Abstract
Lactobacillus buchneri 40788 and the fibrolytic enzymes β-glucanase and xylanase were applied to chopped sugarcane to study their effects on the nutritive value of silage. Sugarcane was mechanically harvested after 14 mo of growth and treated without (control) or with L. buchneri at a theoretical application rate of 5 × 10(4) cfu/g, 1 × 10(5) cfu/g, or 1 × 10(5) cfu/g plus enzymes. Forage was packed into farm-scale bag silos (40 t/silo) and stored for 92 d. Fifty-six bulls (32 Nellore and 24 Charolais × Nellore) were housed in 20 collective pens and fed diets comprising (dry matter [DM] basis) 458 g/kg sugarcane silage and 542 g/kg concentrates for an 84-d period. Treated silages had higher concentrations of acetic acid and lower concentrations of ethanol. Total mixed rations (TMR) containing inoculated silages exhibited significantly lower neutral detergent fiber (NDF) concentration and, consequently, higher in vitro DM digestibility (IVDMD). Thus, animals fed TMR containing treated silages spent less time chewing per day and per kilogram of DM intake (DMI), even at higher DMI levels. Nonetheless, the intake of NDF was similar across treatments (0.77 to 0.79 kg/100 kg BW) but markedly lower than the value reported for traditional forages. Average daily gain was significantly greater for animals fed TMR based on inoculated silages due to the higher DMI (14% on average) and the higher energy content of the diets, as indicated by the higher feed efficiency (12% on average). The dose of inoculants used and the addition of fibrolytic enzymes had no significant effects on silage parameters or animal performance. Therefore, inoculation of L. buchneri during sugarcane ensilage can alter the fermentation pattern by increasing acetic acid yield, reducing silage nutrient losses, and improving feed efficiency by bulls.
feed efficiency; heterolactic bacteria; Saccharum officinarum L.; voluntary feed intake
FORAGE CROPS
Effects of Lactobacillus buchneri on the nutritive value of sugarcane silage for finishing beef bulls1 1 Funded by FAPESP, CAPES, and CNPq
Patrick SchmidtI; Luiz Gustavo NussioII; Oscar Cézar Müller QueirozIII; Mateus Castilho SantosII; Maity ZopollattoI; Sérgio Gil de Toledo FilhoII; João Luiz Pratti DanielII
IDepartamento de Zootecnia, Universidade Federal do Paraná, Curitiba, PR, Brasil
IIDepartamento de Zootecnia, Escola Superior de Agricultura "Luiz de Queiroz", Piracicaba, SP, Brasil
IIIDepartment of Animal Sciences, University of Florida, Gainesville, USA
ABSTRACT
Lactobacillus buchneri 40788 and the fibrolytic enzymes β-glucanase and xylanase were applied to chopped sugarcane to study their effects on the nutritive value of silage. Sugarcane was mechanically harvested after 14 mo of growth and treated without (control) or with L. buchneri at a theoretical application rate of 5 × 104 cfu/g, 1 × 105 cfu/g, or 1 × 105 cfu/g plus enzymes. Forage was packed into farm-scale bag silos (40 t/silo) and stored for 92 d. Fifty-six bulls (32 Nellore and 24 Charolais × Nellore) were housed in 20 collective pens and fed diets comprising (dry matter [DM] basis) 458 g/kg sugarcane silage and 542 g/kg concentrates for an 84-d period. Treated silages had higher concentrations of acetic acid and lower concentrations of ethanol. Total mixed rations (TMR) containing inoculated silages exhibited significantly lower neutral detergent fiber (NDF) concentration and, consequently, higher in vitro DM digestibility (IVDMD). Thus, animals fed TMR containing treated silages spent less time chewing per day and per kilogram of DM intake (DMI), even at higher DMI levels. Nonetheless, the intake of NDF was similar across treatments (0.77 to 0.79 kg/100 kg BW) but markedly lower than the value reported for traditional forages. Average daily gain was significantly greater for animals fed TMR based on inoculated silages due to the higher DMI (14% on average) and the higher energy content of the diets, as indicated by the higher feed efficiency (12% on average). The dose of inoculants used and the addition of fibrolytic enzymes had no significant effects on silage parameters or animal performance. Therefore, inoculation of L. buchneri during sugarcane ensilage can alter the fermentation pattern by increasing acetic acid yield, reducing silage nutrient losses, and improving feed efficiency by bulls.
