Abstract
The effects of inactive dry yeast (Saccharomyces cerevisiae) from sugar cane were studied in 18 primiparus Saanen dairy goats (51.07±1.43) on dry matter intake and digestibility, milk production and quality. Animals were distributed in a completely randomized design during 90 days (from day 60 of milking). Diets were composed of soybean meal; soybean meal + dry yeast; or dry yeast, as protein sources, and ground corn, mineral supplement and corn silage (40%). Animals fed the dry yeast diet showed lower intake of dry matter (DM), organic matter (OM), crude protein, ether extract and neutral detergent fiber. Diets did not influence milk yield; however the milk production efficiency (kg of milk produced/kg of crude protein ingested) was better in goats fed the dry yeast diet. Acidity, somatic cell counts and milk urea nitrogen values were not affected by treatments. Animals fed the soybean + dry yeast diet had higher fat and total solids than those fed the dry yeast diet. The digestibility of DM, OM and total carbohydrate was lower for soybean only and soybean + dry yeast diets. Total digestible nutrients were higher for dry yeast and soy bean diets than soybean + dry yeast diet. Dry yeast from sugar cane is a good alternative protein source for feeding lactating dairy goats and can be recommended because it maintains the production performance.
digestibility; goats; milk quality; Saccharomyces cerevisiae
SHORT COMMUNICATION
Performance of dairy goats fed diets with dry yeast from sugar cane as protein source1 1 Project financed by CNPq.
Luciano Soares de LimaI; Claudete Regina AlcaldeII; Hanna Sakamoto FreitasI; Bruna Susan de Labio MolinaIII; Francisco de Assis Fonseca de MacedoII; José Augusto HorstIV
IPós-Graduação em Zootecnia da Universidade Estadual de Maringá/UEM, Maringá - PR
IIDepartamento de Zootecnia da Universidade Estadual de Maringá/UEM, Maringá - PR
IIIGraduação em Zootecnia - Universidade Estadual de Maringá/UEM, Maringá - PR
IVAssociação Paranaense dos Criadores de Bovinos da Raça Holandesa/APCBRH, Curitiba - PR
ABSTRACT
The effects of inactive dry yeast (Saccharomyces cerevisiae) from sugar cane were studied in 18 primiparus Saanen dairy goats (51.07±1.43) on dry matter intake and digestibility, milk production and quality. Animals were distributed in a completely randomized design during 90 days (from day 60 of milking). Diets were composed of soybean meal; soybean meal + dry yeast; or dry yeast, as protein sources, and ground corn, mineral supplement and corn silage (40%). Animals fed the dry yeast diet showed lower intake of dry matter (DM), organic matter (OM), crude protein, ether extract and neutral detergent fiber. Diets did not influence milk yield; however the milk production efficiency (kg of milk produced/kg of crude protein ingested) was better in goats fed the dry yeast diet. Acidity, somatic cell counts and milk urea nitrogen values were not affected by treatments. Animals fed the soybean + dry yeast diet had higher fat and total solids than those fed the dry yeast diet. The digestibility of DM, OM and total carbohydrate was lower for soybean only and soybean + dry yeast diets. Total digestible nutrients were higher for dry yeast and soy bean diets than soybean + dry yeast diet. Dry yeast from sugar cane is a good alternative protein source for feeding lactating dairy goats and can be recommended because it maintains the production performance.
Key Words: digestibility, goats, milk quality, Saccharomyces cerevisiae
Introduction
Supply of protein is important in dairy ruminant production systems because this is the second limiting nutrient. Thus, the search for new sources of protein should provide livestock with alternative foods.
Yeasts are unicellular organisms that reproduce asexually by budding and develop in the alcoholic fermentation (Yara et al., 2006). In Brazil, the ethanol from sugar cane is usually produced using strains from Saccharomyces cerevisiae, and the industrial process produces a large amount of yeast that can be sold live, inactive (dry) and as derivatives.
The inactive dry yeast has 26 to 32% of cell wall that consists of complex carbohydrates (beta-glucans and mannans). It is rich in B vitamins (Yamada et al., 2003), among which are vitamins B1, B2, B6, pantothenic acid, niacin, folic acid and biotin. Depending on the strains used in the fermentation process and extraction techniques, dry yeast can provide about 42% crude protein (Butolo, 2002). Therefore, this feedstuff can be a good source of protein for livestock animals.
Early experiments were performed to evaluate the use of dry yeast as dietary protein source in diets for broiler chicks (Generoso et al., 2008), pigs (Junqueira et al., 2008), cattle (Messana et al., 2009), sheep (Aguiar et al., 2007) and meat goats (Lima et al., 2011). However, data on dairy goats are not found.
