ABSTRACT
This study aimed to assess the effects of palm kernel cake on semen quality and biochemical parameters of Santa Inês lambs. A total of 40 animals with 24.10±2.72 kg body weight and five months old were assigned in a completely randomized design into four groups and 10 replicates. The animals were subjected to four levels of palm kernel cake (0, 15, 30, and 45%) based on dry matter. The trial lasted 90 days foregone by 15 days for adaptation. Blood samples were collected every 45 days from jugular vein using vacuum tubes without anticoagulant. Total serum cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, and very-low-density lipoprotein were assessed. Once the animals reached puberty at a mean age of 225 days, the semen samples were collected by electroejaculator once a week for three sequence weeks and assessed for volume, color, aspect, wave motion, motility, sperm concentration, sperm vigor, total of spermatozoa per ejaculate, viable spermatozoa per mL, and sperm morphology. The data were subjected to analysis of variance and followed by regression analysis. Non-parametric data were analysed by Kruskal-Wallis test. Total cholesterol, high-density lipoprotein, triglycerides, and very-low-density lipoprotein were linearly increased. There was no difference for low-density lipoprotein. Diets did not affect mass motility, sperm motility, vigor, total spermatozoa per ejaculate, viability sperm per mL, and minor and total sperm defects. Sperm concentration increased linearly. Negative quadratic effects were observed for major sperm defects. Supplementation of diets with palm kernel cake up to 45% on dry matter enhance biochemical parameters and do not impair the qualitative variables of lamb sperm.
Key Words:
animal nutrition; animal breeding; fatty acids; small ruminants
Introduction
Nutrition plays a vital role in the reproductive performance of sheep, since it affects the hypothalamic-pituitary-gonadal axis. There is a forceful link between nutrition and reproduction in male animals, in which underfeeding negatively affects hormonal status and reproductive function because of the severe negative energy balance. This in turn results in delayed puberty, low testicular development, and reduced semen production as a response to disorders in gonadotropinreleasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) synthesis (Zabuli et al., 2009Zabuli, J.; Tanaka, T.; Lu, Wengeng; Kuroiwa, T. and Kamomae, H. 2009. Responses of gonadotropin secretion to short-term dietary supplementation in ovariectomized goats with different body weights. Animal Reproduction Science 116:274-281.).
However, male reproductive performance and sperm quality are basically sustained by the proper functioning of the endocrine system, which leads and stimulates androgen biosynthesis from cholesterol, whose concentration in blood may establish testosterone secretion modulated by steroidogenic acute regulatory protein and translocator protein. Within the cells, this protein transfers cholesterol from the outer to the inner mitochondrial membrane, being the main step for testosterone biosynthesis. Disorders in StAR function are related to testicular dysfunction and underfeeding condition (Awad et al., 2015Awad, N. S.; Soliman, M. M.; Mohamed, A. A.; Sabry, A. M.; Shahaby, A. F. and El-Tarras, A. E. 2015. Effect of altitude on some male fertility related traits in Saudi ovine and caprine species. Annals of Animal Science 15:641-653.).
Cholesterol is synthetized as response to the energy metabolism in the liver, using glucose and aminoacids as key precursors. Nutritional status and composition of dietary fatty acids seems to be a key condition to the levels of cholesterol in the blood. Moreover, dietary polyunsaturated fatty acids (PUFA) enhance energy density and optimize reproductive parameters in male sheep. However, high levels of cholesterol in animals and higher accumulation in Sertoli cells may thereby reduce normal and physiologycal testicular function, decrease sperm concentration, impair sperm motility, and undervalue the male fertility (Fair et al., 2014Fair, S.; Doyle, D. N.; Diskin, M. G.; Hennessy, A. A. and Kenny, D. A. 2014. The effect of dietary n-3 polyunsaturated fatty acids supplementation of rams on semen quality and subsequent quality of liquid stored semen. Theriogenology 81:210-219.; Morgan et al., 2014Morgan, D. H.; Ghribi, O.; Hui, L.; Geiger, J. D. and Chen, X. 2014. Cholesterol-enriched diet disrupts the blood-testis barrier in rabbits. American Journal of Physiology and Endocrinology and Metabolism 307:1125-1130.).
The source of fatty acids suggested is the byproduct from agro-industrial processing, in which palm kernel cake stands out due the contents of fatty acids such as lauric, myristic, oleic, palmitic, stearic, and linoleic (Oliveira et al., 2015bOliveira, R. L.; Neto, S. G.; Lima, F. H. S.; Medeiros, A. N.; Bezerra, L. R.; Pereira, E. S.; Bagaldo, A. R.; Pellegrini, C. B. and Correia, B. R. 2015b. Composition and fatty acid profile of milk from cows supplemented with pressed oilseed cake. Animal Science Journal 87:1225-1232.) and their inclusion in the diet may increase GnRH pulses, resulting in reproductive performance. The n-3 and n-6 fatty acids are the main polyunsatured fatty acids acting on reproductive axis to promote testicular development and production of hormones (Sartoni and Guardieiro, 2010Sartori, R. and Guardieiro, M. M. 2010. Fatores nutricionais associados à reprodução da fêmea bovina. Revista Brasileira de Zootecnia 39:422-432.). Moreover, supplemental feed can be a major cost incurred to maintain husbandry. Then, alternative feeds may provide nutrients needed by animals to enhance their reproduction at a lower cost than traditional feeds. Therefore, the objective of this study was to assess the effects of palm kernel cake on biochemical and semen parameters of sheep.
Material and Methods
The experimental period lasted 90 days foregone by 15 days for adaptation to the diets. This study was approved by the Ethics Committee on the Use of Animals under case number 23007.013783/2014-31.
