Abstract
The objective of this study was to estimate the dehydration curve and occurrence of fungi and mycotoxins in Tifton 85 hay. The experimental design was randomized blocks in split plots with four replications and five levels of nitrogen (0, 25, 50, 75 and 100 kg ha-1) in the form of urea, two regrowth ages (28 and 35 days) and drying hay in the sun and in shed. After harvesting, the grass was sampled four times (0, 8, 23 and 32 hours) to dry in the sun and twelve times (0, 3, 18, 27, 42, 51, 66, 75, 90, 99, 114 and 123 hours) for drying in shed, turned over daily. The fungi were identified after seeding and growth in three steps of haymaking (cutting, baling and after 30 days of storage). To verify the presence of mycotoxins, 20 samples, composed of hay stored for 30 days, were collected, of which 10 samples were from sun-dried hay and 10 from hay dried in shed. It took 32 hours to produce hay at field conditions, with water loss rates up to 6.10 g g-1 DM-h during the first 8 hours after cutting. The average loss of water in this period was 2.0 g g-1 DM-h. Hays dried in the shed took 123 hours and the dehydration rates were less than 0.5 g g-1 DM-h due to environmental conditions. There was a predominance of three genera of fungi: Fusarium, Penicillium and Aspergillus. The largest population was the Fusarium, followed by Penicillium, at the steps of baling and storage. The fumonisin mycotoxin was found at a higher concentration and there was no difference in fumonisin concentration in the hay dried in the sun and in shed. Although it brings no hazard to animal health, the production of aflatoxin and zearalenone was significantly higher in the samples of sun-dried hay.
contaminants; dry matter; forage conservation; microorganisms; toxins
FORAGE CROPS
Dehydration curve, fungi and mycotoxins in Tifton 85 hay dehydrated in the field and in shed
Loreno Egidio TaffarelI; Eduardo Eustáquio MesquitaI; Deise Dalazen CastagnaraI; Patrícia Barcellos CostaI; Marcela Abbado NeresI; Marcelina Bottoni HornII; Paulo Sérgio Rabello de OliveiraI; Cristiane Claudia MeinerzI
ICentro de Ciências Agrárias - Universidade Estadual do Oeste do Paraná UNIOESTE - Campus Marechal Cândido Rondon - PR, Brazil
IICentro de Ciências Agrárias - Universidade Federal de Santa Catarina e NUTRIFARMA - Taió - SC, Brazil
ABSTRACT
The objective of this study was to estimate the dehydration curve and occurrence of fungi and mycotoxins in Tifton 85 hay. The experimental design was randomized blocks in split plots with four replications and five levels of nitrogen (0, 25, 50, 75 and 100 kg ha-1) in the form of urea, two regrowth ages (28 and 35 days) and drying hay in the sun and in shed. After harvesting, the grass was sampled four times (0, 8, 23 and 32 hours) to dry in the sun and twelve times (0, 3, 18, 27, 42, 51, 66, 75, 90, 99, 114 and 123 hours) for drying in shed, turned over daily. The fungi were identified after seeding and growth in three steps of haymaking (cutting, baling and after 30 days of storage). To verify the presence of mycotoxins, 20 samples, composed of hay stored for 30 days, were collected, of which 10 samples were from sun-dried hay and 10 from hay dried in shed. It took 32 hours to produce hay at field conditions, with water loss rates up to 6.10 g g-1 DM-h during the first 8 hours after cutting. The average loss of water in this period was 2.0 g g-1 DM-h. Hays dried in the shed took 123 hours and the dehydration rates were less than 0.5 g g-1 DM-h due to environmental conditions. There was a predominance of three genera of fungi: Fusarium, Penicillium and Aspergillus. The largest population was the Fusarium, followed by Penicillium, at the steps of baling and storage. The fumonisin mycotoxin was found at a higher concentration and there was no difference in fumonisin concentration in the hay dried in the sun and in shed. Although it brings no hazard to animal health, the production of aflatoxin and zearalenone was significantly higher in the samples of sun-dried hay.
Key Words: contaminants, dry matter, forage conservation, microorganisms, toxins
Introduction
The production of hay and silage of high quality for use in the period of forage scarcity (Ribeiro et al., 2001) can help raise productivity levels in milk and meat in Brazil (Gonçalves et al., 2003).
Although there are difficulties in climate for production of hay of high nutritional value in the summer (Reis et.al., 2001b), the haymaking allows for high yields of tropical forages (Pereira et al., 2006), especially those of the genus Cynodon like Tifton 85 (Cecato et al., 2001).
However, the quality of the hay is dependent on the characteristics of plants, on the climate conditions during drying and on the storage system employed (Reis et al., 2001a), which are important for the nutritional value, chemical composition, the presence or absence of pathogens, and interference with the loss of carbohydrates, proteins, vitamins and minerals during the production process (Cecava, 1995; Rotz & Shinners, 2007).