Key Words: feed efficiency, heterolactic bacteria, Saccharum officinarum L., voluntary feed intake
Introduction
Fresh chopped sugarcane is widely used for feeding beef and dairy cattle because its harvesting period coincides with the period of pasture shortage in Brazil. However, to avoid daily harvesting, chopping, and hauling and to prevent crop loss by accidental fire, this forage could be ensiled. In addition, considering that sugarcane is a semi-perennial tropical grass, its field lifespan may be prolonged by uniform harvesting and post-harvesting management.
Ensiling sugarcane results in the conversion of most of the water-soluble carbohydrates (WSC) into fermentation end-products, which are characterized by high levels of volatile organic compounds, mainly ethanol (Kung Jr. and Stanley, 1982; Pedroso et al., 2005). Although the gross energy is almost completely recovered during alcoholic fermentation, large amounts of DM and net energy are lost (McDonald et al., 1991; Daniel and Nussio, 2011).
Furthermore, ethanol is metabolized to acetate in the rumen with concomitant methane formation (Yoshii et al., 2005), which has a negative impact on the environment. Due to the undesirable characteristics of the natural fermentation of sugarcane, additives have been recommended to inhibit epiphytic yeast populations and mitigate alcohol synthesis in sugarcane silages (Pedroso et al., 2008).
A heterofermentative lactic acid bacterium, Lactobacillus buchneri, has been studied as an inoculant to improve the preservation of sugarcane silage during both anaerobic storage and air exposure (Pedroso et al., 2008; Ávila et al., 2009). L. buchneri is known to produce acetic acid (Oude Elferink et al., 2001; Pahlow et al., 2003), which is a powerful antifungal agent (Danner et al., 2003) capable of decreasing ethanol production and improving the aerobic stability of silages (Ranjit et al., 2002; Reich and Kung Jr., 2010). Conversely, high concentrations of acetic acid in silages have the potential to reduce feed intake (Dinius et al., 1968; Hutchinson and Wilkins, 1971).
Several studies have reported increased DM recovery when L. buchneri was applied to sugarcane silages (Schmidt, 2009; Zopollatto et al., 2009). However, experiments involving animals fed sugarcane silage are scarce. The objectives of this study were to evaluate the effects of dose of L. buchneri (strain 40788) and its association with fibrolytic enzymes on the fermentation of sugarcane silage ensiled in farm-scale silos and on the performance of finishing beef bulls. We hypothesized that L. buchneri alone or associated with fibrolytic enzymes may improve the nutritive value of sugarcane silages for finishing beef bulls.
Material and Methods
Sugarcane variety RB 85-5536 was mechanically harvested (Colhiflex Mentamit®, Cajurú, Brazil) from one field after 14 mo of growth (first cut) in 2002-2003 crop year, to a theoretical cut of 8 mm, and packed into 4 bag silos (2.7 m i.d., Pacifil, Estância Velha, Brazil). At harvest, the content of soluble solids in the sugarcane juice was 21.8ºbrix, indicating that the crop was mature. The mean chemical composition (DM basis) of fresh sugarcane was 332 g/kg DM, 23 g/kg ash, 502 g/kg NDF, 290 g/kg ADF, and 41 g/kg CP, and in vitro DM digestibility (IVDMD) was 615 g/kg.