Forty growing lambs, with 24.10±2.72 kg of average body weight and five months old were assigned in a completely randomized design into four groups and 10 replicates. The treatments consisted of four concentrate rations with levels of palm kernel. The control group fed a basal ration, the second group fed diet with 15% of palm kernel cake included, the third group fed a diet with 30% of palm kernel cake, and the fourth fed 45% of palm kernel cake. The animals were kept under massai grass (Panicum híbrido vr. Massai) pasture and fed the concentrate in creep feeding in the morning.
Diets were formulated to be isonitrogenous and to meet the nutritional requirements of sheep (NRC, 2007NRC - National Research Council. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington, DC.) (Table 2). Chemical analysis (Table 1) for dry matter (DM), mineral matter (MM), crude protein (CP), and ether extract (EE) were performed according to procedure of AOAC (1990)AOAC - Association of Official Analytical Chemistry. 1990. Official methods of analysis. 15th ed. AOAC International, Washington, DC.. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined using the method proposed by Van Soest et al. (1991)Van Soest, P. J.; Robertson, J. B. and Lewis, B. A. 1991. Methods for dietary fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74:3583-3597..
Total carbohydrates (TC) were obtained using the following equation, as proposed by Sniffen et al. (1992)Sniffen, C. J.; O’Connor, J. D. and Van Soest, P. J. 1992. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Animal Science 70:3562-3577.: TC = 1000 - (CP + EE + MM); non-fiber carbohydrates (NFC) were estimated through the formula suggested by Hall (2000)Hall, M. B. 2000. Neutral detergent-soluble carbohydrates. Nutritional relevance and analysis. Gainesville, EUA. as indicated: NFC = 1000 - (NDF + CP + EE + MM). Hemicellulose (HEM) and cellulose (CEL) were assessed by the sequencial method, according to methodologies suggested by Silva and Queiroz (2002)Silva, D. J. and Queiroz, A. C. 2002. Análise de alimentos: métodos químicos e biológicos. 3.ed. UFV, Viçosa, MG. using the following equations: HEM = NDF - ADF and CELL = ADF - Lig (lignin), respectively. Total digestible nutrients (TDN) were determined using equation according to NRC (2001)NRC - National Research Council. 2001. Nutrient requirements of dairy cattle. 6th ed. National Academy of Science, Washington, DC.: TDN = 87.84 - (ADF × 0.70).
Body weight of the animals was recorded every 15 days to adjust diet offer according to the individual live weights. Then, body condition score was determined.
Blood samples were collected every 45 days in the morning from jugular vein after 12 h of fast using vacuum tubes without anticoagulant. Plasma was separated by centrifugation at 3500 × g for 15 min. Total serum cholesterol (TC), triglycerides (TG), and high-density lipoproteins (HDL) were assessed using enzymatic colorimetric test and absorbance was measured at spectrophotometer standardized for 500 nm using monoreagent kit. Low-density lipoproteins (LDL) and very low-density lipoproteins (VLDL) were estimated using the Friedewald et al. (1972)Friedewald, W. T.; Levy, R. I. and Fredrickson, D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry Journal 18:499-502. equation: LDL-c = TC - HDL-c - (TG/5) and VLDL-c = TG/5 respectively.
Semen analysis was performed on day 75, when the animals reached puberty at a mean age of 225 days. The samples were collected by electroejaculator once a week for three consecutive weeks. Semen parameters were immediately assessed for volume, color, aspect, wave motion, motility, sperm concentration, sperm vigor, and sperm morphology according to CBRA (2013)CBRA - Colégio Brasileiro de Reprodução Animal. 2013. Manual para exame andrológico e avaliação de sêmen animal. 3.ed. Belo Horizonte. procedures. The total number of sperm was estimated by multiplying sperm concentration mL−1 by the total volume of the ejaculate as the following formula: Total number of sperm = sperm mL−1 (106) × volume (mL); total number of viable spermatozoa was calculated by multiplying total number of sperm per ejaculate by percentage of motility according to methodology described by Martin-Rillo (1996)Martin-Rillo, S. 1996. Bora semen evaluation in practice. Reproduction Domestics Animal 31:519-526..
Data were subjected to Shapiro-Wilk and Levene's tests to verify homogeneity of variances and normality, respectively. Then, analysis of variance was carried out using the General Linear Model procedure. Multiple regression equations were developed for the different levels of palm kernel cake. Data that were not normally distributed were analysed by Kruskal-Wallis non-parametric tests. All statistical procedures were performed using SAS (Statistical Analysis System, version 9.0).
The effect of concentrate levels were evaluated according to the following model:
in which Yij is dependent variable; μ is the general constant; αi is the fixed effect of the concentrate i; and eij is the random error.
Results
Concentrations of serum cholesterol linearly increased in function of the levels of palm kernel cake in the diets (Figure 1). The means found in this study ranged from 46.12±8.32 to 54.78±5.83 mg dL−1 (Table 3). This effect may be related to the dietary lipid content; therefore, the addition of palm kernel cake increased levels of ether extract from 47.80 to 57.60 g kg−1 in the DM.
Serum concentration of total cholesterol (mg dL−1) of lambs fed concentrate containing levels of palm kernel cake.