The inadequate drying of the plants subjected to haymaking leads to a reduction of nutritional value of preserved forage, promotes fungal growth, which may be associated with the production of mycotoxins and increase of fibrous components and protein degradation, and results in decreased animal performance (Coblentz et al., 2000) due to the reduced feed intake.
The moisture favors the development of fungi which are present in the developmental environment of grasses. Under field conditions, Fusarium (Amaral & Nussio, 2011), Aspergillus (Domingues, 2006) and Penicillium (Cast, 2003) are found in different populations; however, it is relevant to study them because they are responsible for the production of the majority of known mycotoxins (Sweenwey & Dobson, 1998).
Mycotoxins are metabolites secreted by fungi, which can be produced in a wide variety of raw materials before, during and after harvest and storage. They are resistant to technological treatments and may be present in foods for animals and humans. The ingestion of mycotoxins can reduce the growth and reproductive performance of animals (Oswald, 2011).
Thus, the objective of this research was to estimate the dehydration curve and to verify the growth of fungi and the mycotoxin production in Tifton 85 hay subjected to nitrogen (N) fertilization dried in the field conditions (in the sun) and in shed (under shade).
Material and Methods
The experiment was carried out at the Experimental Farm Antonio Carlos dos Santos Pessoa, belonging to Universidade Estadual do Oeste do Paraná, campus Marechal Cândido Rondon, having as coordinates 24th 33' 40" S latitude, 54th 04' 12" W longitude and 420 m altitude. The local climate, according to Koppen, is classified as a subtropical Cfa with well-distributed rainfall throughout the year and hot summers. The average temperature of the coldest quarter varies between 17 and 18 ºC, and for the hottest quarter, between 28 and 29 ºC. The annual average temperature varies between 22 and 23 ºC. The total average annual rainfall for the region ranges from 1600 to 1800 mm, with the trimester of heavier rainfall showing totals ranging from 400 to 500 mm (Caviglione et al., 2000) (Figure 1 and Table 1).
The soil of the experimental area is classified as an eutrophic Oxisol (EMBRAPA, 2006) and has the following chemical characteristics: P (Mehlich extractor) - 8.15 mg dm-3; organic matter - 23.92 g dm-3; pH (CaCl2) - 0.01 mol L-1; H + Al - 4.30 cmolc dm-3; Al3+ (KCl 1 mol L-1) - 0.05 cmolc dm-3; K (Mehlich extractor) - 0.23 cmolc dm-3; Ca2+ (KCl 1 mol L-1) - 3.62 cmolc dm-3; Mg2+ (KCl 1 mol L-1) - 1.69 cmolc dm-3; sum of bases - 5.54 cmolc dm-3; cation exchange capacity - 9.84 cmolc dm-3; base saturation - 56.30%; Al - 0.89%; Cu (Mehlich extractor) - 6.30; Mn (Mehlich extractor) - 1.4; Zn (Mehlich extractor) - 63.00; Fe (Mehlich extractor) - 25.10; and clay - 650 g kg-1.
The experiment was conducted in a hay production field deployed in 2004, with Cynodon sp. cv. Tifton 85. The experimental design was a completely randomized block in a 5 × 2 × 2 split plot factorial arrangement. The variables were the five nitrogen doses (0, 25, 50, 75 and 100 kg ha-1), two regrowth ages (28 and 35 days) and two drying conditions (sun and shade), while the subplots were sampling times. For the drying curves in the sub-plots of Tifton 85 dried in the sun four samples were made (0, 8, 23 and 32 hours after harvest), while for Tifton 85 dehydrated in shed, twelve samples were collected (0, 3, 18, 27, 42, 51, 66, 75, 90, 99, 114 and 123 hours after cutting) (Table 2). To evaluate the occurrence of fungi, samples were collected at the time of cutting, at baling and 30 days after storage.
The experiment began on October 30, 2010, with cut for uniformity of the grass, and ended on January 11, 2011. The cuts for production of sun-dried hay were carried out on November 26, 2010 (28 days of regrowth) and on January 10, 2011 (35 days of regrowth); cuts whose dehydration was performed in shed occurred on the 6th (35 days of regrowth) and 24th (28 days of regrowth) of December, 2010.
The forage in drying process was revolved manually each day, at 10h00 and 15h00 The experimental periods of drying in the sun and in shed were different and so the analyses of each age and drying condition were carried out separately (Figures 2 and 3).
For the determination of the drying curves, 300 g samples of each plot were collected at times established. The samples were selected and left to dry, packed in paper bags, weighed and placed in a forced-ventilation oven at a temperature of 55 ºC for 72 hours. The last harvest was done at the time of baling (32 hours for drying in the sun and 123 hours to dry in the shed). After sampling, the material was stored in raffia braid bags which allowed for good ventilation, and were stored under identical conditions in the same shed and protected from rain and sunshine.
