ABSTRACT
In this experiment, the beneficial effects of mannan-oligosaccharide (MOS) on immunology and intestinal microbiology of Nile tilapia juveniles was demonstrated. Prior to this, three levels of MOS in Nile tilapia diets (1, 8, and 15 g.kg−1) were tested, and hematological parameters, serum lysozyme, and intestinal microbiology were analyzed. The fish blood was sampled at day zero (basal sample) and after 45 days of trial, and the intestinal microbiota was evaluated at the end of the experiment. After 45 days of trial, fish fed 8 and 15 g.kg−1 of MOS presented an increase in both aerobic and lactic acid bacteria numbers in their guts. The MOS feeding also increased the counts of total leukocytes, monocytes, and lymphocytes of fish, but a decrease in neutrophils was also observed. Additionally, the serum lysozyme was higher in all fish fed MOS. The dietary MOS is able to modulate the intestinal microbiota, increasing the number of beneficial bacteria, and immunostimulates the Nile tilapia juvenile, giving rise to white blood cells and serum lysozyme.
Key Words:
aquaculture; hematology; intestinal microbiota; lysozyme; prebiotic
Introduction
Aquaculture has shown a consistent growth around the world over the last two decades, and one of the major contributors is the tilapia culture. Tilapia species are produced in large scale in more than 130 countries because they are recognized for their toughness, high meat quality, and low protein requirement ( Fitzsimmons et al., 2011Fitzsimmons, K.; Martinez-Garcia, R. and Gonzalez-Alanis, P. 2011. Why tilapia is becoming the most important food fish on the planet. Better science, better fish, better life. p.9-18. In: Proceedings of the 9th International Symposium on Tilapia in Aquaculture. Shanghai Ocean University, Shanghai. AquaFish Collaborative Research Support Program, Corvallis. ). Together with the rapid expansion of the tilapia aquaculture and subsequent intensification of production systems, the stress caused by the increase of stock densities, handling, and the use of artificial feeds gave rise to several disease outbreaks ( Mauel et al., 2007Mauel, M.; Soto, E.; Moralis, J. and Hawke, J. 2007. A piscirickettsiosis-like syndrome in cultured Nile tilapia in Latin America with Francisella spp. as the pathogenic agent. Journal of Aquatic Animal Health 19:27-34. ; Mian et al., 2009Mian, G. F.; Godoy, D. T.; Leal, C. A. G.; Yuhara, T. Y.; Costa, G. M. and Figueiredo, H. C. P. 2009. Aspects of the natural history and virulence of S. agalactiae infection in Nile tilapia. Veterinary Microbiology 136:180-183. ; Iwama et al., 2011Iwama, G. K.; Pickering, A.; Sumpter, J. and Schreck, C. 2011. Fish stress and health in aquaculture. Cambridge University Press, Cambridge. ).
Previously, several strategies were employed to avoid the use of antibiotics, most of them as preventive approaches including non-antibiotics dietary additives for fish ( Pohlenz and Gatlin, 2014Pohlenz, C. and Gatlin, D. M. 2014. Interrelationships between fish nutrition and health. Aquaculture 431:111-117. ). One possible alternative to avoid fish diseases and subsequent use of treatment procedures is the use of prebiotics such as the mannanoligosaccharide (MOS).
Mannan-oligosaccharide is a glycoprotein rich in mannose, usually isolated from the cell wall of Saccharomyces cerevisiae . The addition of MOS to fish feeding has been reported to modulate the intestinal microbiota of the host by promoting the colonization of the intestinal tract by benefic bacteria and eliminating some pathogenic microorganisms by adsorption, resulting in enhanced resistance against pathogens by the host ( Gómez and Balcázar, 2008Gómez, G. D. and Balcázar, J. L. 2008. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunology & Medical Microbiology 52:145-154. ). Moreover, the mannose present in MOS particles can be recognized by innate immune receptors, immunomodulating the host ( Torrecillas et al., 2014Torrecillas, S.; Montero, D. and Izquierdo, M. 2014. Improved health and growth of fish fed mannan oligosaccharides: Potential mode of action. Fish & Shellfish Immunology 36:525-544. ). Positive effects of dietary MOS on immune parameters have been observed in Oncorhynchus mykiss ( Staykov et al., 2007Staykov, Y.; Spring P.; Denev, S. and Sweetman, J. 2007. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout ( Oncorhynchus mykiss ). Aquaculture International 15:153-161. ), Labeo rohita ( Andrews et al., 2009Andrews, S. R.; Sahu, N. P.; Pal, A. K. and Kumar, S. 2009. Haematological modulation and growth of Labeo rohita fingerlings: effect of dietary mannan oligosaccharide, yeast extract, protein hydrolysate and chlorella. Aquaculture Research 41:61-69. ), Sciaenops ocellatus ( Zhou et al., 2010Zhou, Q. C.; Buentello, J. A. and Gatlin, D. M. 2010. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum ( Sciaenops ocellatus ). Aquaculture 309:253-257. ), and Dicentrarchus labrax ( Torrecillas et al., 2015Torrecillas, S.; Montero, D. M.; Caballero, J.; Robaina, L.; Zamorano, M. J.; Sweetman, J. and Izquierdo, M. 2015. Effects of dietary concentrated mannan oligosaccharides supplementation on growth, gut mucosal immune system and liver lipid metabolism of European sea bass ( Dicentrarchus labrax ) juveniles. Fish & Shellfish Immunology 42:508-516. ).