The forage placed in one bag was sprayed with water (Control), whereas the remaining three bags were treated as follows: (LLB) a low dose of L. buchneri 40788 (final application rate of 5 × 104 cfu/g of fresh forage); (HLB) a high dose of L. buchneri 40788 (final application rate of 1 × 105 cfu/g of fresh forage); and (HLBE) a high dose of L. buchneri 40788 plus fibrolytic enzymes (final application rate of 1 × 105 cfu/g plus β-glucanase at 32,340 IU/t and xylanase at 40,165 IU/t of fresh forage; Lallemand Animal Nutrition, Milwaukee, WI). Aqueous solutions of the additives were sprayed onto the forages at a rate of 2.2 L/t. Approximately 40 t were packed into each bag.
After 92 d of storage, the bags were opened and the silages were used to prepare four diets for feeding beef bulls. The diets comprised (DM basis) 458 g/kg sugarcane silage, 314 g/kg dried citrus pulp, 203 g/kg corn gluten feed, 14 g/kg urea, and 11 g/kg mineral and vitamin mix. The mineral and vitamin mix contained 40 g/kg Na; 65 g/kg S; 9 ppm Co; 1,000 ppm Cu; 600 ppm Mn; 2,500 ppm Zn; 50 ppm I; 10 ppm Se; 350,000 IU/kg vitamin A; 30,000 IU/kg vitamin D; 1,800 IU/kg vitamin E; and 25 g/kg monensin. Dietary requirements of Ca and P were met with concentrate ingredients (NRC, 1996). The four diet treatments were named in the same way as the silages described above.
Fifty-six bulls (32 Nellore and 24 Charolais × Nellore, 15 to 18 mo old) from the University of São Paulo herd were stratified by breed and BW and randomly housed in 20 covered pens (21 m2, concrete floor); two or three animals were housed in each pen. Fresh water was available at all times, and the animals were cared for using accepted protocols (FASS, 2010). The bulls were de-wormed with ivermectin (200 µg/kg BW; Ivomec Merial®, Paulínia, Brazil) and acclimated to the facilities for 21 d. During the adaptation period, the bulls were fed a diet containing 542 g/kg concentrates (as above) and 458 g/kg sugarcane silages (1/4 from each treatment, DM basis). Body weight was recorded after 12 h of fasting at the beginning and end of the study (84-d period). Initial BW was 426±54 kg (mean ± SD) for Nellore and 513±43 kg for Charolais × Nellore bulls.
Silages were feed-out, mixed with concentrates, and animals were fed TMR once a day targeting 100 g/kg of orts in the next day. Feed intake was determined by the difference between the amount of feed offered and refused every day. The DMI, average daily gain (ADG), and feed efficiency (ADG:DMI) were estimated for the 84-d feeding period. Ingestive behavior was recorded on day 48 of the experiment by visual observation of the animals throughout a 24-h period. Eating and ruminating activities were recorded at 10-min intervals and a 24-h pattern was estimated considering a constant behavior between observations (Maekawa et al., 2002). Chewing (eating + ruminating) per kilogram of DM and NDF was calculated using measurements of DMI and NDF intake recorded on the same day.
Silage, TMR, and orts were sampled weekly (n = 12) and dried in an air-forced oven at 55 ºC for 72 h. Aliquots of silages were also used to determine the particle size (Lammers et al., 1996) and prepare aqueous extracts (Kung Jr. et al., 1984).
Dried samples were ground through a 1-mm screen (Wiley mill), and sub-samples were analyzed for the following: DM in an air-forced oven at 105 ºC for 24 h (AOAC, 1980); CP by the Dumas method (Wiles et al., 1998); ash (AOAC, 1980); and NDF and ADF (sequential and expressed inclusive of residual ash; Van Soest et al., 1991). Hemicellulose was calculated as NDF minus ADF. The IVDMD was determined using the Tilley and Terry method as modified by Goering and Van Soest (1970).