To quantify the fungi the same experimental design of haymaking process was used. To carry out seeding for fungal growth, first the particle size was reduced in each sample of hay with the aid of scissors. After, each sample was diluted in sterile distilled water at the ratio of 1 g/102 mL and then seeded in PDA culture medium (potato 200 g, 20 g dextrose, 15 g agar and 1000 mL of distilled water), where it was isolated by inducing mycelial growth. The genera were identified with the aid of specific identification keys (Guarro et al., 1999) by transferring fungal colonies with the aid of stylus or needle to a microscope slide with lactophenol cotton blue stain, covered with coverslip and observed by optical microscopy for identification of the fungi.
The samples to verify the presence of mycotoxins were collected from hay stored for 30 days by means of a composite sample originated from the replicates of each dose of nitrogen at each age, in a total of 20 samples, of which 10 came from sun drying and 10 came from the shed drying. Mycotoxins were identified by the ELISA test (enzyme immunoassay - Neogen) in the Nutrilab laboratory - Nutrifarma Laboratory Analysis - Bromatology, Microbiology and Micotoxilogy, located in the municipality of Taió-SC, Brazil.
The data on dry matter (DM) content over the dehydration times were subjected to regression analysis to obtain the drying curves. The choice of regression models was based on the significance of the parameters via a partial t test and the degree of explanation of regression to the data of DM (R2), according to the statistical significance of the factors included in the initial model.
The data of drying curves, quantification of fungi and aflatoxin were subjected to ANOVA and tested by the Fisher F test (Pimentel-Gomes & Garcia, 2002; Pimentel-Gomes, 2009). When there was significant effect of dehydration time, it was studied by regression analysis. For the choice of model, the highest coefficient of determination (R2) was considered. The data of the fumonisin and zearalenone mycotoxins did not present a normal distribution of probabilities, found by the Shapiro-Wilk test, and so an independent and unpaired comparison of the mycotoxins was carried out using non-parametric analysis, by the Mann-Whitney test (Snedecor & Cochran, 1989). Means were compared using the Tukey test at 5% of probability.
Results and Discussion
Nitrogen levels were not significant on the dry matter of the Tifton 85 subjected to dehydration for hay production in any cuts or regrowth age studied. However, the times after cut were significant for dehydration.
For cuts and ages whose dehydration was in the sun, the dry matter (DM) obtained during the dehydration period was adjusted to a polynomial regression model of 3rd order (Figure 2).
Until about eight hours after the cuts, there was a rapid loss of water, favored by the high moisture content of the grass cut, because the stomata were opened at the first hour after cutting and because there was vapor pressure deficit between the air and the dehydrating forage (Collins & Cloblentz, 2007). There were favorable weather conditions for a fast dehydration, although there was a little rain on the second day, after collecting the hay at the age of 35 days (Table 1).
In the period between 8 and 23 hours (Figure 2), the reduction in dehydration intensity is attributed to dew, decay in temperature, reduction of radiation and increase in the average and minimum relative humidity (Table 1). A similar situation was observed by Calixto Júnior et al. (2007), who conducted a study on the production process of stargrass (Cynodon nlemfuensis Vanderyst) hay during 48 hours after harvested (or after cutting for haymaking). The fluctuations that occurred in the rate of dehydration of tifton 85 after cutting times were continuous, probably according to the environment around the plant, similar to that described by Rotz (1995).
The DM curves whose dehydration was performed in shed had adjust by linear regression. The occurrence of precipitation during dehydration (Figure 1) contributed to increase the dehydration period to 123 hours (Figure 3). The dewatering rate was slower with increments of 0.21% of DM per hour after the cut, which can be explained by the precipitation and maintenance of higher air humidity (Table 1). The dehydration rates can achieve progressive levels near zero, due to the balance between the water vapor pressure contained in the plant and the surrounding environment, and can remain unchanged in moisture content indefinitely if the environment is favorable to this (Rotz, 1995). In the shed, there were no re-wetting cycles, because the forage in haymaking was protected from dew and moisture of the soil and this is in agreement with the findings of Calixto Júnior et al. (2007).
The DM content of hay at 35 days of regrowth dried in the shed did not reach the level of 850 g kg-1 of hay. The recommendation is that hay harvested with moisture contents of 250 g kg-1 of hay be kept loose; between 180 and 220 g kg-1 of hay should be chopped, and if harvested with moisture content of 200, 180 or 160 g kg-1 the hay must be kept in small, medium and large bales, respectively (Cecava, 1995).
The hays dried in shed require a longer time for dehydration, due to the lack of direct solar energy to remove moisture (Rotz, 1995) and hay production using other forms of energy is usually impractical or anti-economic. The hay moisture favors fungal growth and heating of the material when baled (Rotz, 2003). The fungi can lead to reduction of intake by the animals and to production of mycotoxins, while heating makes a portion of the proteins unavailable, especially if the heat temperatures reach above 40 ºC, due to the Maillard reaction (Collins & Cloblentz, 2007).