Thus, the objective of the present study was to evaluate the effects of MOS on Nile tilapia ( Oreochromis niloticus ) intestinal microbiota, hematological parameters, and serum lysozyme.
Material and Methods
Research on animals was conducted according to the institutional committee on animal use (case number 22.517/10).
The present experiment was conducted in Jaboticabal (São Paulo State, Brazil – 21°15′17" S, 48°19′20" W). Masculinized Nile tilapia (~1 g), GIFT strain, were obtained from a commercial farm. Initially, the fish were maintained in 310-L fiberglass tanks with continuous aeration and water flow. During this grow-out period, fish fed the control diet ( Table 1 ) twice a day until apparent satiation.
After eight weeks, treatments were started by including MOS in the feed. Fish (~101.42±2.71 g) were randomly distributed into 12 fiberglass tanks of 310 L (30 fish per tank) and maintained in the same conditions as described above. The experimental diets were prepared according to NRC (1993)NRC - National Research Council. 1993. Nutrient requirements of warmwater fishes and shellfishes. National Academy, Washington, D.C. for Nile tilapias ( Table 1 ). The experimental diets were prepared according to the formulation expressed in Table 1 , using the basal formulation as in the-grow out stage. ActiveMOS (Biorigin®, Lençois Paulistas, Brazil) was added to the diets at zero (control), 1, 8, and 15 g.kg−1, and inert Kaolin was added to balance the diets, assuring similar levels of crude energy and crude protein. All the ingredients were ground and then mixed three times. The resultant mixture was extruded in a single screw extruder machine (Ex Micro, Exteec, Brazil) into 1.0 mm pellets and then dried by forced ventilation at room temperature. Fish were fed using the experimental diets twice a day for 45 days. Growth and survival rates were measured during this period. Water parameters including pH, dissolved oxygen, and temperature were measured weekly.
Before starting the experiment, 15 fish were randomly caught, anesthetized in clove oil solution (0.1 mL of clove oil per liter of water), and had the blood sampled, corresponding to the basal sampling. After 45 days of MOS feeding, five fish from each tank (15 per treatment) also had the blood sampled following the same procedures. Blood was collected by puncture in the caudal vein, using 3-mL syringes with a 22 gauge needle and without anticoagulant. Each blood sample was divided into three aliquots for subsequent analysis. For hematological parameters, a 100-μL aliquot was transferred to a 2-mL microtube containing 15 μL of anticoagulant (0.65% NaCl, sodium heparin 100 IU.mL−1). Then, hematocrit was determined by the microhematocrit method ( Goldenfarb et al., 1971Goldenfarb, P. B.; Bowyer, F. P.; Hall, E. and Brosious, E. 1971. Reproducibility in the hematology laboratory: the microhematocrit determination. American Journal of Clinical Pathology 56:35-39. ), hemoglobin by the cyanmethemoglobin method ( Collier, 1944Collier, H. B. 1944. Standardization of blood haemoglobin determinations. Canadian Medical Association Journal 50:550. ), and red blood cells (RBC) were counted in a Neubauer chamber after diluting 10 μL of blood in 2 mL of a citrate-formaldehyde solution. Blood smears were prepared with drops of blood directly from the syringe without anticoagulant to be air-dried and stained with May Grünwald-Gyemsa-Writh ( Tavares-Dias and Moraes, 2006Tavares-Dias, M. and Moraes, F. R. 2006. Características hematológicas da Tilapia rendalli Boulenger, 1896 (Osteichthyes: Cichlidae) capturada em “Pesque-Pague” de Franca, São Paulo, Brasil. Bioscience Journal 19:107-114. ). This was done in preparation for total counts of thrombocytes and leukocytes and differential counts of leukocytes ( Hrubec and Smith, 2000Hrubec, T. and Smith, S. 2000. Hematology of fish. Schalm's veterinary hematology. Lippincott Williams and Wilkins, Philadelphia. p.1120-1125. ). Hematimetric equations ( Wintrobe, 1934Wintrobe, M. M. 1934. Variations in the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematologica 51:32-49. ) were used to determine mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC).