Aqueous extracts from silages were analyzed for pH, VFA (Palmquist and Conrad, 1971), lactic acid (Pryce, 1969), and WSC (Dubois et al., 1956). Ethanol concentration was determined using a biochemistry analyzer (YSI 2700 Biochemistry Analyzer, Yellow Springs, OH) that uses membrane-immobilized ethanol oxidase (EC 1.1.3.13) (Taylor et al., 1999).
Statistical analysis was performed by the MIXED procedure of SAS (Statistical Analysis System, version 9.2) using the following model: yij = µ + αi + βj + εij, in which µ = overall mean; αi = random effect of block (i = 1 to 5); βj = fixed effect of treatment (j = 1 to 4); and εij = residual error. The block effect included the variations of animal breed and initial BW. Pens were considered as the experimental units. Degrees of freedom of treatment were partitioned into three single degree of freedom orthogonal contrasts: additive application effect (Control vs. LLB+HLB+HLBE), additive dose effect (LLB vs. HLB+HLBE), and enzyme application effect (HLB vs. HLBE). Contrasts were declared significant at P<0.05.
Results and Discussion
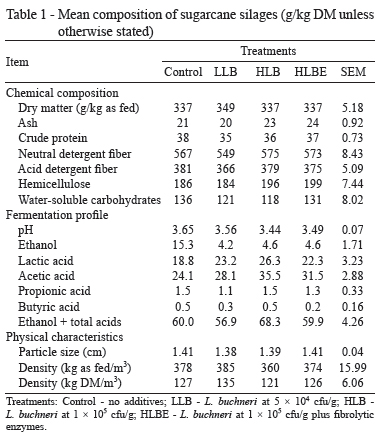
There were no great alterations in chemical entities among silages (Table 1), although inoculated silages had higher concentrations of acetic acid and lower concentrations of ethanol. L. buchneri is known to produce acetic acid (Oude Elferink et al., 2001), which is a powerful antifungal agent (Danner et al., 2003) capable of inhibiting yeast and reducing ethanol formation and DM loss during silage fermentation (Ranjit et al., 2002; Pedroso et al., 2008; Ávila et al., 2009). In the present study, L. buchneri increased the acetic acid content by 31.5% and decreased the ethanol concentration by 70.8%. Nevertheless, ethanol concentrations were much lower than those reported in the literature (Zopollatto et al., 2009; Daniel et al., 2013), probably because of partial volatilization during silage feedout.
Unexpectedly, the DM contents of silages were equivalent to that of fresh sugarcane before ensiling. The conversion of WSC to fermentation end-products generates gases and water (McDonald et al., 1991), which ultimately increase the moisture content. Ensiling sugarcane under laboratory conditions normally decreases DM concentrations (Schmidt, 2009). In farm-scale silos, however, this effect is not always evident if the losses of DM and moisture occur at similar magnitudes (Pedroso et al., 2006). Furthermore, most of the volatile compounds may have been lost during the oven-drying of the silage samples in the laboratory (McDonald and Dewar, 1960; Daniel and Nussio, 2011), thus leading to underestimation of the DM content and reinforcing the extensive occurrence of water loss from the silo working face.
Inoculant dose and addition of fibrolytic enzymes led to silages with similar composition. Several authors have reported seeing no effect of fibrolytic enzymes on tropical silages (Loures et al., 2005; Avellaneda-Cevallos et al., 2009). Sugarcane crop typically contains a high content of WSC. Therefore, the small amount of sugars provided by cell wall hydrolysis would not be expected to change sugar abundance and, thus, the fermentation process.
Rations containing inoculated silages exhibited (numerically) lower NDF and, consequently, higher IVDMD (Table 2). Similarly, the higher DM content may indicate that nutrients are better preserved in treated silages (Schmidt, 2009). These alterations suggest the higher nutritive value of sugarcane inoculated with additives as indicated by animal performance.