The prolonged period of dehydration (Figure 3) can also lead to loss of soluble carbohydrates, proteins, lipids and vitamins A and B (Cecava, 1995); however the non-exposure to sunlight does not transform the ergosterol into vitamin D (Rotz & Shinners, 2007). The direct radiation is more important for hay production than the deficit of water vapor pressure between the plant and the environment and than the wind speed because of the effect evaporation (Wright et al., 2000).
The average loss of water during the first 8 hours of the hay dried in the sun were greater than 6.10 g g-1 of DM h-1 and the average at 32 hours approached 2 g g-1 of DM h-1 (Figure 2). The linear rates of dehydration in the shed for 28 and 35 days were 0.50 and 0.39 g g-1 DM h-1, respectively. The dehydration rate of the sun-dried hay was higher than those found by Moser (1995) and Collins & Cloblentz (2007), who reported that the initial rate of water loss can be 1 g g-1 DM h-1, mainly by the rapid evaporation of the leaf, both for grasses and legumes. Both rates of dehydration of Tifton 85 hay dried in the sun and in shed were higher than those found by Formiga et al. (2011) with black Jurema hay (Mimosa tenuiflora Wild. Poir.), whose dehydration rates ranged like stalk diameter, from 0.03 to 0.05 g g-1 DM h-1 of hay.
The decline in dehydration rate occurs when the stomata close (about two hours after harvest) by reducing the osmotic pressure of the cells. But at this stage about 70-80% water still remains, which is reduced by cuticle evaporation, and is also affected by the leaf structure, characteristics and structure of the plant cuticle. A third stage of dehydration begins when the moisture of the plant reaches about 450 g kg-1 of hay, and at this stage, it is less influenced by the management and more sensitive to climate conditions, especially air humidity (Moser, 1995). This probably occurred in the dehydration process of the hay in the shed (Figure 3).
The forage dried under field conditions (Figure 2) reached more than 800 g kg-1 DM of hay 32 hours after cutting. This result is in agreement with data reported by Jobim et al. (2001), who affirmed that the leaves of Tifton-85 need 24 hours to reach approximately 900 g kg-1 DM, while the stalk fraction needs about 30 hours of sun exposure to achieve near 800 g kg-1 DM, because of the stalk diameter. Pinheiro & Peça (2004) said the deficit between the humidity of the environment and plant and wind speed are the variables that most influence the drying process. Forage not conditioned, well distributed and with up to 3 kg m-2 increases the drying rate at approximately 50% (Wright et al., 1997).
The prolonged time of dehydration in the shed probably resulted in loss of protein and soluble carbohydrates and changed the composition of the fibers. In plants harvested in the growth phase and rapidly dehydrated there can be losses of 3-4% in DM due to the breathing process, which continues until the moisture percentage is below 40%. When a slow dehydration happens, as in the rainy season, there are increased losses of DM and nutrients (Cecava, 1995).
The fungi genera identified were Fusarium, Penicillium and Aspergillus, in addition to Rhizopus and Cladosporium, in two forage cuts and three stages of hay production (cutting, baling and after 30 days of storage).
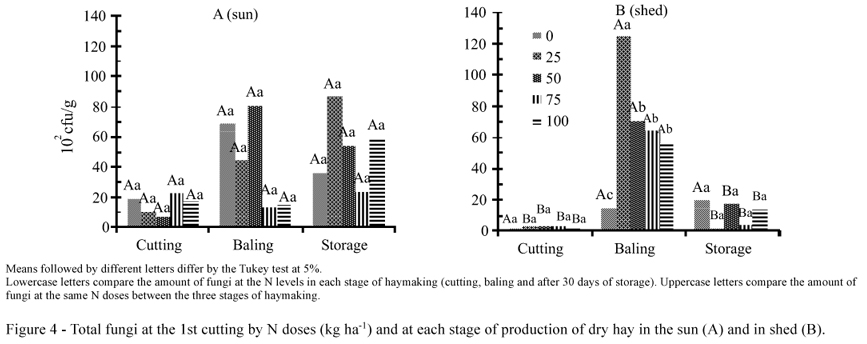
For the interaction of N doses and haying stages, there were significant differences in hay dried in shed at doses of 25, 50, 75 and 100 kg N ha-1 in the baling stage, in the 1st cut compared with other stages of haymaking (Figure 4 - B). There were also differences in the 2nd cut, in the hay dried in the sun at dose of 25 kg N ha-1 during baling (Figure 5 - A) and in hay dried in the shed, at doses of zero, 25 and 100 kg N ha-1 in relation to the same doses at the cutting stage (Figure 5 - B). There were no differences in the fungal population in the 1st cut dried in the sun, neither between stages of haymaking nor between N doses (Figure 4 - A).
The highest fungi population occurred in the 1st cut at the baling phase in the hay dried in shed (Figure 6 - B). In the 2nd cut, the largest population of Fusarium occurred at the baling and storage stages in the hay dried in the sun (Figure 7 - A) and in the storage in the hay dried in the shed (Figure 7 - B). For Fusarium fungi, there were no differences in the 1st cut between stages of haymaking for the hay dried in the sun (Figure 6 - A).