This trial was performed with the objective of evaluating performance and milk quality of primiparous Saanen goats fed diets with inactive dry yeast (Saccharomyces cerevisiae) from sugar cane as an alternative protein source.
Material and Methods
The study was performed at the Fazenda Experimental de Iguatemi, from the Universidade Estadual de Maringá, south Brazil. Eighteen primiparus Saanen goats (51.07±1.43) randomly distributed in a completely randomized design were used to evaluate the effects of replacement of soybean meal by inactive dry yeast in diets. The experimental period was composed of 90 days (from the 60th day of milking). Animals were allocated to single pens. Goats were weighed at the beginning of the trial and every 15 days after the morning milking and before feeding.
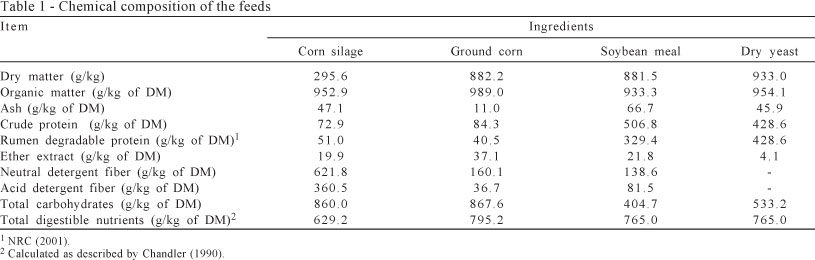
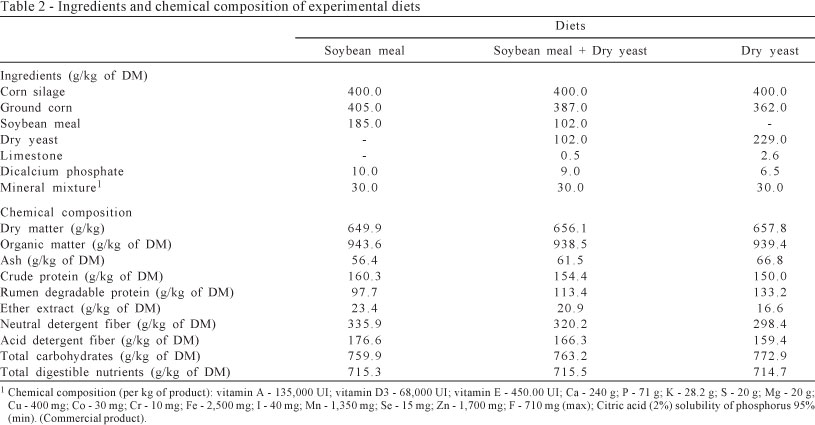
Diets were composed of soybean meal, soybean meal + dry yeast and dry yeast as protein source. The other ingredients were ground corn, mineral supplement and corn silage with 40:60 of forage to concentrate ratio (Tables 1 and 2).
Diets were formulated according to the AFRC (1998) for a milk production of 3.0 kg/d and were offered twice a day: 50% at 9 a.m. and 50% at 4 p.m. The supplied feeds and orts were weighed daily to calculate voluntary intake. Approximately 100 g/kg were allowed in orts. Water was offered ad libitum. Goats were milked twice daily (7:30 a.m. and 3:00 p.m.) and the milk yield of individual goats was recorded at each milking.
Samples of feeds and orts were taken once every two weeks and then frozen (-20 ºC). Samples of feces (50 g) were collected for five consecutive days at intervals of 26 hours, and then stored at -20 ºC until use. These samples were pooled for each animal after drying (55 ºC) for subsequent analysis.
Samples (feed , orts and feces) were oven-dried (55 °C for 72 h), then ground in a knife mill to pass through a 1-mm screen sieve (Wiley mill model 4, Arthur H. Thomas, Philadelphia, PA). Dry matter was evaluated according to method no. 934.01 of AOAC (1998). Organic matter was determined by combustion in a muffle furnace according to method no. 942.05 of AOAC (1998). Total nitrogen (TN) determination used a Tecnal TE-036/1 (Tecnal, Piracicaba, São Paulo, Brazil) following method no. 988.05 of AOAC (1998) and crude protein (CP) was estimated as TN × 6.25. Ether extraction in diets was conducted with Tecnal TE-044/1 according to the method no. 920.39 of AOAC (1998). The neutral detergent fiber (NDF) was evaluated as described by Mertens (2002) using a heat-stable α-amylase, without using sodium sulphite. Procedures for NDF determination were adapted to the Ankom200 filter bag technique (Ankom, 2011). The acid detergent fiber (ADF) content was determined according to AOAC (1998) method no. 973.18.