Biochemical parameters of lambs fed diet containing levels of palm kernel cake in the concentrate
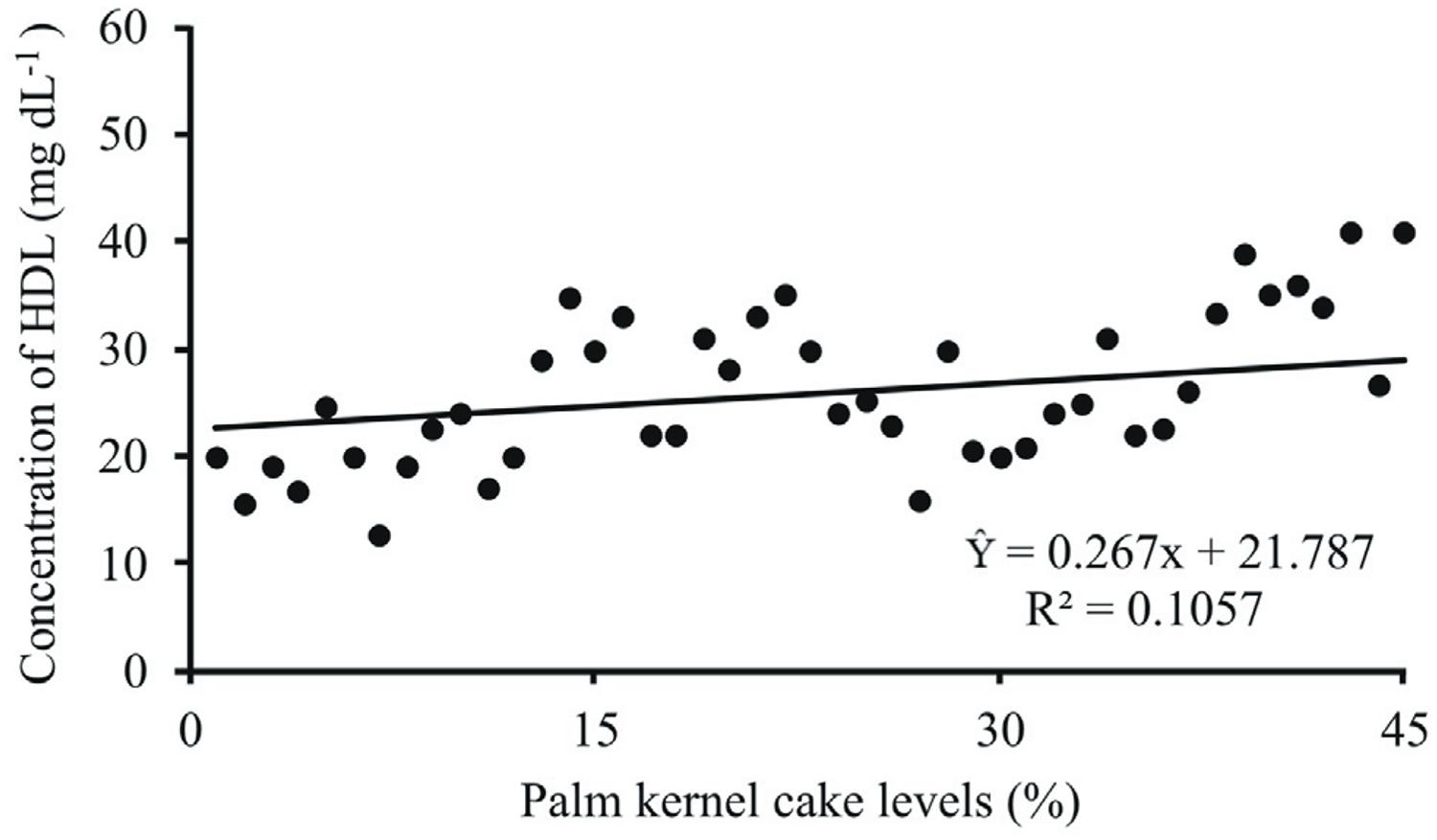
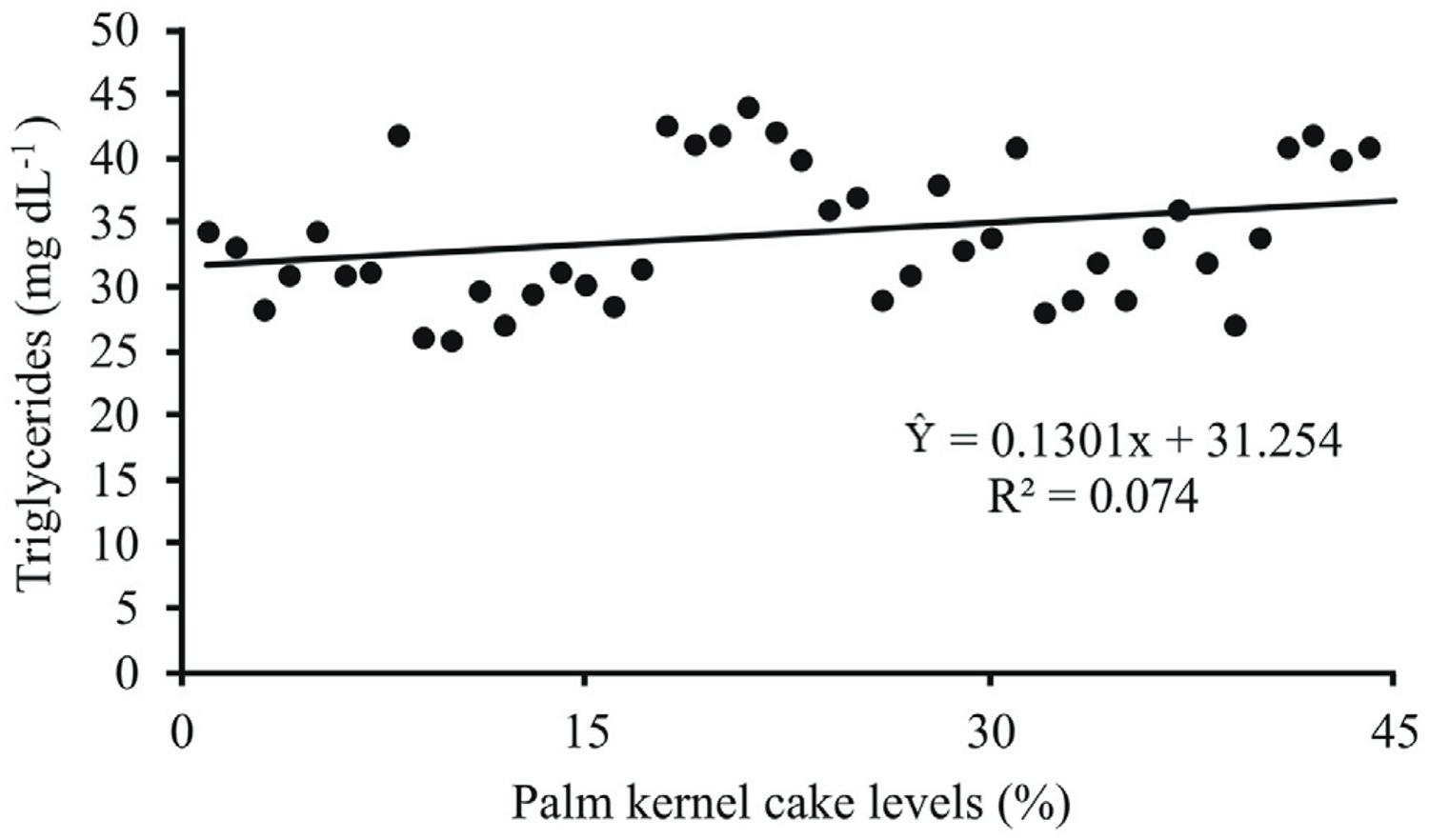
Serum HDL levels had a linear increase as contents of palm kernel cake increased in the diet (P<0.05) (Figure 2). Concentrations of triglycerides linearly increased (P<0.05) in function of the levels of palm kernel cake (Figure 3). The highest values of triglycerides were recorded in groups in experimental diets containing high content of EE. A similar behavior was observed for VLDL (Figure 4).
Serum concentrations of high-density lipoprotein (HDL) (mg dL−1) of lambs fed diets containing levels of palm kernel cake in the concentrate.
Serum triglycerides concentrations (mg dL−1) of lambs fed diets containing levels of palm kernel cake.
Serum concentrations of very low-density lipoprotein (VLDL) (mg dL−1) of lambs fed palm kernel cake under grazing.
Levels of palm kernel cake did not affect (P>0.05) the concentration of LDL. The minimum and maximum means found in this study were 8.40±1.38 and 17.70±6.93 mg dL−1, respectively. The LDL:HDL ratio was significantly affected (P<0.05) over the treatments, possibly due to the behavior observed for LDL and HDL.
The ejaculate volume was not affected (P>0.05) when palm kernel cake contents were increased and the overall mean for whole groups was 0.90±0.34 mL. No difference was observed (P>0.05) in mass motility. In all groups fed levels of palm kernel cake, the percentage of progressive sperm motility did not differ significantly.
Sperm vigor was not affected by levels of palm kernel cake (Table 4). This variable is positively correlated with semen viability in many species, including ovines.
The diets affected (P<0.05) sperm concentration, which increased linearly from the control group to that fed diets with 45% of palm kernel cake (Figure 5). It may be related to the level of ether extract as well as semen volume, although this variable did not vary significantly in this study.
Discussion