There were no differences between the Penicillium population over the haymaking stages, both for the hay dried in the sun and in shed (Figures 6 and 7).
For the genus Aspergillus, the greatest population occurred in baling and storage steps in the hay dried in the shed (Figures 6 - B and 7 - B), probably due to the higher moisture content of hay because these fungi do not grow at a moisture content below 15% (Domingues, 2006).
In a study with alfalfa, the highest incidence of fungi was found in forage not subjected to wilting and that remained piled, and this difference also prevailed during storage for 60 days (Nascimento et al., 2000). These authors also identified a higher prevalence of fungi immediately after cutting, with identification of various species, where Penicillium was prevailing at 15 and 30 days of storage, followed by Aspergillus in hay dried 50% and 60% in the sun, and then by Fusarium, Cladosporium and others.
In the present study, in both drying conditions, the hay remained dispersed and daily subjected to turning, and the greatest number of fungi occurred during the baling and storage stages. The genus Fusarium was prevalent, followed by Penicillium, Aspergillus, Cladosporium and Rhizopus. The slow drying allows for the colonization of hay by saprophytic fungi such as Fusarium, Alternaria and Cladosporium (Nascimento et al., 2000) and that may explain the larger population of Fusarium during 30 days of storage in the hay dried in the shed.
Fungi are producers of mycotoxins, and Fusarium is the producer of fumonisin (Pozzi et al., 2002) and zearelenone (Trés et al., 2011); Aspergillus produces aflatoxins and ochratoxins; and Penicillium produces ochratoxin (Kawashima & Soares, 2006).
Twenty composite samples were analyzed for the presence of aflatoxin, fumonisin and zearelenone (Table 3).
For aflatoxin, the average of sun-dried samples (5.38±0.00173 µg kg-1) was higher than the average of samples dried in the shed (3.07±0.00167 µg kg-1) and although the levels of aflatoxin were low, the results were significant (P = 0.0071) for higher presence of aflatoxin in the sun-dried hay. However, the recommendations of the Laboratory of Mycotoxicological Analysis of Universidade Federal de Santa Maria suggest maximum limits (ML) of 20.00 µg g-1 for calves and adult males and zero for lactating cows (LAMIC, 2011).
Aflatoxin is produced by the fungi Aspergillus flavus and Aspergillus parasiticus and lasts for a long time after formed in the food. It is heat-resistant, withstanding temperatures of up to 220 ºC. The aflatoxins B1, B2, G1 and G2 are known, and the aflatoxin B1 is one of the most carcinogenic substances (Domingues, 2006). Aflatoxins are produced mainly with relative humidity between 80 and 85%, water activity higher than 70% and temperatures between 24-35 ºC (Malmann & Dilkin, 2007). The hypothesis for the higher levels of mycotoxins in hay dried in the sun was the daytime-night thermal variation, which stimulated the fungi to produce mycotoxins before the grass reached a moisture content below 15% (CAST, 2003).
The average level of fumonisin in sun-dried hay samples was 90.00 µg kg-1 and those of the hay dried in shed was 60.00 µg kg-1 and the comparison was not significant (P = 0.8038) (Table 3). The explanation is that Fusarium can produce toxins in the stage of pre-harvest, and is regarded as the flora of grains and remainders of dead plants that serve as substrate for them to stay in the field, and infect a subsequent crop, producing toxins (Bullerman & Tsai, 1994).
High levels of fumonisin are associated with hot and dry periods followed by periods of high humidity and mild temperatures, insect damage and improper storage conditions such as humidity above 18% (Barros, 2011). Fumonisin production is restricted to pre-harvest and drying and the levels tend to increase gradually along with the maturation period and, except in extreme conditions, the conventional storage is not a concern (Schiabel, 2004).
There are no practical methods available to significantly reduce fumonisin contamination and so good management practices are recommended at pre-harvest, harvest (including time of harvest and control of temperature and humidity during transport and storage) and post-harvest, which should be routine to avoid contamination and development of fungi and release of mycotoxins (FAO/WHO, 2000).
No legal limits for fumonisins have been established, although the Mycotoxin Commitee of the Americam Association of Veterinary Laboratory Diagnosticians recommends 50,000 µg kg-1 for cattle (Munkvold & Desjardins, 1997), while Mallmann & Dilkin (2007) recommend 60,000 µg kg-1. The Food and Drug Administration recommends caution from 30,000 µg kg-1 for ruminants over three months old and for those to be slaughtered it should not exceed 60,000 µg kg-1 of the food; for horses it should not exceed 5,000 µg kg-1. Foods with these concentrations should not exceed 50% of the total diet (OARDC, 2011a). Based on these instructions, the levels of fumonisin in the studied hay are above the recommended for horses and in this species it is associated with leukoencephalomalacia (CAST, 2003). Cows and sheep are more resistant to poisoning by fumonisins, with mild liver damage (Barros, 2011).