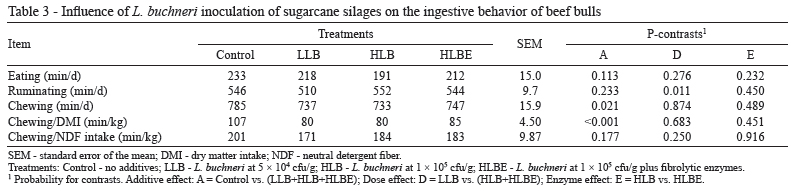
Ingestive behavior was recorded to verify the possible adverse effects of silage inoculation on the eating pattern because high levels of acetic acid have been associated with decreased intake (Dinius et al., 1968). In the present study, silage inoculation with L. buchneri did not significantly affect eating (P = 0.113) or rumination (P = 0.233; Table 3). However, because of the higher content of NDF, animals fed TMR that contained control silage spent more time chewing per day (P = 0.021) and per kilogram of DMI (P<0.001), even with a lower DMI (see below). A dose effect was observed for ruminating (P = 0.011), but the reason for this was unclear.As anticipated, chewing/kg NDF intake was unchanged by treatments (P = 0.177). Therefore, enzyme application was unable to alter the ability of fiber to stimulate chewing (P = 0.916).
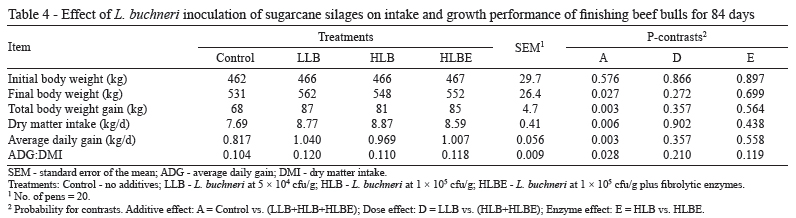
Bulls fed TMR containing sugarcane silage inoculated with L. buchneri had higher DMI (P = 0.006; Table 4). Several studies have shown that when cattle or sheep are fed silages treated with L. buchneri, DMI is not affected (Driehuis et al., 1999; Taylor et al., 2002; Ranjit et al., 2002; Kung et al., 2003). However, in the present study, the inoculants were effective in mitigating NDF accumulation caused by sugar oxidation during the ensiling process and improved the DMI. Indeed, the control treatment limited DMI with rumen fill from dietary fiber. Nonetheless, the NDF intake was similar across treatments (0.77 to 0.79 kg/100 kg BW; P = 0.555) but substantially lower than the value reported for traditional forages (1.0 to 1.2 kg/100 kg BW; Mertens, 1994; Krizsan et al., 2010). Feed intake constraint was consistent with the high chewing activity. Low fiber digestibility is a possible explanation for the lower feed intake and higher chewing activity associated with sugarcane-based diets compared with conventional roughage (Corrêa et al., 2003; Costa et al., 2005).
The average daily gain (ADG) was significantly greater for animals fed TMR based on inoculated silages (P = 0.003) (Table 4) due to the higher DMI (14% on average) and the higher energy content of the diets, as indicated by the superior feed efficiency (12% on average). In addition to increased ADG, animals fed TMR that contained inoculated silages exhibited higher final BW and total BW gain across the 84-d finishing period. The application rate of the inoculants and the addition of fibrolytic enzymes did not affect animal performance (P>0.119). An earlier study carried out by Pedroso et al. (2006) reported an increase of 31.9% in the ADG of Holstein heifers fed a TMR that contained sugarcane inoculated with L. buchneri 40788 (1.24 kg/d) compared with animals fed a TMR that was based on untreated silage (0.94 kg/d). In the current trial, silage DM was determined by oven drying; thus, the DM content may have been underestimated, leading to underestimation of DMI and overestimation of feed efficiency (McDonald and Dewar, 1960; Daniel and Nussio, 2011).