The concentrations of serum cholesterol found in this study reveal the effect of the dietary lipid content. Nunes et al. (2011)Nunes, A. S.; Oliveira, R. L.; Borja, M. S.; Bagaldo, A. R.; Macome, F. M.; Jesus, I. B.; Silva, T. M.; Barbosa, L. P. and Garcez Neto, A. F. 2011. Consumo, digestibilidade e parâmetros sanguíneos de cordeiros submetidos a dietas com torta de dendê. Revista Archivos de Zootecnia 60:903-912. reported that including palm kernel cake up to 19.5% in diet for lambs increases the levels of serum cholesterol.
Oliveira et al. (2015a)Oliveira, R. L.; Faria, M.; Silva, R.; Bezerra, L.; Carvalho, G.; Pinheiro, A.; Simionato, J. and Leão, A. 2015a. Fatty acid profile of milk and cheese from dairy cows supplemented a diet with palm kernel cake. Molecules 20:15435-15448. described palm kernel cake as an important source of saturated fatty acids such as lauric (C12:0), myristic (C14:0), palmitic (C16:0), and stearic (C18:0) acids with 47.75%, 16.89%, 7.99% and 2.85%, respectively. Furthermore, it contains the following unsaturated fatty acids: myristoleic (C14:1) (0.02%), palmitoleic (C16:1) (0.04%), oleic (C18:1) (13.84%), and polyunsaturated fatty acids (PUFA) with 2.88 and 0.13% of linoleic (C18:2) and linolenic (C18:3) acids, respectively. Concentrations of serum cholesterol in lambs are influenced by dietary saturated fatty acids (Abdel-Fattah et al., 2013Abdel-Fattah, M. S.; Hashem, A. L. S.; Shaker, Y. M.; Ellamei, A. M. and Amer, H. Z. 2013. Effect of weaning age on productive performance and some plasma biochemical parameters of barki lambs in Siwa Oasis, Egypt. Global Veterinaria 10:189-202.). González and Silva (2006)González, F. H. D. and Silva, S. C. 2006. Introdução à bioquímica clínica veterinária. 2.ed. UFRGS, Porto Alegre. reported variations from 52 to 76 mg dL−1 as reference values of cholesterol, while Sitmo (2014)Sitmo, M. S. 2014. Effect of gender on some plasma biochemical parameters of sheep from Southern AI Jabal Al Akhdar in Libya. Journal of American Science 10:74:77. suggested 64 to 104 mg dL−1 for adult males and 44 to 126 mg dL−1 for females.