For zearalenone, the average sun-dried samples had 79.89 µg kg-1 and those dried in the shade showed 40.33 µg kg-1 and the difference was significant (P = 0.0257). The temperature differences of day and night that occurred during the drying process may have influenced the production of zearalenone, because the forage dried in the sun has been exposed to temperatures above 30 ºC during the day and around 15 ºC at night, and with moisture of up to 20% (Figure 1 and Table 1). The Fusarium fungi are more susceptible to mycotoxin production when subjected to heat shock, mainly with alternating temperatures, especially the daytime and night, and with moisture content exceeding 22% (Maboni et al., 2011).
Zearelenona is a secondary metabolite that is produced by several species of Fusarium, especially F. graminearum and F. culmorum. These species are known to colonize maize, sorghum, barley, oats, wheat and other grasses and tend to raise during cold periods or periods of high humidity and harvest season, both in temperate and hot regions of the world (Milicevic et al., 2010).
Zearalenone is also known as RAL and F-2 toxin, and is a powerful estrogenic metabolite that causes infertility, abortion and reproductive failure in dairy cows (Trés et al., 2011); in heifers, it can reduce fertility when fed at up to 12.500 µg kg-1 diet (OARDC, 2011b). The toxin is heat-stable and is not destroyed by long-term storage, heating or by addition of propionic acid or other controllers of fungi. The LAMIC (2011) recommends a maximum of 250 µg kg-1 in feed supplied to ruminants.
Mycotoxins are found at low concentrations in most foods contaminated by toxigenic fungi and high rates are rarely observed. Therefore, cases of acute poisoning with clinical symptoms or evident pathology are infrequent, and chronic effects that do not call the immediate attention of the veterinaries and nutritionists prevail (Cruz, 2010).
Conclusions
Tifton 85 hay with regrowth age of 28 and 35 days dried in the sun requires less than 40 hours to reach 85% of dry matter. For production of Tifton 85 hay dried in shed, over 120 hours are necessary. In the baling and storage stages of the hay dried in the sun and in shed there is a higher population of total fungi, and the predominant genus was Fusarium. The hay dried in the sun provides higher levels of mycotoxins, aflatoxin and zearalenone, as compared with the hay dried in the shed.
Acknowledgements
Prof. Dr. Newton Escocard Tavares de Oliveira, for his help in the experimental design; Prof. Dr. José Renato Stangarlin and Prof. Dr. Odair José Kuhn, for their assistance in fungi identification; Nutrifarma Enterprise and Veterinarian Jaime Gris, for their aid with mycotoxin analysis.
Received April 2, 2012 and accepted December 14, 2012.
Corresponding author: taffarelle@gmail.com
- AMARAL, R.C.; NUSSIO, L.G. Fungos e micotoxinas. In: SIMPÓSIO DE PRODUÇÃO E UTILIZAÇÃO DE FORRAGENS CONSERVADAS, 4., 2011 Maringá. Anais... Maringá: UEM, 2011. p.221-249.
- BARROS, C.S.L. Intoxicação por fumonisina em suínos e eqüinos Laboratório de Patologia Veterinária, Universidade Federal de Santa Maria, 2011. Available at: <www.ufsm.br/lpv/aulas/II%20ENDIVET/endivet-palestra_5.pdf>. Accessed on: July 1, 2011.
- BULLERMAN, L.B.; TSAI, W.Y.L. Incidence and levels the fumonisins in corn and corn-based foods and feeds. Journal of Food Protection, v.57, n.6, p.541-546, 1994.
- CALIXTO JÚNIOR, M.; JOBIM, C.C.; CANTO, M.W. Taxa de desidratação e composição químico-bromatológica do feno de grama-estrela (Cynodon nlemfuensis Vanderyst) em função de níveis de adubação nitrogenada. Semina: Ciências Agrárias, v.28, n.3, p.493-502, 2007.
- CAVIGLIONE, J.H.; KIIHL, L.R.B.; CARAMORI, P.H. et al. Cartas climáticas do Paraná Londrina: IAPAR, 2000. Available at: <http://www.iapar.br/modules/conteudo/conteudo.php?conteudo=677>. Accessed on: Apr. 10, 2011.
- CECAVA, M.J. Making hay and haylage. In: PERRY, T.W.; CECAVA, M.J. (Eds.) Beef cattle feeding and nutrition 2.ed. San Diego: Academic Press, 1995. p.104-116.
- CECATO, U.; SANTOS, G.T.; MACHADO, M.A. et al. Avaliação de cultivares do gênero Cynodon com e sem nitrogênio. Acta Scientiarum, v.23, n.4, p.781-788, 2001.
- COBLENTZ, W.K.; TURNER, J.E.; SCARBROUGH, D.A. et al. Storage characteristics and nutritive value changes in bermudagrass hay as affected by moisture content and density of rectangular bales. Crop Science, v.40, p.1375-1383, 2000.