It is important to emphasize that the dietary level of forage adopted in the current study was within the practical range under Brazilian conditions (Millen et al., 2009) but is much greater than the concentration of roughage used in feedlots in the U.S. (Vasconcelos and Galyean, 2007). Therefore, one can expect less improvement in animal performance resulting from the use of silage additives when TMR containing less forage are fed.
Conclusions
Inoculating sugarcane with L. buchneri 40788 at ensiling can alter the fermentation process by increasing acetic acid production. Due to the antifungal properties of acetic acid, the total mixed ration containing treated silages have higher nutritive value. Bulls fed a total mixed ration containing sugarcane silages inoculated with
L. buchneri 40788 eat greater amounts of dry matter and gain more body weight than bulls feeding untreated silage. Furthermore, silages treated with L. buchneri 40788 improve feed efficiency. The dose of L. buchneri and the addition of fibrolytic enzymes have no significant effects on silage parameters or animal performance.
Received March 13, 2013 and accepted October 17, 2013.
Corresponding author: jldaniel@usp.br
- AOAC -Association of Official Analytical Chemistry. 1980. Official methods of analysis. 13th ed. AOAC International, Arlington, VA.
- Avellaneda-Cevallos, J. H.; Montañez-Valdez, O. C.; Gonzáles-Muñoz, S.; Pinos-Rodrigues, J. and Barcena-Gama, R. 2009. Effect of exogenous fibrolytic enzymes on dry matter and cell wall in vitro digestibility of guinea grass hay. Journal of Applied Animal Research 36:199-202.
- Ávila, C. L. S.; Pinto, J. C.; Figueiredo, H. C. P. and Schwan, R. F. 2009. Effects of an indigenous and a commercial Lactobacillus buchneri strain on quality of sugar cane silage. Grass and Forage Science 6:384-394.
- Corrêa, C. E. S.; Pereira, M. N.; Oliveira, S. G. and Ramos, M. H. 2003. Performance of Holstein cows fed sugarcane or corn silages of different grain textures. Scientia Agricola 60:621-629.
- Costa, M. G.; Campos, J. M. S.; Valadares Filho, S. C.; Valadares, R. F. D.; Mendonça, S. S.; Souza, D. P. and Teixeira, M. P. 2005. Desempenho produtivo de vacas leiteiras alimentadas com diferentes proporções de cana-de-açúcar e concentrado ou silagem de milho na dieta. Revista Brasileira de Zootecnia 34:2437-2445.
- Daniel, J. L. P. and Nussio, L. G. 2011. Contribution of silage volatile compounds for the animal nutrition. p.279-306. In: Proceedings of the 2nd International Symposium on Forage Quality and Conservation, São Pedro. FEALQ, Piracicaba.
- Daniel, J. L. P.; Weiß, K.; Custódio, L.; Sá Neto, A.; Santos, M. C.; Zopollatto, M. and Nussio, L. G. 2013. Occurrence of volatile organic compounds in sugarcane silages. Animal Feed Science and Technology 185:101-105.
- Danner, H.; Holzer, M.; Mayrhuber, E. and Braun, R. 2003. Acetic acid increases stability of silage under aerobic conditions. Applied and Environmental Microbiology 69:562-567.
- Dinius, D. A.; Hill, D. L. and Noner, C. H. 1968. Influence of supplemental acetate feeding on the voluntary intake of cattle fed green corn and corn silage. Journal of Dairy Science 51:1505-1507.
- Driehuis, F.; Oude Elferink, S. J. W. H. and Van Wikleaar, P. G. 1999. Lactobacillus buchneri improves the aerobic stability of laboratory and farm scale whole crop maize but does not affect feed intake and milk production of dairy cows. p.106-107. In: Proceedings of the 12th International Silage Conference. SLU, Uppsala, Sweden.
- Dubois, M.; Gilles, K. A.; Hamilton, J. K.; Rebers, P. A. and Smith, F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28:350-356.