Content of palm kernel cake affected serum HDL. Possibly, this is a result of the increase in the levels of total cholesterol observed in this study. In addition to the increase in EE, the increase in NDF of concentrate may have influenced the HDL as well as cholesterol. In fact, many studies have demonstrated that these variables in lambs are sensitive to increase in NDF. The minimum HDL and cholesterol recorded with the control group was probably due to the lower level of NDF; therefore, there is a physiological interdependence between HDL and cholesterol. Fredenrich and Bayer (2003)Fredenrich, A. and Bayer, P. 2003. Reserve cholesterol transport, high density lipoprotein and HDL cholesterol: Recent data. Annals of Epidemiology 14:265-273. and Olswold and Andrade (2003)Olswold, C. and Andrade, M. 2003. Localization of genes involved in the metabolic syndrome using multivariate linkage analysis. BMC Genetics Journal 4:2-5. observed that HDL are positively associated with levels of serum cholesterol.
Studies carried out by Hawkins et al. (1995)Hawkins, D. E.; Niswender, K. D. and Oss, G. M. 1995. An increase in serum lipids increases luteal lipid content and alters the disappearance rate of progesterone in cows. Journal of Animal Science 73:541-545. and Beynen et al. (2000)Beynen, A. C.; Schonewille, J. T. and Terpstra, A. H. M. 2000. Influence of amount and type of dietary fat on plasma cholesterol concentrations in goats. Small Ruminant Research 35:141-147. showed that diets containing lipid increased the level of HDL. This is the major lipoprotein fraction carrying cholesterol from periferic tissues to liver. Moreover, HDL plays a key role in the male reproductive system, which is associated with sperm capacitation and the acrosome reaction (Therien et al., 1997Therien, I.; Soubeyrand, S. and Manjunath, P. 1997. Major proteins of bovine seminal plasma modulate sperm capacitation by high- density lipoprotein. Biology Reproduction 57:1080-1088.). Sitmo (2014)Sitmo, M. S. 2014. Effect of gender on some plasma biochemical parameters of sheep from Southern AI Jabal Al Akhdar in Libya. Journal of American Science 10:74:77. reported means of HDL in rams ranging from 46.10 to 71 mg dL−1.
Levels of palm kernel cake increased the concentration of triglycerides and the LDL. This suggests that high content of ether extract plays a major role in these interdependent components. According to Jafaroghli et al. (2014)Jafaroghli, M.; Abdi-Benemar, H.; Zamiri, M. J.; Khalili, B.; Farshad, A. and Shadparvar, A. A. 2014. Effects of dietary n-3 fatty acids and vitamin C on semen characteristics, lipid composition of sperm and blood metabolites in fat-tailed Moghani rams. Animal Reproduction Science 147:17-24., triglycerides are carried in the blood by VLDL; therefore, the increased concentration of VLDL may have ocured because of the increases in serum triglycerides.
Oleaginous fruit contains triglycerides stored as energy source and inclusion of such ingredient in diets for ruminants may increase the concentrations of circulating triglycerides. When fat-rich feeds are added to diets with contents of ether extract up to 5% in the dry matter, it results in increases in the triglyceride levels (Palmquist and Jenkins, 1980Palmquist, D. L. and Jenkins, T. C. 1980. Fat in lactation rations: review. Journal of Dairy Science 63:1-14.). Moreover, this molecule is the main source of energy and lipids for sperm mobility. The amount of lipids in the gametes of ruminants is about 76 ng approximately, containing around 58% of triglycerides. The competence, quality, and cryo-resistence of sperms are related to the amount of triglyceride content, which additionally plays a role in development, during and after fertilization (Santos et al., 2008Santos, J. E. P.; Billy, T. R.; Thatcher, W. W.; Staples, C. R. and Silvestre, F. T. 2008. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reproduction in Domestic Animals 43:1627-3591.).
When testing levels of propylene on blood parameters in rams, Santos et al. (2015)Santos, R. P.; Sousa, L. F.; Sousa, J. T. L.; Andrade, M. E. B.; Júnior, G. L. M. and Silva, S. P. 2015. Parâmetros sanguíneos de cordeiros em crescimento filhos de ovelhas suplementadas com níveis crescentes de propilenoglicol. Revista Brasileira de Ciências Agrárias 10:473-478. found that values of VLDL increased linearly as triglycerides increased, supporting the results observed in this study. According to the same authors, these metabolites present the same pattern of response in animals fed the same diet. The referred study reported means of VLDL varying from 5.93 to 8.87 mg dL−1 in nine-month rams.
The results observed for the concentration of LDL may be related to the saturated fatty acids contained in the palm kernel cake. Some fatty acids such as myristic (C14:0) and palmitic (C16:0) are related to levels of LDL in the blood and these acids present a high pronounced hypercholesterolemic effect (French et al., 2000French, P.; Stanton, C.; Lawless, F.; O’Riordan, E. G.; Monahan, F. J.; Caffrey, P. J. and Moloney, A. P. 2000. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. Journal of Animal Science 78:2849-2855.). This lipoprotein is essential for sperm resistance during the cryogenics process, since it provides phospholipid molecules to plasma membrane for stability and inhibition of cryo-injury. Furthermore, LDL binds to some proteins of seminal plasma, inhibiting sperm toxicity. In addition, levels up to 8% enhance motility, plasmatic membrane integrity, and acrosome membrane (Moussa et al., 2002Moussa, M.; Martinet, V; Trimeche, A.; Tainturier, D. and Anton, M. 2002. Low density lipoprotein extracted from hen egg yolk by an easy method: cryoprotective effect on frozen-thawed bull semen. Theriogenology 57:1695-1706. and Hu et al., 2011Hu, J. H.; Jiang, Z. L.; Li, R. K.; Zhang, S. S.; Zan, L. S.; Li, L. S.; Li, W. K. and Li, X. 2011. The advantages of low-density lipoprotein in the cryopreservation of bull semen. Cryobiology 62:83-87.).