- COLLINS, M.; COBLENTZ, W.K. Post-harvest physiology. In: BARNES, R.F.; MOORE, K.J.; NELSON, C. et al. (Eds.) Forages: The science of grassland agriculture 6.ed. Ames, Iowa: Blackwell Publishing, 2007. p.583-599.
- COUNCIL FOR AGRICULTURAL SCIENCE AND TECNOLOGY - CAST. Mycotoxins: risks in plant, animal and human systems Nş 139. Ames, Iowa: CAST Task Force Report, 2003. 200p.
- CRUZ, L.C.H.da. Micologia veterinária 2.d. Rio de Janeiro: Revinter, 2010. 350p.
- DOMINGUES, P.F. Higiene dos alimentos Departamento de Higiene Veterinária e Saúde Pública. FMVZ/UNESP/Botucatu, 2006. Available at: <http://www.fmvz.unesp.br/Eventos/Especializacao/disciplinas/Aula6_Alimentos.pdf>. Accessed on: July 11, 2011.
- EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA EMBRAPA. Centro Nacional de Pesquisa de Solos. Sistema brasileiro de classificação de solos 2.ed. Rio de Janeiro, 2006. 306p.
- FOODS AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS FAO. WORLD HEALTH ORGANIZATION - WHO. Codex Alimentarius Commission. Position paper on fumonins Roma, 2000. Available at: <ftp://ftp.fao.org/codex/ccfac32/fa00_22e.pdf>. Accessed on: Ago. 7, 2011.
- FORMIGA, L.D.A.S.; PEREIRA FILHO, J.M.; NASCIMENTO JÚNIOR, N.G. et al. Diâmetro do caule sobre a desidratação, composição química e produção do feno de Jurema preta (Mimosa tenuiflora Wild. Poir.). Revista Brasileira de Saúde e Produção Animal, v.12, n.1, p.22-31, 2011.
- GONÇALVES, G.D.; SANTOS, G.T.; JOBIM, C.C. et al. Determinação do consumo, digestibilidade e frações protéicas e de carboidratos do feno de Tifton 85 em diferentes idades de corte. Revista Brasileira de Zootecnia, v.32, n.4, p.804-813, 2003.
- GUARRO, J.; GENÉ, J.; STCHIGEL, A.M. Developments in fungal taxonomy. Clinical Microbiology Reviews, v.12, n.3, p.454-500, 1999.
- JOBIM, C.C.; LOMBARDI, L.; GONÇALVES, G.D. et al. Desidratação de cultivares de Cynodon spp durante o processo de fenação. Acta Scientiarum, v.23, n.4, p.795-799, 2001.
- KAWASHIMA, L.M.; SOARES, L.M.V. Incidência de fuminisina B1, aflatoxinas B1, B2, G1 e G2, ocratoxina e a zearalenona em produtos de milho. Ciência e Tecnologia de Alimentos, v.26, n.3, p.516-521, 2006.
- LABORATÓRIO DE ANÁLISES MICOTOXICOLOGICAS - LAMIC. Limites máximos (ppb) de micotoxinas recomendados pelo LAMIC para animais de produção 2011. Available at: <http://www.lamic.ufsm.br/legislacao.html>. Accessed on: July 11, 2011.
- MABONI, F.; FICK, F.; MÜRMANN, L. et al. Avaliação dos níveis de contaminação de sorgo por zearalenona no sul do país Available at: <http://www.lamic.ufsm.br/papers/115z.pdf>. Accessed on: July 11, 2011.
- MALLMANN, C.A.; DILKIN, P. Micotoxinas e micotoxicoses em suínos Santa Maria: Ed. do Autor, 2007. 240p.
- MILICEVIC, D.R.; SKRINJAR, M.; BALTIC, T. Real and perceived risks por mycotoxin contamination in foods and feeds: challenges for food safety control. Toxins, n.2, p.572-592, 2010.
- MOSER, L.E. Post Harvest Physiological Changes in Forage Plants. In: MOORE, K.J.; KRAL, D.M.; VINEY, M.K. (Eds). Post harvest physiology and preservation of forages Madison: American Society of Agronomy, 1995. p.1-19.
- MUNKVOLD, G.P.; DESJARDINS, A.E. Fumonisin in maize: can we reduce their occurrence? Plant Disease, v.81, p.556-565, 1997.
- NASCIMENTO, J.M.; COSTA, C.; SILVEIRA, A.C. et al. Influência do método de fenação e tempo de armazenamento sobre a composição bromatológica e ocorrência de fungos no feno de alfafa (Medicago sativa, L. cv. Flórida 77). Revista Brasileira de Zootecnia, v.29, n.3, p.669-677, 2000.
- OHIO Agricultural Research and Development Center - OARDC. Fumonisins Available at: <http://www.oardc.ohio-state.edu/ohiofieldcropdisease/t01_pageview2/Fumonisins.htm>. Accessed on: July 11, 2011a.