The remaining blood sample was transferred to 5-mL glass tubes and incubated for 45 min at room temperature to obtain the serum, which was collected using a micropipette and maintained at −20 °C for later analysis. The lysozyme concentration was then determined using a modification of Abreu et al. (2009)Abreu, J.; Marzocchi-Machado, C.; Urbaczek, A.; Fonseca, L. and Urbinati, E. C. 2009. Leukocytes respiratory burst and lysozyme level in pacu ( Piaractus mesopotamicus Holmberg, 1887). Brazilian Journal of Biology 69:1133-1139. . An aliquot of 60 μL of serum sample was diluted in 40 μL of phosphate buffer solution in 96-well plates. Then, 100 μL of PBS containing 1 μg μL−1 of Micrococcus lysodeikticus were added to the mixture. The optical density was measured at 5 and 10 min at 540 nm in spectrophotometer to estimate lysozyme concentration.
After 45 days of MOS feeding, one fish from each tank was euthanized with highly concentrated clove oil solution (0.2 mL.L−1) and sampled for microbiological analysis. Fish was rinsed with 70% ethanol, and the gut was removed. The lumen was opened and rinsed twice with 0.9% saline solution for the removal of intestinal content. Then, the whole intestinal lumen was gently scraped with a swab, and the material removed was placed into 15-mL tubes containing 10 mL of 0.85% NaCl solution to be gently homogenized for 3 min. Each sample was diluted to 10−7 in 0.85% NaCl solution. For the determination of aerobic bacteria, 100 μL of each suspension were spread onto triplicate nutrient agar plates (Himedia, China) and incubated at 36 °C for 72 h. For the determination of anaerobic bacteria, the same procedure was conducted with the plates placed inside anaerobic jars with a Gas Pak system and incubated under the same conditions. For the lactic acid bacteria (LAB) culture, 100 μL of each dilution were spread onto triplicate MRS agar plates (DeMan, Rogosa and Sharpe agar, Sigma, USA) and also incubated at 36 °C for 72 h. Each bacterial population (given in units per mL) was calculated from plates containing 30 to 300 cfu.
Results were expressed as mean ± standard error. The statistical analyses were performed with the software R V3.0.3. All data were checked for homoscedasticity and normality with Levene's test and Cramer-Von Mises' test, respectively, and were transformed to fit normal distribution using log(x + 1.5). A one-way ANOVA was performed following the model below:
in which Yij is the response of animal j of treatment group i; μ is the overall mean; τi is the fixed effect of treatment i (dietary levels of MOS); and εij is the random error of the response of animal j of treatment group i. The resultant means were compared using Tukey's multiple range test (α<0.05).
Results
Throughout the experiment, the water parameters were maintained at pH of 7.53±0.15; dissolved oxygen at 4.15±1.04 mg L−1; and temperature at 28.12±0.89 °C with no disease outbreaks or natural mortality being detected.
Regarding the erythrocytic parameters ( Table 2 ), no statistical differences were observed for Ht, Hb, VCM, MCH, MCHC, and RBC (P>0.05). In relation to the white cell parameters ( Table 2 ), no differences were observed for thrombocytes and basophils (P>0.05). The fish fed 8 and 15 g.kg−1 of MOS presented more leukocytes than the basal and control groups (P = 0.0016). The basal fish presented a lower number of monocytes than fish sampled after 45 days of experiment, and all fish fed MOS presented more monocytes than the control group (P<0.0001). A similar pattern was observed for lymphocytes, but only the groups fed 8 and 15 g.kg−1 MOS presented more lymphocytes than the control (P = 0.0022). On the other hand, all fish fed MOS presented a lower number of neutrophil than the control and basal groups (P = 0.0017) ( Figure 1 ).
Changes in white blood cells of Nile tilapia juvenile before and after 45 days of mannan-oligosaccharide (MOS) feeding.
Hematological parameters of Nile tilapia juveniles before and after 45 days of mannan-oligosaccharide (MOS) feeding
The serum lysozyme ( Figure 2 ) for fish sampled after 45 days of trial was significantly higher than that of basal fish, and the fish fed MOS presented a higher concentration of serum lysozyme than the control group (P<0.0001).
Changes in serum lysozyme of Nile tilapia juvenile before and after 45 days of mannan-oligosaccharide (MOS) feeding.