The predominance of LDL results in higher deposition of cholesterol in the periferic tissues due to its inability to catabolize the excess of LDL. Low LDL:HDL ratio indicates a positive effect of diet on maintainance of cholesterol levels within the adequated range in animals (Filipponi et al., 2007Filipponi, D.; Hobbs R. M.; Ottolenghi, S.; Rossi, P.; Jannini, E. A.; Pandolfi, P. P. and Dolci, S. 2007. Repression of kit expression by Plzf in germ cells. Molecular and Cellular Biology 27:81-6770.).
Furthermore, seminal parameters were recorded in lambs. The mean value found for mass motility was 3.00±1.5 and is classified by CBRA (2013)CBRA - Colégio Brasileiro de Reprodução Animal. 2013. Manual para exame andrológico e avaliação de sêmen animal. 3.ed. Belo Horizonte. as normal level for lambs. The results found in this study suggest that supplementation of palm kernel cake did not alter volume and mass motility.
The diet did not affect progressive sperm motility. This variable is affected by diets containing low crude protein content. According to Martin and Walkden-Brown (1995)Martin, G. B. and Walkden-Brown, S. W. 1995. Nutritional influences on reproduction in mature male sheep and goats. Journal of Reproduction and Fertility 49:49-437., the energy component of the diet and certain protein contents are likely to be responsible for affecting sperm motility. In this study, diets were balanced to ensure that lambs received similar amount of crude protein and different levels of EE. Possibly, the results found for motility are related to the same level of crude protein content in the diet. According to Vasconcelos et al. (2003)Vasconcelos, J. L.; Sangsritavong, S.; Tsai, S.J. and Wiltbank, M. C. 2003. Acute reduction in serum progesterone concentrations after intake in dairy cows. Theriogenology 60:795-807., energy and protein act on the hypothalamus-pituitary-gonadal axis by increasing the GnRH and LH pulse frequencies and the tonic secretion of FSH as well. As the spermatogenic process responds rapidly to changes in crude protein, sperm motility is rapidly affected as well.
Sperm vigor is positively correlated with semen quality in many species and affects male reproductive performance. For an effective fertilization, it is essential that the sperm presents a vigor around 3 and intact membrane (CBRA, 2013CBRA - Colégio Brasileiro de Reprodução Animal. 2013. Manual para exame andrológico e avaliação de sêmen animal. 3.ed. Belo Horizonte.). Besides nutrition, other factors such as genetics, environmental conditions, and animal age can influence this variable (Cunha et al., 2012Cunha, M. G. G.; Gonzalez, C. I. M.; Carvalho, F. F. R. and Soares, A. T. 2012. Effect of diets containing whole cottonseed on the quality of sheep. ActaScientiarum. Animal Sciences 34:305-311.). Adeyemi et al. (2015)Adeyemi, K. D.; Ebrahimi, M.; Samsudin, A. A.; Sabow, A. B. and Sazili, A. Q. 2015. Carcass traits, meat yield and fatty acid composition of adipose tissues and Supraspinatus muscle in goats fed blend of canola oil and palm oil. Journal of Animal Science and Technology 57:3-14. suggested that levels below 6% of ether extract content in diets may negatively affect sperm vigor; however, in this study, such result was not observed.
Possibly, the increase of sperm concentration in this study is related to the increase of ether extract in the diet. Rege et al. (2000)Rege, J. E. O.; Toe, F.; Mukasa-Mugerwa, E.; Tembely, S.; Anindo, D.; Baker, R. L. and Lahlou-Kassi, A. 2000. Reproductive characteristics of Ethiopian highland sheep: II Genetic parameters of semen characteristics and their relationship with testicular measurements in ram lambs. Small Ruminants Research 37:173-187. found a strong influence of diets containing source of fatty acids on sperm concentration. Pacheco et al. (2009)Pacheco, A.; Madella-Oliveira, A. F.; Quirino, C. R. and Landim, A. V. 2009. Características seminais de carneiros da raça Santa Inês na pré-puberdade, puberdade e na pós-puberdade. ArsVeterinaria 25:90-99. found differences on the sperm concentration of lambs fed diets containing up to 4.5% of ether extract in dry matter. Balanced nutrition is of paramount importance for sperm concentration. In addition, energy level provides a high amount of sperm. Concentration is likely to be a better factor to estimate the number of cells and fertilization rates. Another factor that affects sperm concentration is the amount of seminal fluid released by sexual accessory glands and the number of cells produced during spermatogenesis process. Depending upon the age, genetics, and nutritional status, sperm concentration may vary from 1 to 3 × 109 spermatozoa mL−1 (Cheah and Yang, 2011Cheah, Y. and Yang, W. 2011. Functions of essential nutrition for high quality spermatogenesis. Advances in Bioscience and Biotechnology 2:182-197.; CBRA, 2013CBRA - Colégio Brasileiro de Reprodução Animal. 2013. Manual para exame andrológico e avaliação de sêmen animal. 3.ed. Belo Horizonte.).
