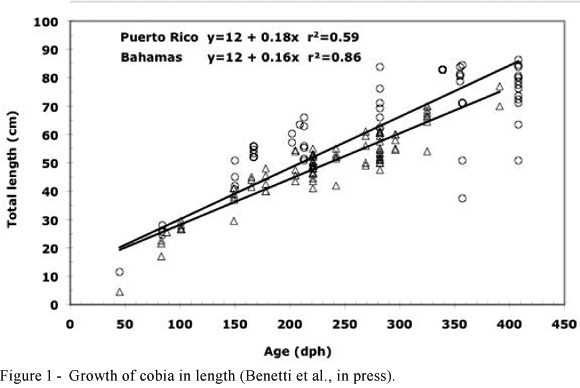
The fish stocked at the warmer, lower density Puerto Rico site grew faster than those at the cooler, higher density Bahamas site. Cobia grew to averages of 6.035 kg (Specific Growth Rate (SGR) = 2.10%/day) in 363 days at the Puerto Rico site (PR) and 3.545 kg (SGR = 2.04%/day) in 346 days at the Bahamas site (BA). Results of cobia growth in length and weight are expressed in Figures 1 and 2, respectively. Fish in the two cages had similar length-weight relationships. Growth in length is best expressed by the equations: y = 12 + 0.18x; r2 = 0.59 at PR and y = 12 + 0.16x; r2 = 0.86 at BA. The Laird-Gompertz model was used to represent growth in weight to best express the rate of decline in growth rate with age (a = 0.006194 PR and a = 0.006323 BA), which occurred at the onset of precocious maturation for this species at 2.0-4.5 kg in 300 days post hatch (dph). The exponents (b) of length-weight relationships calculated (3.31 at PR and 3.20 at BA) demonstrate that cultured cobia exhibit greater condition factors than their wild counterparts (b = 2.8) and explain the morphological differences observed between wild and culture cobia. Although the Laird-Gompertz model is generally used to express growth rates in weight of early developmental stages of fish (Kramer and Zweiffel, 1970; Cushing, 1995), this model best fitted the data presented to show the inflexion point during the onset of maturation, at approximately 10 months of age (300 dph). Indeed, the cobia were observed to engage in courtship behavior and began spawning naturally in the cages starting at approximately 10 months of age, by which time there was a noticeable decrease in growth rates (Figure 2).
The final stocking densities in the two cages were approximately 5 kg/m3 in the Puerto Rico cage and 15 kg/m3 in the Bahamas cage. Average water temperatures were 27.8 and 25.5ºC at the Puerto Rico and Bahamas sites, respectively. Salinities at the two sites averaged 34.6 ppt and 37.2 ppt, respectively and dissolved oxygen, 5.3 mg/L and 6.1 mg/L, respectively. Results showed that the growth rates of cobia can vary widely and imply a negative effect of lower temperature and increasing stocking density. These trials also demonstrated, for the first time, that growout of cobia is technically feasible in submerged open ocean cages.
Economic performance modelling
Together with a Ph.D. candidate in Operations Research at the Georgia Institute of Technology, we are developing a model for assessing the economic viability of offshore cobia aquaculture in the United States. The model is based on the Network Flow Optimization approach that has been used to model large-scale shrimp production (Yu & Leung, 2005).
Initial results from modeling efforts have provided valuable insights, and a complete analysis will be provided on the final report. Generally, our research indicates that offshore cobia aquaculture can be profitable, given economy of scale, suitably sited farms, and warm water conditions (Benetti, 2010).
Conclusions
Cobia, Rachycentron canadum, is considered one of the most promising candidates for warm-water marine fish aquaculture in the world because of its excellent characteristics, a growing market, and because it is highly adaptable and tolerant of a wide range of environmental and culture conditions.
Cobia aquaculture production is well established and is steadily expanding in Asia, primarily in Taiwan, Vietnam and China, and in other Southeast and Indo-Pacific Asian countries including the Philippines, Indonesia, Iran and Reunion Island. In recent years it also began developing in the Americas and the Caribbean. Current commercial project locations include the United States, Puerto Rico, the Bahamas, Belize, the Dominican Republic, Mexico, Panama, and Brazil.
During the last eight years, the University of Miami's Aquaculture Program / University of Miami Experimental Hatchery (UMEH) has been involved in basic and applied research to develop Hatchery-to-Market Aquaculture Technology for commercial production of cobia. This technology is widely applied, and further improvement research is ongoing. Commercial cobia aquaculture has a bright future and will likely become a very significant industry in the Western Central Atlantic, particularly in countries like Brazil that have exceptional culture conditions for warm-water fish culture along its long coastline, as well as excellent logistics and established ancillary industries.