- OHIO Agricultural Research and Development Center - OARDC. Zearalenone Available at: <http://www.oardc.ohio-state.edu/ohiofieldcropdisease/t01_pageview2/Zearalenone.htm>. Accessed on: July 11, 2011b.
- OSWALD, I. Micotoxinas e imunidade Available at: <http://www.knowmycotoxins.com/pt/documents/IsabellePortugueseFinal18.12000.pdf>. Accessed on: July 11, 2011.
- PEREIRA, O.G.; GOBBI, K.F.; PEREIRA, D.H. et al. Conservação de forragens como opção para o manejo de pastagens. In: REUNIÃO ANUAL DA SOCIEDADE BRASILEIRA DE ZOOTECNIA, 43., 2006, João Pessoa. Anais... João Pessoa: SBZ, 2006. p.507-539.
- PIMENTEL-GOMES, F.P. Curso de estatística experimental 15.ed. Piracicaba: FEALQ, 2009. 451p.
- PIMENTEL-GOMES, F.; GARCIA, C.H. Estatística aplicada a experimentos agronômicos e florestais: exposição com exemplos e orientações para uso de aplicativos 11.ed. Piracicaba: FEALQ, 2002. 309p.
- PINHEIRO, A.C.; PEÇA, J.O. Forage drying models for oats and vetches under Mediterranean climate conditions. Mathematics and Computers in Simulation, n.65, p.87-100, 2004.
- POZZI, C.R.; ARCARO, J.R.P.; ARCARO JUNIOR, I. et al. Aspectos relacionadas à ocorrência e mecanismo de ação de fumonisinas. Ciência Rural, v.32, n.5, p.901-907, 2002.
- REIS, R.A.; MOREIRA, A.L.; PEDREIRA, M.S. Técnicas para produção e conservação de fenos de forrageiras de alta qualidade. In: SIMPÓSIO SOBRE PRODUÇÃO E UTILIZAÇÃO DE FORRAGENS CONSERVADAS, Maringá, 2001. Anais... Maringá: UEM/CCA/DZO, 2001a. 319p.
- REIS, R.A.; RODRIGUES, L.R.A.; RESENDE, K.T. et al. Avaliação de fontes de amônia para o tratamento de fenos de gramíneas tropicais. 2. Compostos nitrogenados. Revista Brasileira de Zootecnia, v.30, n.3, p.682-686, 2001b.
- RIBEIRO, K.G.; PEREIRA, O.G.; VALADARES FILHO, S.C. et al. Caracterização das frações que constituem as proteínas e os carboidratos, e respectivas taxas de digestão, do feno de capim-Tifton 85 de diferentes idades de rebrota. Revista Brasileira de Zootecnica, v.30, n.2, p.589-595, 2001.
- ROTZ, C.A. Field curing of forage. In: MOORE, K.J.; KRAL, D.M.; VINEY, M.K. (Eds). Post-harvest physiology and preservation of forages Madison: American Society of Agronomy, 1995. p.39-66.
- ROTZ, C.A. How to maintain forage quality during harvest and storage. Advances in Dairy Tecnology, v.15, p. 227-239, 2003.
- ROTZ, C.A.; SHINNERS, K.J. Hay harvest and storage. In: BARNES, R.F.; MOORE, K.J.; NELSON, C. et al. (Eds). Forages: The science of grassland agriculture 6.ed. Ames, Iowa: Blackwell Publishing, 2007. p.601-616.
- SCHIABEL, V.C. Genética e toxicidade de Fusarium verticillioides em grãos de milho (Zea mays L.) sob plantio direto e convencional 2004. 95f. (Dissertação em Agronomia) - Universidade Estadual de Londrina, Londrina.
- SNEDECOR, G.W.; COCHRAN, W.G. Statistical methods 6.ed. Ames: Iowa State University, 1989. 593p.
- SWEENWEY, M.J.; DOBSON, A.D.W. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. International Journal of Food Microbiology, v.43, p.141-158, 1998.
- TRÉS, J.E.; PEREIRA, R.C.G., OLIVEIRA, J.P. et al. Influência da zearalenona sobrea reprodução de novilhas mestiças. Revista Brasileira de Medicina Veterinária, v.33, n.1, p.48-50, 2011.
- WRIGHT, D.A; FROST, J.P.; PATTERSON, D.C. et al. The influence of weight of ryegrass per unit area and treatment at and after mowing on rate of drying. Grass and Forage Science, v.52, p.86-98, 1997.
- WRIGHT, D.A; FROST, J.P.; KILPATRICK, D.J. The influence of weather factors on the drying rate of cut perennial ryegrass herbage under controlled conditions. Grass and Forage Science, v.55, p.331-342, 2000.
Publication Dates
-
Publication in this collection
07 June 2013 -
Date of issue
June 2013
History
-
Received
02 Apr 2012 -
Accepted
14 Dec 2012