While palm kernel cake affected major defects, the minor and total defects were not affected. Sperm defects in sheep are largely caused by pathological changes in the sexual organs, degeneration of the epithelium of the seminal ducts, cysts, and spermiostasis. A prolonged interruption in the sexual activity of sheep may lead to a temporary loss in the semen quality in the first ejaculates. In addition, age is one of the key factors that increase percentage of sperm defects (Andreevskii, 1940Andreevskii, V. Y. 1940. Reasons for sperm defects of rams. Iskusstv ennoeosemeneniesel’khoz. Zhivotn Journal 1:36-45.). In this study, the age of animals may have caused the observed result of major defects. The overall means found in this study were 3.99±1.69% for minor defects, 3.90±2.45% for major defects, and 7.89±3.12% for total defects (Table 5). These values are considered normal by CBRA (2013)CBRA - Colégio Brasileiro de Reprodução Animal. 2013. Manual para exame andrológico e avaliação de sêmen animal. 3.ed. Belo Horizonte..
Morphological characteristics of sperm of lambs fed diets containing palm kernel cake under grazing
The major defects affected by diet containing palm kernel cake (Figure 6) in this study seem to be one of the variables influenced by the ether extract and the age of animals. Pacheco et al. (2009)Pacheco, A.; Madella-Oliveira, A. F.; Quirino, C. R. and Landim, A. V. 2009. Características seminais de carneiros da raça Santa Inês na pré-puberdade, puberdade e na pós-puberdade. ArsVeterinaria 25:90-99. found 21.20%; 19.80%, and 4.40% of major defects in pre-pubertal, pubescent, and post-puberscent sheep, respectively.
Conclusions
The supplementation of diets with palm kernel cake up to 45% in dry matter enhance biochemical parameters and does not impair the qualitative variables of lamb sperm. Hence, it can be used as a feed alternative for lambs.
Acknowledgments
The first author is grateful to Universidade Federal do Recôncavo da Bahia and Universidade Zambeze for supporting permanence in Brazil. Furthermore, he wishes to thank FAPESB for funding this research.
References
- Abdel-Fattah, M. S.; Hashem, A. L. S.; Shaker, Y. M.; Ellamei, A. M. and Amer, H. Z. 2013. Effect of weaning age on productive performance and some plasma biochemical parameters of barki lambs in Siwa Oasis, Egypt. Global Veterinaria 10:189-202.
- Adeyemi, K. D.; Ebrahimi, M.; Samsudin, A. A.; Sabow, A. B. and Sazili, A. Q. 2015. Carcass traits, meat yield and fatty acid composition of adipose tissues and Supraspinatus muscle in goats fed blend of canola oil and palm oil. Journal of Animal Science and Technology 57:3-14.
- Andreevskii, V. Y. 1940. Reasons for sperm defects of rams. Iskusstv ennoeosemeneniesel’khoz. Zhivotn Journal 1:36-45.
- AOAC - Association of Official Analytical Chemistry. 1990. Official methods of analysis. 15th ed. AOAC International, Washington, DC.
- Awad, N. S.; Soliman, M. M.; Mohamed, A. A.; Sabry, A. M.; Shahaby, A. F. and El-Tarras, A. E. 2015. Effect of altitude on some male fertility related traits in Saudi ovine and caprine species. Annals of Animal Science 15:641-653.
- Beynen, A. C.; Schonewille, J. T. and Terpstra, A. H. M. 2000. Influence of amount and type of dietary fat on plasma cholesterol concentrations in goats. Small Ruminant Research 35:141-147.
- Cheah, Y. and Yang, W. 2011. Functions of essential nutrition for high quality spermatogenesis. Advances in Bioscience and Biotechnology 2:182-197.
- CBRA - Colégio Brasileiro de Reprodução Animal. 2013. Manual para exame andrológico e avaliação de sêmen animal. 3.ed. Belo Horizonte.
- Cunha, M. G. G.; Gonzalez, C. I. M.; Carvalho, F. F. R. and Soares, A. T. 2012. Effect of diets containing whole cottonseed on the quality of sheep. ActaScientiarum. Animal Sciences 34:305-311.
- Fair, S.; Doyle, D. N.; Diskin, M. G.; Hennessy, A. A. and Kenny, D. A. 2014. The effect of dietary n-3 polyunsaturated fatty acids supplementation of rams on semen quality and subsequent quality of liquid stored semen. Theriogenology 81:210-219.
- Filipponi, D.; Hobbs R. M.; Ottolenghi, S.; Rossi, P.; Jannini, E. A.; Pandolfi, P. P. and Dolci, S. 2007. Repression of kit expression by Plzf in germ cells. Molecular and Cellular Biology 27:81-6770.
- Fredenrich, A. and Bayer, P. 2003. Reserve cholesterol transport, high density lipoprotein and HDL cholesterol: Recent data. Annals of Epidemiology 14:265-273.
- French, P.; Stanton, C.; Lawless, F.; O’Riordan, E. G.; Monahan, F. J.; Caffrey, P. J. and Moloney, A. P. 2000. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. Journal of Animal Science 78:2849-2855.
- Friedewald, W. T.; Levy, R. I. and Fredrickson, D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry Journal 18:499-502.
- González, F. H. D. and Silva, S. C. 2006. Introdução à bioquímica clínica veterinária. 2.ed. UFRGS, Porto Alegre.
- Hall, M. B. 2000. Neutral detergent-soluble carbohydrates. Nutritional relevance and analysis. Gainesville, EUA.
- Hawkins, D. E.; Niswender, K. D. and Oss, G. M. 1995. An increase in serum lipids increases luteal lipid content and alters the disappearance rate of progesterone in cows. Journal of Animal Science 73:541-545.
- Hu, J. H.; Jiang, Z. L.; Li, R. K.; Zhang, S. S.; Zan, L. S.; Li, L. S.; Li, W. K. and Li, X. 2011. The advantages of low-density lipoprotein in the cryopreservation of bull semen. Cryobiology 62:83-87.