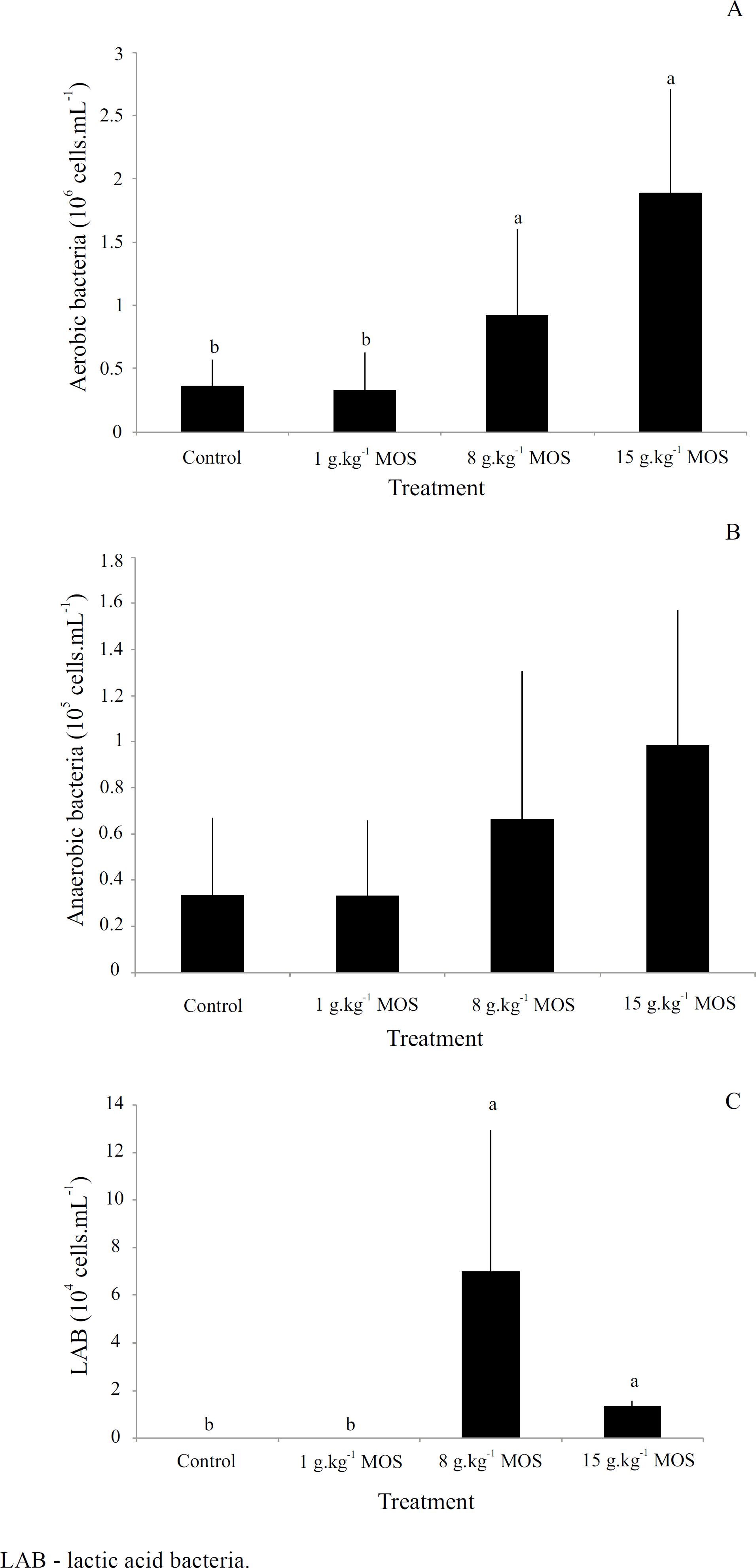
After 45 days of feeding, the number of aerobic bacteria for fish fed 8 and 15 g.kg−1 of MOS was higher than for fish fed control and 1 g.kg−1 diets (P = 0.0003), but this presented no statistically significant effect for the anaerobic bacteria count (P = 0.8500). The number of LAB were higher for groups fed 8 and 15 g.kg−1 of MOS, but these bacteria were not found in fish fed control or 1 g.kg−1 MOS diets ( Figure 3 ).
Changes in intestinal microbiota of Nile tilapia juvenile before and after 45 days of mannan-oligosaccharide (MOS) feeding.
Discussion
Several researchers have described positive effects in gut health and innate immunity of fish feeding MOS ( Lara-Flores et al., 2003Lara-Flores, M.; Olvera-Novoa, M. A.; Guzmán-Méndez, B. Z. E. and López-Madrid, W. 2003. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus , and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia ( Oreochromis niloticus ). Aquaculture 216:193-201. ; Pirarat et al., 2006Pirarat, N.; Kobayashi, T.; Katagiri, T.; Maita, M. and Endo, M. 2006. Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia ( Oreochromis niloticus ). Veterinary Immunology and Immunopathology 113:339-347. ; Aly et al., 2008aAly, S. M.; Ahmed, Y. A.-G.; Ghareeb, A. A.-A. and Mohamed, M. F. 2008a. Studies on Bacillus subtilis and Lactobacillus acidophilus , as potential probiotics, on the immune response and resistance of Tilapia nilotica ( Oreochromis niloticus ) to challenge infections. Fish & Shellfish Immunology 25:128-136. ; Aly et al., 2008bAly, S. M.; Mohamed, M. F. and John, G. 2008b. Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica ( Oreochromis niloticus ). Aquaculture Research 39:647-656. https://doi.org/10.1111/j.1365-2109.2008.01932.x
https://doi.org/10.1111/j.1365-2109.2008...