Acknowledgments
UMEH research has been supported by NOAA Grant NA08OAR4170826, including partial or total support for Graduate Students/Research Assistants Aaron Welch, Ronald Hoenig, John Stieglitz, and partial funding for Research Associate Bruno Sardenberg.
Corresponding author: dbenetti@rsmas.miami.edu
- BENETTI, D.D. Continuing and advancing the development of cobia (Rachycentron canadum) aquaculture technology from hatchery to market Progress Report NOAA Grant NA08OAR4170826. 2010. 14p.
- BENETTI, D.D.; ORHUN, M.R.; ZINK, I. et al. Aquaculture of cobia (Rachycentron canadum) in the Americas and the Caribbean. In: LIAO, I.C.; LEAÑO, E.M. (Eds.) Cobia aquaculture: research, development and commercial production. Manila, Philippines: Asian Fisheries Society; Louisiana: World Aquaculture Society; Keelung, Taiwan: The Fisheries Society of Taiwan; and Keelung, Taiwan: National Taiwan Ocean University, 2007. p.57-78.
- BENETTI, D.D.; ORHUN, M.R.; SARDENBERG, B. et al. Advances in hatchery and grow-out technology of cobia Rachycentron canadum (Linnaeus). Aquaculture Research, v.39, p.701-711, 2008a.
- BENETTI, D.D.; SARDENBERG, B.; WELCH, A. et al. Intensive larval husbandry and fingerling production of cobia Rachycentron canadum Aquaculture, v.281, p.22-27, 2008b.
- BENETTI, D.D.; O'HANLON, B.; RIVERA, J.A. et al. Growth rates of cobia (Rachycentron canadum) in open ocean cages in the Caribbean. Aquaculture (in press)
- BRIGGS, J.C. Fishes of world-wide (circumtropical) distribution. Copeia, v.3, p.171-180, 1960.
- BROCK, D.; SATO, V.; BROCK, J. et al. The application of hydrogen peroxide as a treatment for the ectoparasite Amyloodinium oceallatum (Brown 1931) on the pacific threadfin Polydactylus sexfilis Journal of the World Aquaculture Society, v.32, n.2, p.250-254, 2001.
- COLLETTE, B.B., RACHYCENTRIDAE. In: CARPENTER, K.E.; NIEM, V.H. (Eds.) The living marine resources of the western central pacific Bony fishes part 2 (Mugilidae to Carangidae). Rome: FAO, 1999. v.4.
- CUSHING, D.H. Population production and regulation in the sea: a fisheries perspective. Cambridge: University Press, 1995.
- DITTY, J.G.; SHAW, R.F. Larval development, distribution, and ecology of cobia Rachycentron canadum (family: Rachycentridae) in the northern Gulf of Mexico. Fishery Bulletim, v.90, n.4, p.668-677, 1992.
- HASSLER, W.W.; RAINVILLE. R.P. Techniques for hatching and rearing cobia, Rachycentron canadum, through larval and juvenile stages Univ. N.C. Sea Grant Prog. UNC-SG-75-30. 1975.
- HOLT, G.J.; FAULK, C.K.; SCHWARZ, M.H. A review of the larviculture of cobia, Rachycentron canadum, a warm water marine fish. Aquaculture, v.268, p.181-187, 2007.
- KRAMER, D.; ZWEIFEL, J.R. Growth of anchovy larvae (Engraulis mordax Girard) in the laboratory as influenced by temperature. CCOFI Report, v.14, p.84-87, 1970.
- LIAO, I.; HUANG, T.; TSAI, W. et al. Cobia culture in Taiwan: current status and problems. Aquaculture, v.237, n.1-4, p.155-165, 2004.
- RESLEY, M.; WEBB, K.; HOLT, J. Growth and survival of juvenile cobia, Rachycentron canadum, at different salinities in a recirculating aquaculture system. Aquaculture, v.253, p.398-407. 2006.
- SHAFFER, R.V.; NAKAMURA, E.L. Synopsis of biological data on the cobia Rachycentron canadum (Pisces: Rachycentridae) NOAA Technical Report, NMFS 82, FAO Fisheries Synopsis. 153. 1989. 21p.
- YEH, S.P.; YANG, T.; CHU, T.W. Marine fish seed industry in Taiwan Workshop on offshore technologies for aquaculture. Haifa, 1998.
- YEH, S. Cobia culture in Taiwan (Rachycentron canadum). The Advocate, v.3, n.2, p.67-68, 2000.
- YU, R.; LEUNG, P.S. optimal harvesting strategies for a multi-cycle and multi-period shrimp operation: a practical network model. Mathematics and Computers in Simulation, v.68, p.339-354, 2005.
Cobia (Rachycentron canadum) hatchery-to-market aquaculture technology: recent advances at the University of Miami Experimental Hatchery (UMEH)
Publication Dates
-
Publication in this collection
09 Aug 2010 -
Date of issue
July 2010