- Jafaroghli, M.; Abdi-Benemar, H.; Zamiri, M. J.; Khalili, B.; Farshad, A. and Shadparvar, A. A. 2014. Effects of dietary n-3 fatty acids and vitamin C on semen characteristics, lipid composition of sperm and blood metabolites in fat-tailed Moghani rams. Animal Reproduction Science 147:17-24.
- Martin-Rillo, S. 1996. Bora semen evaluation in practice. Reproduction Domestics Animal 31:519-526.
- Martin, G. B. and Walkden-Brown, S. W. 1995. Nutritional influences on reproduction in mature male sheep and goats. Journal of Reproduction and Fertility 49:49-437.
- Morgan, D. H.; Ghribi, O.; Hui, L.; Geiger, J. D. and Chen, X. 2014. Cholesterol-enriched diet disrupts the blood-testis barrier in rabbits. American Journal of Physiology and Endocrinology and Metabolism 307:1125-1130.
- Moussa, M.; Martinet, V; Trimeche, A.; Tainturier, D. and Anton, M. 2002. Low density lipoprotein extracted from hen egg yolk by an easy method: cryoprotective effect on frozen-thawed bull semen. Theriogenology 57:1695-1706.
- NRC - National Research Council. 2001. Nutrient requirements of dairy cattle. 6th ed. National Academy of Science, Washington, DC.
- NRC - National Research Council. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington, DC.
- Nunes, A. S.; Oliveira, R. L.; Borja, M. S.; Bagaldo, A. R.; Macome, F. M.; Jesus, I. B.; Silva, T. M.; Barbosa, L. P. and Garcez Neto, A. F. 2011. Consumo, digestibilidade e parâmetros sanguíneos de cordeiros submetidos a dietas com torta de dendê. Revista Archivos de Zootecnia 60:903-912.
- Oliveira, R. L.; Faria, M.; Silva, R.; Bezerra, L.; Carvalho, G.; Pinheiro, A.; Simionato, J. and Leão, A. 2015a. Fatty acid profile of milk and cheese from dairy cows supplemented a diet with palm kernel cake. Molecules 20:15435-15448.
- Oliveira, R. L.; Neto, S. G.; Lima, F. H. S.; Medeiros, A. N.; Bezerra, L. R.; Pereira, E. S.; Bagaldo, A. R.; Pellegrini, C. B. and Correia, B. R. 2015b. Composition and fatty acid profile of milk from cows supplemented with pressed oilseed cake. Animal Science Journal 87:1225-1232.
- Olswold, C. and Andrade, M. 2003. Localization of genes involved in the metabolic syndrome using multivariate linkage analysis. BMC Genetics Journal 4:2-5.
- Pacheco, A.; Madella-Oliveira, A. F.; Quirino, C. R. and Landim, A. V. 2009. Características seminais de carneiros da raça Santa Inês na pré-puberdade, puberdade e na pós-puberdade. ArsVeterinaria 25:90-99.
- Palmquist, D. L. and Jenkins, T. C. 1980. Fat in lactation rations: review. Journal of Dairy Science 63:1-14.
- Rege, J. E. O.; Toe, F.; Mukasa-Mugerwa, E.; Tembely, S.; Anindo, D.; Baker, R. L. and Lahlou-Kassi, A. 2000. Reproductive characteristics of Ethiopian highland sheep: II Genetic parameters of semen characteristics and their relationship with testicular measurements in ram lambs. Small Ruminants Research 37:173-187.
- Santos, J. E. P.; Billy, T. R.; Thatcher, W. W.; Staples, C. R. and Silvestre, F. T. 2008. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reproduction in Domestic Animals 43:1627-3591.
- Santos, R. P.; Sousa, L. F.; Sousa, J. T. L.; Andrade, M. E. B.; Júnior, G. L. M. and Silva, S. P. 2015. Parâmetros sanguíneos de cordeiros em crescimento filhos de ovelhas suplementadas com níveis crescentes de propilenoglicol. Revista Brasileira de Ciências Agrárias 10:473-478.
- Sartori, R. and Guardieiro, M. M. 2010. Fatores nutricionais associados à reprodução da fêmea bovina. Revista Brasileira de Zootecnia 39:422-432.
- Silva, D. J. and Queiroz, A. C. 2002. Análise de alimentos: métodos químicos e biológicos. 3.ed. UFV, Viçosa, MG.
- Sitmo, M. S. 2014. Effect of gender on some plasma biochemical parameters of sheep from Southern AI Jabal Al Akhdar in Libya. Journal of American Science 10:74:77.
- Sniffen, C. J.; O’Connor, J. D. and Van Soest, P. J. 1992. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Animal Science 70:3562-3577.
- Therien, I.; Soubeyrand, S. and Manjunath, P. 1997. Major proteins of bovine seminal plasma modulate sperm capacitation by high- density lipoprotein. Biology Reproduction 57:1080-1088.
- Van Soest, P. J.; Robertson, J. B. and Lewis, B. A. 1991. Methods for dietary fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74:3583-3597.
- Vasconcelos, J. L.; Sangsritavong, S.; Tsai, S.J. and Wiltbank, M. C. 2003. Acute reduction in serum progesterone concentrations after intake in dairy cows. Theriogenology 60:795-807.
- Zabuli, J.; Tanaka, T.; Lu, Wengeng; Kuroiwa, T. and Kamomae, H. 2009. Responses of gonadotropin secretion to short-term dietary supplementation in ovariectomized goats with different body weights. Animal Reproduction Science 116:274-281.
Publication Dates
-
Publication in this collection
Aug 2017
History
-
Received
13 Nov 2016 -
Accepted
08 June 2017