; Ngamkala et al., 2010Ngamkala, S.; Futami, K.; Endo, M.; Maita, M. and Katagiri, T. 2010. Immunological effects of glucan and Lactobacillus rhamnosus GG, a probiotic bacterium, on Nile tilapia Oreochromis niloticus intestine with oral Aeromonas challenges. Fisheries Science 76:833-840. ; Pirarat et al., 2011Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N. and Maita, M. 2011. Modulation of intestinal morphology and immunity in nile tilapia ( Oreochromis niloticus ) by Lactobacillus rhamnosus GG. Research in Veterinary Science 91:e92-e97. ), but it is the first time that this is described in Nile tilapia. Mannan-oligosaccharide is a polymer rich in mannose, a complex carbohydrate that can be used as a substrate to beneficial microorganisms, especially LAB ( Katakura et al., 2010Katakura, Y.; Sano, R.; Hashimoto, T.; Ninomiya, K. and Shioya, S. 2010. Lactic acid bacteria display on the cell surface cytosolic proteins that recognize yeast mannan. Applied Microbiology and Biotechnology 86:319-326. ). Lactic acid bacteria are present in invertebrate and vertebrate animals and have an important role in the maintenance of health in mucous environments by producing antimicrobial substances that act against pathogens or competing for cell-surface and mucin-binding sites ( Liu et al., 2013Liu, W.; Ren, P.; He, S.; Xu, L.; Yang, Y.; Gu, Z. and Zhou, Z. 2013. Comparison of adhesive gut bacteria composition, immunity, and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains. Fish & Shellfish Immunology 35:54-62. ). In the present study, a significant increase of aerobic bacteria and LAB in the intestine of fish fed 8 and 15 g.kg−1 MOS was observed but not for fish fed the 1 g.kg−1 MOS and control diets, suggesting the effectiveness of dietary MOS as prebiotic for Nile tilapia. Previous studies using MOS as feed additive also reported alterations in the intestinal microbiota of Oncorhynchus mykiss ( Dimitroglou et al., 2009Dimitroglou, A.; Merrifield, D. L.; Moate, R.; Davies, S. J.; Spring, P.; Sweetman, J. and Bradley, G. 2009. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Animal Science 87:3226-3234. ), as well as showed an increase in LAB number in Sparus aurata guts ( Dimitroglou et al., 2010Dimitroglou, A.; Merrifield, D. L.; Spring, P.; Sweetman, J.; Moate, R. and Davies, S. J. 2010. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream ( Sparus aurata ). Aquaculture 300:182-188. ). However, in review of the related literature, this is the first study showing beneficial changes to the intestinal microflora of Nile tilapia fed MOS.
In the present study, no significant effects were observed on erythrocytic parameters from dietary MOS, as also observed in Huso huso ( Mansour et al., 2012Mansour, M. R.; Akrami, R.; Ghobadi, S. H.; Denji, K. A.; Ezatrahimi, N. and Gharaei, A. 2012. Effect of dietary mannan oligosaccharide (MOS) on growth performance, survival, body composition, and some hematological parameters in giant sturgeon juvenile ( Huso huso Linnaeus, 1754). Fish Physiology and Biochemistry 38:829-835. ), O. niloticus ( Sado et al., 2008Sado, R. Y.; Bicudo, A. J. A. and Cyrino, J. E. P. 2008. Feeding dietary mannan oligosaccharides to juvenile Nile tilapia, Oreochromis niloticus , has no effect on hematological parameters and showed decreased feed consumption. Journal of the World Aquaculture Society 39:821-826. ), Piaractus mesopotamicus ( Sado et al., 2014Sado, R. Y.; Bicudo, A. J. A. and Cyrino, J. E. P. 2014. Hematology of juvenile pacu, Piaractus mesopotamicus (Holmberg, 1887) fed graded levels of mannan oligosaccharides (MOS). Latin American Journal of Aquatic Research 42:30-39. ), and Channa striata ( Talpur et al., 2014Talpur, A. D.; Munir, M. B.; Mary, A. and Hashim, R. 2014. Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead ( Channa striata ) fingerlings. Aquaculture 426-427:14-20. ). However, Sado et al. (2014)Sado, R. Y.; Bicudo, A. J. A. and Cyrino, J. E. P. 2014. Hematology of juvenile pacu, Piaractus mesopotamicus (Holmberg, 1887) fed graded levels of mannan oligosaccharides (MOS). Latin American Journal of Aquatic Research 42:30-39. observed a higher RBC in P. mesopotamicus fed 0.4 and 0.8% of MOS while Talpur et al. 2014Talpur, A. D.; Munir, M. B.; Mary, A. and Hashim, R. 2014. Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead ( Channa striata ) fingerlings. Aquaculture 426-427:14-20. ) observed higher RBC, Ht, and Hb in Channa striata fed 0.2% of MOS.