The dietary rumen degradable protein (RDP) content was calculated according to the NRC (2001) model. The values considered were 70, 48, 65 and 100 g RDP/100g CP for corn silage, ground corn, soybean meal and dry yeast, respectively.
Total carbohydrates (TC) and total digestible nutrients (TDN) were estimated according to equations described by Sniffen et al. (1992): TC (g/kg DM) = 1000 - (CP + EE + ash) and TDN = dCP + (2.25 × dEE) + dTC, where dCP = digestible crude protein, dEE = digestible ether extract and dTC = digestible total carbohydrates.
Indigestible NDF (iNDF) was used as an internal marker to estimate fecal output and apparent nutrient digestibility. For iNDF analysis, 0.5 g (1 mm) of period samples (fecal, orts and feeds) were incubated in situ (144 h) in the rumen goat within nylon bags (F57 Ankom) followed by neutral detergent analysis (Mertens, 2002) by an Ankom200 Fiber Analyzer (Ankom Technology Corp., Fairport, NY).
Milk was sampled monthly from two consecutive milkings, preserved with 2-bromo-2-nitropropane-1,3-diol at 4 ºC for chemical composition determination. Other samples of milk were taken monthly from four consecutive milkings and were frozen (-20 ºC) to determine milk urea nitrogen.
The protein, fat, lactose and total solids contents in milk were estimated by infrared spectroscopy (Bentley model 2000; Bentley Instrument Inc., Chaska, MN). Yield of fat-corrected milk was calculated according to the equation reported by Gravert (1987): FCM (3.5%) = 0.433MY + 16.218 FY, where: FCM: fat-corrected milk; MY: milk yield (kg/day); FY: fat yield (kg/day).
Milk somatic cells counts were obtained using an electronic counter (Somacount 500, Chaska, MN) as described by Voltolini et al. (2001). At the same time milk acidity using the Dornic solution was measured according to the AOAC (1998; method no 947.05). The milk urea nitrogen content was evaluated by enzymatic-colorimetric method (Bergmeyer, 1985).
The data obtained were analyzed by variance analysis (α = 0.05) and means were compared using the Tukey test through the SAEG system (version 9.1), with the general model: Yij = µ + Di + ej; where: Yij = the dependent variable, µ = general constant; Di = effect of diet i, i = soybean meal, soybean + dry yeast, and dry yeast; and ej = random error.
Results and Discussion
Dry matter and organic matter intakes of the dry yeast diet were lower (P<0.05) than soybean + dry yeast (Table 3). Goats are able to choose food and they do it selecting feed on the basis of apprehension ease and sensorial characteristics (Provenza et al., 2003). Dry yeast has very fine texture and peculiar smell from sugar cane and it may have contributed to the reduction in dry matter intake. However, these differences seem to be more related to slight variation for body weight of animals within each diet because when the dry matter intake was calculated as percentage of body weight there was no difference (P>0.05) between diets.
Rodrigues et al. (2007) evaluated the intake of Alpine goats (57.14 kg BW) fed diets containing levels of crude protein and net energy and found 1.98 kg of dry matter intake on average. Zambom et al. (2008) observed dry matter intake of 2.21 kg in Saanen goats (75.7 kg BW) fed diets with soybean hulls replacing ground corn and corn silage as roughage (40%). These results are similar to results from this trial and are also related to body weight of animals.
Crude protein, ether extract and neutral detergent fiber intakes were lower (P<0.05) for dry yeast diet compared with other diets. It may be the result of the difference observed in dry matter intake and especially of diets composition (Table 2), where ether extract and neutral detergent fiber contents were reduced by dry yeast inclusion. Even though the dry yeast diet showed lower crude protein intake, the intake obtained met the requirements for maintenance and milk production.
There were no differences (P>0.05) between diets as to total carbohydrates intake (Table 3), and the average value was similar to the observed by Fonseca et al. (2006) in dairy goats (1.38 kg/day) that were fed diets with protein levels.
Diets did not influence (P>0.05) milk yield; notwithstanding, the milk production efficiency (kg of milk produced/kg of crude protein ingested) was better (P<0.05) in goats fed the dry yeast diet (Table 4). Considering that diets did not influence the total digestible nutrients intake (Table 3), this can be related to the content of rumen degradable protein (RDP) of the diets. Protein is the second limiting nutrient for milk production and dry yeast diet provided more RDP, which contributes to microbial growth and thus contributing to milk production.
The fat milk content was higher (P<0.05) in goats fed the soybean + dry yeast diet compared with dry yeast diet. However, milk protein and lactose were not affected (P>0.05) by diets (Table 4).