- FASS -Federation of Animal Science Societies. 2010. Guide for the care and use of agricultural animals in teaching and research. 3rd ed. Champaign, IL.
- Goering, H. K. and Van Soest, P. J. 1970. Forage fiber analyses (apparatus, reagents, procedures and some applications).
- Agriculture Handbook No. 379. USDA Agricultural Research Service, Washington, D.C.
- Hutchinson, K. J. and Wilkins, R. J. 1971. The voluntary intake of silage by sheep II. The effects of acetate on silage intake. Journal of Agricultural Science 77:539-543.
- Krizsan, S. J.; Ahvenjärvi, S. and Huhtanen, P. 2010. A meta-analysis of passage rate estimated by rumen evacuation with cattle and evaluation of passage rate prediction models. Journal of Dairy Science 93:5890-5901.
- Kung Jr., L. and Stanley, R. W. 1982. Effect of stage of maturity on the nutritive value of whole-plant sugarcane preserved as silage. Journal of Animal Science 54:689-696.
- Kung Jr., L.; Grieve, D. B.; Thomas, J. W. and Huber, J. T. 1984. Added ammonia or microbial inoculant for fermentation and nitrogenous compounds of alfalfa ensiled at various percents of dry matter. Journal of Dairy Science 67:299-306.
- Kung Jr., L.; Taylor, C. C.; Lynch, M. P. and Neylon, J. M. 2003. The effect of treating alfalfa with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for lactating dairy cows. Journal of Dairy Science 86:336-343.
- Lammers, B. P.; Buckmaster, D. R. and Heinrichs, A. J. 1996. A simple method for the analysis of particle sizes of forage and total mixed rations. Journal of Dairy Science 79:922-928.
- Loures, D. R. S.; Nussio, L. G.; Paziani, S. F.; Pedroso, A. F.; Mari, L. J.; Ribeiro, J. L.; Zopollatto, M.; Schmidt, P.; Junqueira, M. C.; Packer, I. U. and Campos, F. P. 2005. Composição bromatológica e produção de efluente de silagens de capim-Tanzânia sob efeitos do emurchecimento, do tamanho de partícula e do uso de aditivos biológicos. Revista Brasileira de Zootecnia 34:726-735.
- Maekawa, M.; Beauchemin, K. A. and Christensen, D. A. 2002. Effect of concentrate level and feeding management on chewing activities, saliva production, and ruminal pH of lactating dairy cows. Journal of Dairy Science 485:1165-1175.
- McDonald, P. and Dewar, W. A. Determination of dry matter and volatiles in silage. 1960. Journal of the Science of Food and Agriculture 11:566-570.
- McDonald, P.; Henderson, A. R. and Heron, S. J. 1991. Biochemistry of silage. 2nd ed. Chalcombe Publication, Marlow.
- Mertens, D. R. 1994. Regulation of forage intake. p.450-493. In: Forage quality, evaluation and utilization. Fahey Jr., G. C., ed. American Society of Agronomy, Madison.
- Millen, D. D.; Pacheco, R. D. L.; Arrigoni, M. D. B.; Galyean, M. L. and Vasconcelos, J. T. 2009. A snapshot of management practices and nutritional recommendations used by feedlot nutritionists in Brazil. Journal of Animal Science 87:3427-3439.
- NRC -National Research Council. 1996. Nutrient requirements of beef cattle. 7th ed. National Academic Press, Washington, D.C.
- Oude Elferink, S. J. W. H.; Krooneman, J.; Gottschal, J. A.; Spoelstra, S. F.; Faber, F. and Driehuis, F. 2001. Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri Applied and Environmental Microbiology 67:125-132.
- Palmiquist, D. and Conrad, H. 1971. Origin of plasma fatty acids in lactating cows fed high grain or high fat diets. Journal of Dairy Science 54:1025.