On the other hand, after 45 days of MOS feeding, an increase in leukocyte, monocyte, and lymphocyte numbers was observed, which is concomitant to a decrease in the number of neutrophils. A higher content of leukocytes in peripheral blood usually indicates organic response to infections and parasitical infestations ( Grant, 2015Grant, K. R. 2015. Fish hematology and associated disorders. Veterinary Clinics of North America: Exotic Animal Practice 18:83-103. ), but some substances or microorganisms are able to modulate, directly or indirectly, some of the innate immune response, both cellular and humoral, resulting in higher protection of fish against diseases ( Anderson, 1992Anderson, D. P. 1992. Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annual Review of Fish Diseases 2:281-307. ; Bricknell and Dalmo, 2005Bricknell, I. and Dalmo, R. A. 2005. The use of immunostimulants in fish larval aquaculture. Fish & Shellfish Immunology 19:457-472. ; Galina et al., 2009Galina, J.; Yin, G.; Ardó, L. and Jeney, Z. 2009. The use of immunostimulating herbs in fish. An overview of research. Fish Physiology and Biochemistry 35:669-676. ; Dimitroglou et al., 2011Dimitroglou, A.; Merrifield, D. L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D. and Davies, S. J. 2011. Microbial manipulations to improve fish health and production – a Mediterranean perspective. Fish & Shellfish Immunology 30:1-16. ). Different results found by Mansour et al. (2012)Mansour, M. R.; Akrami, R.; Ghobadi, S. H.; Denji, K. A.; Ezatrahimi, N. and Gharaei, A. 2012. Effect of dietary mannan oligosaccharide (MOS) on growth performance, survival, body composition, and some hematological parameters in giant sturgeon juvenile ( Huso huso Linnaeus, 1754). Fish Physiology and Biochemistry 38:829-835. presented a decrease in Huso huso lymphocytes after 46 days of feeding 2 g.kg−1 of MOS with no changes in the other white cell counts. Also in contrast with our data, Sado et al. (2014)Sado, R. Y.; Bicudo, A. J. A. and Cyrino, J. E. P. 2014. Hematology of juvenile pacu, Piaractus mesopotamicus (Holmberg, 1887) fed graded levels of mannan oligosaccharides (MOS). Latin American Journal of Aquatic Research 42:30-39. observed no differences in white cell profile when giving MOS to Piaractus mesopotamis with.
The lysozyme is an important protein in the innate immune system of fish. It acts as an enzyme, hydrolyzing the peptidoglycan present on the cell wall of Gram-positive bacteria, and also as an opsonin, activating the complementary system and promoting the phagocytosis ( Ellis, 1999Ellis, A. E. 1999. Immunity to bacteria in fish. Fish & Shellfish Immunology 9:291-308. ; Magnadóttir, 2006Magnadóttir, B. 2006. Innate immunity of fish (overview). Fish & Shellfish Immunology 20:137-151. ; Saurabh and Sahoo, 2008Saurabh, S. and Sahoo, P. 2008. Lysozyme: an important defence molecule of fish innate immune system. Aquaculture Research 39:223-239. ). In the present study, MOS feeding during 45 days successfully increased the serum lysozyme levels in Nile tilapia juveniles, which can also be considered an immunostimulation. Akrami et al. (2012)Akrami, R.; Chitsaz, H.; Hezarjaribi, A. and Ziaei, R. 2012. Effect of dietary mannan oligosaccharide (MOS) on growth performance and immune response of Gibel carp juveniles ( Carassius auratus gibelio). Journal of Veterinary Advances 2:507-513. also observed an increase in lysozyme levels of Carassus aurata gibelio. However, their supplementary doses were considerably higher than ours (1.5, 3, and 4.5 g.kg−1 MOS), and only the 4.5 g.kg−1 MOS was statistically different from the control.
Conclusions
The presented data shows that mannan-oligosaccharide, as a dietary supplement, exerts a prebiotic effect and is able to immunostimulate Nile tilapia juveniles, presenting potential as a prophylactic additive for Nile tilapia in the substitution of antibiotics. The authors recommend the enrichment of Nile tilapia diets with 8 g.kg−1 of mannanoligosaccharide due to the better results on monocytes, lysozyme, and intestinal lactic acid bacteria.
Acknowledgments
The authors are grateful to Biorigin (Lençóis Paulistas, Brazil), for supplying the ActiveMOS®diets, and the Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp), for the financial support of this research and scholarship (Grant no. 2010/00319-9). They also acknowledge Msc. Tiago H. S. Pires, for the manuscript review. They would also like to thank Michael James Stablein, of the University of Illinois Urbana-Champaign, for his translation and English revision services in this work.