Milk protein is strongly influenced by polymorphisms in the locus αS1-casein in goats (Greppi et al., 2008) and lactose is synthesized and secreted at the same rate as the milk (Pulina et al., 2008). Thus, the levels of these milk components are, usually, constant in the milk. Unlike milk protein and lactose, fat is the milk component most sensitive to changes in nutrition of animals (Pulina et al., 2008), as observed in the current experiment. Furthermore, these differences can be associated with the lower NDF intake showed by goats on the dry yeast diet (Table 3).
As a consequence of the change in milk fat, goats fed dry yeast had lower (P<0.05) milk total solids than goats fed soybean + dry yeast (Table 4). There was no significant treatment effect (P>0.05) on acidity (17.87ºD), somatic cell counts (3.18 log10) or milk urea nitrogen (15.54 mg/dL).
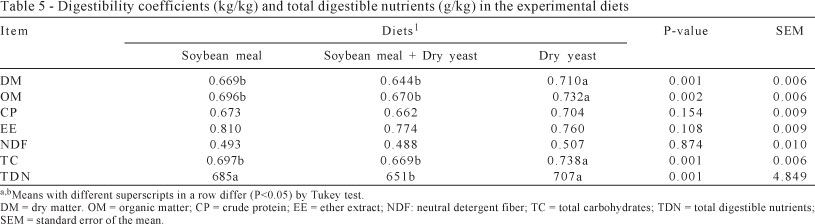
The digestibility coefficients of dry matter, organic matter and total carbohydrates were highest (P<0.05) for the dry yeast diet (Table 5). This diet has more rumen degradable protein than the soybean and soybean + dry yeast diets, which may have provided better utilization of dietary energy and protein improving the digestibility of the rations.
Similar results were reported by Lima et al. (2011), who evaluated the digestibility of diets with inactive dry yeast replacing soybean meal in finishing kids. In this experiment, improvement was observed in the digestibility of dry matter, organic matter and total carbohydrate in diets. To heifers, Martins et al. (2000) also observed higher digestibility of dry matter and organic matter using dry yeast as compared with cottonseed meal.
Changes were not found (P>0.05) for the digestibility of crude protein, ether extract or neutral detergent fiber. However, the total digestible nutrients were higher (P<0.05) for dry yeast and soybean diets compared with the soybean + dry yeast diet (Table 5).
Different effects to dry yeast were observed by Lima et al. (2011), who reported increase in crude protein digestibility, reduction in digestibility of ether extract, no effects on the neutral detergent fiber digestibility and increase in total digestible nutrients of diets with dry yeast in finishing kids. Martins et al. (2000) also reported improvement in the digestibility of crude protein and neutral detergent fiber in heifers fed rations with dry yeast. These differences may be related to the other ingredients of the rations (corn or cassava, hay or corn silage), species, sex and also the physiological stage of animals, which can influence the availability of nutrients.
Conclusions
Dry yeast from sugar cane can be used as protein source for feeding lactating dairy goats because it provides milk quality and production similar to those observed when soybean meal is used.
Received April 4, 2011 and accepted October 11, 2011.
Corresponding author: luciano.delima@hotmail.com
- AGRICULTURAL AND FOOD RESEARCH COUNCIL - AFRC. Technical committee on response to nutrients The nutrition of goats. Wallingford: CAB International, 1998. 118p.
- AGUIAR, S.R.; FERREIRA, M.A.; BATISTA, A.M.V. et al. Desempenho de ovinos em confinamento, alimentados com níveis crescentes de levedura e uréia. Acta Scientiarum.Animal Science, v.29, p.411-416, 2007.
- ANKOM TECHNOLOGY [2011]. Method for determining neutral detergent fiber in feeds Available at: <http://www.ankom.com/media/documents/Method_6_NDF_4013011_A200,A200I.pdf> Accessed on: Sep. 6, 2011.
- ASSOCIATION OF OFFICIAL AGRICULTURAL CHEMISTS - AOAC. Official methods of analysis 16.ed. Gaithersburg, M.P.: Association of Official Analytical Chemists, 1998. 1141p.
- BERGMEYER, H.U. Proteins and peptides, amino acids and related compounds. In: BERGMEYER, H.U. (Ed.) Methods of enzymatic analysis 3.ed. Wiley-VCH, 1985. p.449-453.
- BUTOLO, J.E. Qualidade de ingredientes na alimentação animal Campinas: CBNA, 2002. 180p.
- CHANDLER, P. Energy prediction of feeds by forage testing explorer. Feedstuffs, v.62, p.12, 1990.