- Pahlow, G.; Muck, R. E.; Driehuis, F.; Oude Elferink, S. J. W. H. and Spoelstra, S. F. 2003. Microbiology of ensiling. p.31-94. In: Silage science and technology. Buxton, D. R.; Muck, R. E. and Harrison, J. H., eds. American Society of Agronomy; Crop Science Society of America; Soil Science Society of America, Madison.
- Pedroso, A. F.; Nussio, L. G.; Paziani, S. F.; Loures, D. R. S.; Igarasi, M. S.; Coelho, R. M.; Packer, I. H.; Horii, J. and Gomes, L. H. 2005. Fermentation and epiphytic microflora dynamics in sugarcane silage. Scientia Agricola 62:427-432.
- Pedroso, A. F.; Nussio, L. G.; Barioni, W.; Rodrigues, A. A.; Loures, D. R. S.; Campos, F.; Ribeiro, J. L.; Mari, L. J.; Zopollatto, M.; Junqueira, M.; Schmidt, P.; Paziani, S. F. and Horii, J. 2006. Performance of Holstein heifers fed sugarcane silages treated with urea, sodium benzoate or Lactobacillus buchneri Pesquisa Agropecuária Brasileira 41:649-654.
- Pedroso, A. F.; Nussio, L. G.; Loures, D. R. S.; Paziani, S. F.; Ribeiro, J. L.; Mari, L. J.; Zopollatto, M.; Schmidt, P.; Mattos, W. R. S. and Horii, J. 2008. Fermentation, losses, and aerobic stability of sugarcane silages treated with chemical or bacterial additives. Scientia Agricola 65:589-594.
- Pryce, J. D. 1969. A modification of Barker-Summerson method for the determination of lactic acid. Analyst 94:1151-1152.
- Ranjit, N. K.; Taylor, C. C. and Kung Jr., L. 2002. Effect of Lactobacillus buchneri 40788 on the fermentation, aerobic stability and nutritive value of maize silage. Grass and Forage Science 57:73-81.
- Reich, L. J. and Kung Jr., L. 2010. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Animal Feed Science and Technology 159:105-109.
- Schmidt, P. 2009. Improved efficiency of sugarcane ensiling for ruminant supplementation. p.47-72. In: Proceedings of the 1st International Symposium on Forage Quality and Conservation, São Pedro. FEALQ, Piracicaba.
- Taylor, A. G.; Johnson, C. F.; Kataki, P. K. and Obendorf, R. L. 1999. Ethanol production by hydrated seeds: A highresolution index of seed quality. Acta Horticulturae 504:153-160.
- Taylor, C. C.; Ranjit, N. J.; Mills, J. A.; Neylon, J. M. and Kung Jr., L. 2002. The effect of treating whole-plant barley with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for dairy cows. Journal of Dairy Science 85:1793-1800.
- Van Soest, P. J.; Robertson, J. B. and Lewis, B. A. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74:3583-3597.
- Vasconcelos, J. T. and Galyean, M. L. 2007. Nutritional recommendations of feedlot consulting nutritionists: The 2007 Texas Tech University survey. Journal of Animal Science 85:2772-2781.
- Wiles, P. G.; Gray, I. K. and Kissling, R. C. 1998. Routine analysis of protein by Kjeldahl and Dumas methods: review and interlaboratory study using dairy products. Journal of AOAC International 81:620-632.
- Yoshii, T.; Asanuma, N. and Hino, T. 2005. Effect of ethanol on nitrate and nitrite reduction and methanogenesis in the ruminal microbiota. Animal Science Journal 76:37-42.
- Zopollatto, M.; Daniel, J. L. P. and Nussio, L.G. 2009. Aditivos microbiológicos em silagens no Brasil: revisão dos aspectos da ensilagem e do desempenho de animais. Revista Brasileira de Zootecnia 38(supl. especial):170-189.
Publication Dates
-
Publication in this collection
13 Jan 2014 -
Date of issue
Jan 2014
History
-
Received
13 Mar 2013 -
Accepted
17 Oct 2013