References
- Abreu, J.; Marzocchi-Machado, C.; Urbaczek, A.; Fonseca, L. and Urbinati, E. C. 2009. Leukocytes respiratory burst and lysozyme level in pacu ( Piaractus mesopotamicus Holmberg, 1887). Brazilian Journal of Biology 69:1133-1139.
- Akrami, R.; Chitsaz, H.; Hezarjaribi, A. and Ziaei, R. 2012. Effect of dietary mannan oligosaccharide (MOS) on growth performance and immune response of Gibel carp juveniles ( Carassius auratus gibelio). Journal of Veterinary Advances 2:507-513.
- Aly, S. M.; Ahmed, Y. A.-G.; Ghareeb, A. A.-A. and Mohamed, M. F. 2008a. Studies on Bacillus subtilis and Lactobacillus acidophilus , as potential probiotics, on the immune response and resistance of Tilapia nilotica ( Oreochromis niloticus ) to challenge infections. Fish & Shellfish Immunology 25:128-136.
- Aly, S. M.; Mohamed, M. F. and John, G. 2008b. Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica ( Oreochromis niloticus ). Aquaculture Research 39:647-656. https://doi.org/10.1111/j.1365-2109.2008.01932.x
» https://doi.org/10.1111/j.1365-2109.2008.01932.x - Anderson, D. P. 1992. Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annual Review of Fish Diseases 2:281-307.
- Andrews, S. R.; Sahu, N. P.; Pal, A. K. and Kumar, S. 2009. Haematological modulation and growth of Labeo rohita fingerlings: effect of dietary mannan oligosaccharide, yeast extract, protein hydrolysate and chlorella. Aquaculture Research 41:61-69.
- Bricknell, I. and Dalmo, R. A. 2005. The use of immunostimulants in fish larval aquaculture. Fish & Shellfish Immunology 19:457-472.
- Collier, H. B. 1944. Standardization of blood haemoglobin determinations. Canadian Medical Association Journal 50:550.
- Dimitroglou, A.; Merrifield, D. L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D. and Davies, S. J. 2011. Microbial manipulations to improve fish health and production – a Mediterranean perspective. Fish & Shellfish Immunology 30:1-16.
- Dimitroglou, A.; Merrifield, D. L.; Moate, R.; Davies, S. J.; Spring, P.; Sweetman, J. and Bradley, G. 2009. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Animal Science 87:3226-3234.
- Dimitroglou, A.; Merrifield, D. L.; Spring, P.; Sweetman, J.; Moate, R. and Davies, S. J. 2010. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream ( Sparus aurata ). Aquaculture 300:182-188.
- Ellis, A. E. 1999. Immunity to bacteria in fish. Fish & Shellfish Immunology 9:291-308.
- Fitzsimmons, K.; Martinez-Garcia, R. and Gonzalez-Alanis, P. 2011. Why tilapia is becoming the most important food fish on the planet. Better science, better fish, better life. p.9-18. In: Proceedings of the 9th International Symposium on Tilapia in Aquaculture. Shanghai Ocean University, Shanghai. AquaFish Collaborative Research Support Program, Corvallis.
- Galina, J.; Yin, G.; Ardó, L. and Jeney, Z. 2009. The use of immunostimulating herbs in fish. An overview of research. Fish Physiology and Biochemistry 35:669-676.
- Goldenfarb, P. B.; Bowyer, F. P.; Hall, E. and Brosious, E. 1971. Reproducibility in the hematology laboratory: the microhematocrit determination. American Journal of Clinical Pathology 56:35-39.
- Gómez, G. D. and Balcázar, J. L. 2008. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunology & Medical Microbiology 52:145-154.
- Grant, K. R. 2015. Fish hematology and associated disorders. Veterinary Clinics of North America: Exotic Animal Practice 18:83-103.
- Hrubec, T. and Smith, S. 2000. Hematology of fish. Schalm's veterinary hematology. Lippincott Williams and Wilkins, Philadelphia. p.1120-1125.
- Iwama, G. K.; Pickering, A.; Sumpter, J. and Schreck, C. 2011. Fish stress and health in aquaculture. Cambridge University Press, Cambridge.
- Katakura, Y.; Sano, R.; Hashimoto, T.; Ninomiya, K. and Shioya, S. 2010. Lactic acid bacteria display on the cell surface cytosolic proteins that recognize yeast mannan. Applied Microbiology and Biotechnology 86:319-326.
- Lara-Flores, M.; Olvera-Novoa, M. A.; Guzmán-Méndez, B. Z. E. and López-Madrid, W. 2003. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus , and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia ( Oreochromis niloticus ). Aquaculture 216:193-201.