- FONSECA, C.E.M.; VALADARES, R.F.D.; VALADARES FILHO, S.C. et al. Produção de leite em cabras alimentadas com diferentes níveis de proteína na dieta: consumo e digestibilidade dos nutrientes. Revista Brasileira de Zootecnia, v.35, p.1162-1168, 2006.
- GENEROSO, R.A.R.; GOMES, P.C.; ROSTAGNO, H.S. et al. Composição química e energética de alguns alimentos para frangos de corte em duas idades. Revista Brasileira de Zootecnia, v.37, p.1251-1256, 2008.
- GRAVERT, H.O. Dairy cattle production Nova York: Elsevier Science, 1987. 234p.
- GREPPI, G.F.; RONCADA, P.; FORTIN, R. Protein components of goat's milk. In: PULINA, G.; CANNAS, A. (Eds.) Dairy goats feeding and nutrition 2.ed. Bologna: CAB International, 2008. p.71-94.
- JUNQUEIRA, O.M.; SILZ, L.Z.T.; ARAÚJO, L.F. et al. Avaliação de níveis e fontes de proteína na alimentação de leitões na fase inicial de crescimento. Revista Brasileira de Zootecnia, v.37, p.1622-1627, 2008.
- LIMA, L.S.; ALCALDE, C.R.; FREITAS, H.S. et al. Sugar cane dry yeast in feeding for growing and finishing goat kids. Revista Brasileira de Zootecnia, v.40, p.168-173, 2011.
- MARTINS, A.S.; PRADO, I.N.; ZEOULA, L.M. et al. Digestibilidade aparente de dietas contendo milho ou casca de mandioca como fonte energética e farelo de algodão ou levedura como fonte protéica em novilhas. Revista Brasileira de Zootecnia, v.29, p.269-277, 2000.
- MERTENS, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. Journal of AOAC International, v.85, p.1217-1240, 2002.
- MESSANA, J.D.; BERCHIELLI, T.T.; ARCURI, P.B. et al. Valor nutritivo do resíduo do processamento do caroço de algodão suplementado com levedura e avaliado em bovinos. Revista Brasileira de Zootecnia, v.38, p.2031-2037, 2009.
- NATIONAL RESEARCH COUNCIL - NRC. Nutrients requirements of dairy cattle 7.ed. Washington, D.C.: The National Academies Press, 2001. 381p.
- PROVENZA, F.D.; VILLALBA, J.J.; DZIBA, L.E. et al. Linking herbivore experience, varied diets, and plant biochemical diversity. Small Ruminant Research, v.49, p.257-274, 2003.
- PULINA, G.; NUDDA, A.; BATTACONE, G. Nutrition and quality of goat's milk. In: PULINA, G.; CANNAS, A. (Eds.) Dairy goats feeding and nutrition 2.ed. Bologna: CAB International, 2008. p.1-30.
- RODRIGUES, C.A.F.; RODRIGUES, M.T.; BRANCO, R.H. et al. Consumo, digestibilidade e produção de leite de cabras leiteiras alimentadas com dietas contendo diferentes níveis de proteína bruta e energia líquida. Revista Brasileira de Zootecnia, v.36, p.1658-1665, 2007.
- SNIFFEN, C.J.; O'CONNOR, J.D.; VAN SOEST, P.J. et al. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Animal Science, v.70, p.3562-3577, 1992.
- VOLTOLINI, T.V.; SANTOS, G.T.; ZAMBOM, M.A. et al. Influência dos estádios de lactação sobre a contagem de células somáticas do leite de vaca da raça Holandesa e identificação de patógenos causadores de mastite no rebanho. Acta Scientiarum.Animal Science, v.23, p.961-966, 2001.
- YAMADA, E.A.; ALVIM, I.D.; SANTUCCI, M.C.C. et al. Composição centesimal e valor protéico de levedura residual da fermentação etanólica e de seus derivados. Revista de Nutrição, v.16, p.423-432, 2003.
- YARA, R.; MACCHERONI, W.; HORRI, J. A bacterium belonging to the Burkholderia cepacia complex associated with Pleurotus ostreatus. Journal of Microbiology, v.44, p.263-269, 2006.
- ZAMBOM, M.A.; ALCALDE, C.R.; SILVA, K.T. et al. Desempenho e digestibilidade dos nutrientes de rações com casca do grão de soja em substituição ao milho para cabras Saanen em lactação e no pré-parto. Revista Brasileira de Zootecnia, v.37, p.1311-1318, 2008.
Publication Dates
-
Publication in this collection
14 Feb 2012 -
Date of issue
Jan 2012
History
-
Received
04 Apr 2011 -
Accepted
11 Oct 2011