- Liu, W.; Ren, P.; He, S.; Xu, L.; Yang, Y.; Gu, Z. and Zhou, Z. 2013. Comparison of adhesive gut bacteria composition, immunity, and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains. Fish & Shellfish Immunology 35:54-62.
- Magnadóttir, B. 2006. Innate immunity of fish (overview). Fish & Shellfish Immunology 20:137-151.
- Mansour, M. R.; Akrami, R.; Ghobadi, S. H.; Denji, K. A.; Ezatrahimi, N. and Gharaei, A. 2012. Effect of dietary mannan oligosaccharide (MOS) on growth performance, survival, body composition, and some hematological parameters in giant sturgeon juvenile ( Huso huso Linnaeus, 1754). Fish Physiology and Biochemistry 38:829-835.
- Mauel, M.; Soto, E.; Moralis, J. and Hawke, J. 2007. A piscirickettsiosis-like syndrome in cultured Nile tilapia in Latin America with Francisella spp. as the pathogenic agent. Journal of Aquatic Animal Health 19:27-34.
- Mian, G. F.; Godoy, D. T.; Leal, C. A. G.; Yuhara, T. Y.; Costa, G. M. and Figueiredo, H. C. P. 2009. Aspects of the natural history and virulence of S. agalactiae infection in Nile tilapia. Veterinary Microbiology 136:180-183.
- Ngamkala, S.; Futami, K.; Endo, M.; Maita, M. and Katagiri, T. 2010. Immunological effects of glucan and Lactobacillus rhamnosus GG, a probiotic bacterium, on Nile tilapia Oreochromis niloticus intestine with oral Aeromonas challenges. Fisheries Science 76:833-840.
- NRC - National Research Council. 1993. Nutrient requirements of warmwater fishes and shellfishes. National Academy, Washington, D.C.
- Pirarat, N.; Kobayashi, T.; Katagiri, T.; Maita, M. and Endo, M. 2006. Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia ( Oreochromis niloticus ). Veterinary Immunology and Immunopathology 113:339-347.
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N. and Maita, M. 2011. Modulation of intestinal morphology and immunity in nile tilapia ( Oreochromis niloticus ) by Lactobacillus rhamnosus GG. Research in Veterinary Science 91:e92-e97.
- Pohlenz, C. and Gatlin, D. M. 2014. Interrelationships between fish nutrition and health. Aquaculture 431:111-117.
- Sado, R. Y.; Bicudo, A. J. A. and Cyrino, J. E. P. 2008. Feeding dietary mannan oligosaccharides to juvenile Nile tilapia, Oreochromis niloticus , has no effect on hematological parameters and showed decreased feed consumption. Journal of the World Aquaculture Society 39:821-826.
- Sado, R. Y.; Bicudo, A. J. A. and Cyrino, J. E. P. 2014. Hematology of juvenile pacu, Piaractus mesopotamicus (Holmberg, 1887) fed graded levels of mannan oligosaccharides (MOS). Latin American Journal of Aquatic Research 42:30-39.
- Saurabh, S. and Sahoo, P. 2008. Lysozyme: an important defence molecule of fish innate immune system. Aquaculture Research 39:223-239.
- Staykov, Y.; Spring P.; Denev, S. and Sweetman, J. 2007. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout ( Oncorhynchus mykiss ). Aquaculture International 15:153-161.
- Talpur, A. D.; Munir, M. B.; Mary, A. and Hashim, R. 2014. Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead ( Channa striata ) fingerlings. Aquaculture 426-427:14-20.
- Tavares-Dias, M. and Moraes, F. R. 2006. Características hematológicas da Tilapia rendalli Boulenger, 1896 (Osteichthyes: Cichlidae) capturada em “Pesque-Pague” de Franca, São Paulo, Brasil. Bioscience Journal 19:107-114.
- Torrecillas, S.; Montero, D. and Izquierdo, M. 2014. Improved health and growth of fish fed mannan oligosaccharides: Potential mode of action. Fish & Shellfish Immunology 36:525-544.
- Torrecillas, S.; Montero, D. M.; Caballero, J.; Robaina, L.; Zamorano, M. J.; Sweetman, J. and Izquierdo, M. 2015. Effects of dietary concentrated mannan oligosaccharides supplementation on growth, gut mucosal immune system and liver lipid metabolism of European sea bass ( Dicentrarchus labrax ) juveniles. Fish & Shellfish Immunology 42:508-516.
- Wintrobe, M. M. 1934. Variations in the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematologica 51:32-49.
- Zhou, Q. C.; Buentello, J. A. and Gatlin, D. M. 2010. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum ( Sciaenops ocellatus ). Aquaculture 309:253-257.
Publication Dates
-
Publication in this collection
08 Nov 2018 -
Date of issue
2018
History
-
Received
06 Mar 2017 -
Accepted
22 Feb 2018