ABSTRACT
This study was conducted to evaluate the effects of Curcuma longa on the growth performance, intestinal integrity, and antimicrobial activity of chicks colonized by Salmonella Typhimurium. The study included 672 one-day-old male chicks of the CobbTM lineage, which were divided into eight treatment groups with 14 birds per group and six repetitions, for a total of 48 experimental units per treatment. A randomized 4×2 factorial design scheme (C. longa levels × inoculation by Salmonella Typhimurium) was used. Chicks were orally inoculated with 1.2×104 cfu/mL of Salmonella Typhimurium in 0.5 mL of 0.85% buffered saline solution. Curcuma was added to the feed of chicks at 0, 1, 2, and 3% for 35 days. Data were analyzed by analysis of variance and Tukey's test. Optimal feed conversion was observed in chicks given feed supplemented with 1% C. longa, regardless of infection, and 1% C. longa prevented intestinal colonization by Salmonella Typhimurium. Supplementation and bacterial infection influenced the histomorphometry and pH of the intestine. Bacterial infection reduced the intestinal pH, whereas C. longa supplementation increased the pH, but only in infected chicks. Thus, supplementation with 1% C. longa favors feed conversion, inhibits intestinal colonization by Salmonella Typhimurium, and does not alter intestinal integrity. In contrast, supplementation with 3% Curcuma longa decreases feed intake, affecting the performance of 35-day-old chicks.
Keywords:
avian microbiology; intestinal histomorphometry; performance; poultry
Introduction
Additives are added to animal feed to improve performance and control pathogens in the gastrointestinal tract. However, in some consumer markets in the European Union (Huyghebaert et al., 2011Huyghebaert, G.; Ducatelle, R. and Van Immerseel, F. 2011. An update on alternatives to antimicrobial growth promoters for broilers. The Veterinary Journal 187:182-188. https://doi.org/10.1016/j.tvjl.2010.03.003
https://doi.org/10.1016/j.tvjl.2010.03.0...
) and Brasil (Brasil, 1998Brasil. Ministério da Agricultura Pecuária e Abastecimento. 1998. Portaria 448, de 10 de setembro de 1998. Proibe a fabricação, importação, a comercialização e o emprego de preparações farmacêuticas de uso veterinário, de rações e de aditivos alimentares contendo cloranfenicol, furazolidona e nitrofurazona, em animais cujos produtos sejam destinados à alimentação humana. Diário Oficial da União, Brasília, DF.), the use of antibiotics of growth modulators is prohibited. These restrictions have prompted a search for alternative additives, including phytogenic compounds that exhibit antimicrobial properties.
The intestine contains diverse bacteria that have various beneficial health effects, such as the promotion of maturation and intestinal integrity, antagonistic activity against pathogens through competitive exclusion, and immunomodulatory activity. Regulation of microbiota physiology is important for preventing the pathological effects of undesirable bacteria (Lan et al., 2005Lan, Y.; Verstegen, M. W. A.; Tamminga, S. and Williams, B. A. 2005. The role of the commensal gut microbial community in broiler chickens. World's Poultry Science Journal 61:95-104. https://doi.org/10.1079/WPS200445
https://doi.org/10.1079/WPS200445...
).
Among the various pathogenic bacteria that can adversely affect animal production and/or the humans that consume animal products, Salmonella spp. are epidemiologically complex, because they are widespread in nature and have diverse serotypes, making them capable of infecting different animal species (Chambers and Gong, 2011Chambers, J. R. and Gong, J. 2011. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Research International 44:3149-3159. https://doi.org/10.1016/j.foodres.2011.08.017
https://doi.org/10.1016/j.foodres.2011.0...
). Salmonella Typhimurium, a zoonotic serotype, is a human pathogen, and infection can occur through the consumption of contaminated meat (Hur et al., 2012Hur, J.; Jawale, C. and Lee, J. H. 2012. Antimicrobial resistance of Salmonella isolated from food animals: A review. Food Research International 45:819-830. https://doi.org/10.1016/j.foodres.2011.05.014
https://doi.org/10.1016/j.foodres.2011.0...
; Pickler et al., 2012Pickler, L.; Hayashi, R. M.; Lourenço, M. C.; Miglino, L. B.; Caroni, L. F.; Beirão, B. C. B.; Silva, A. V. F. and Santin, E. 2012. Avaliação microbiológica, histológica e imunológica de frangos de corte desafiados com Salmonella Enteritidis e Minnesota e tratados com ácidos orgânicos. Pesquisa Veterinária Brasileira 32:27-36. https://doi.org/10.1590/S0100-736X2012000100006
https://doi.org/10.1590/S0100-736X201200...
).
Curcuma longa (Zingiberaceae) is a rhizomatous plant containing a volatile oil composed of monoterpenes and sesquiterpenes as the main chemical constituents. These terpenes possess pharmacological activities, such as anti-inflammatory, antimicrobial, anti-parasitic, antioxidant, immunostimulant, and hepatoprotective activities (El-Hakim et al., 2009El-Hakim, A. S. A.; Cherian, G. and Ali, M. N. 2009. Use of organic acid, herbs and their combination to improve the utilization commercial low protein broiler diets. International Journal of Poultry Science 8:14-20. https://doi.org/10.3923/ijps.2009.14.20
https://doi.org/10.3923/ijps.2009.14.20...
; Eevuri and Putturu, 2013Eevuri, T. R. and Putturu, R. 2013. Use of certain herbal preparations in broiler feeds - A review. Vet World 6:172-179. https://doi.org/10.5455/vetworld.2013.172-179
https://doi.org/10.5455/vetworld.2013.17...
) and exhibit potential antimicrobial activities in chicks. In poultry feed, turmeric has been extensively used in different concentrations, dosages, and durations (Khan et al., 2012Khan, R. U.; Naz, S.; Javdani, M.; Nikousefat, Z.; Selvaggi, M.; Tufarelli, V. and Laudadio, V. 2012. The use of Turmeric (Curcuma longa) in poultry feed. World's Poultry Science Journal 68:97-103. https://doi.org/10.1017/S0043933912000104
https://doi.org/10.1017/S004393391200010...
). Studies have shown that some phytogenic additives promote the beneficial modulation of the intestinal microbiota, have trophic effects on intestinal mucous, and stimulate immunomodulators, resulting in improved digestion, nutrient absorption, and performance (Nunes et al., 2009Nunes, A. D.; Vaz, A. C. N.; Raspantini, L. E.; Silva, E. M. and Albuquerque, R. 2009. Desempenho e morfologia intestinal de frangos de corte alimentados com rações contendo aditivos alternativos a antimicrobianos. Brazilian Journal of Veterinary and Animal Science 46:500-506.).
This study was conducted to evaluate the utility of C. longa as a phytogenic additive in feed by determining its effects on the performance, integrity, and intestinal colonization by Salmonella Typhimurium in infected chicks.
Material and Methods
The experimental protocol used in this study was approved by the local Ethics Committee for the Use of Animals (CEUA; approval no. 127/14) and was carried out in Goiânia, GO, Brazil (−16.67926° N, −49.25629° W).
The experimental design was entirely randomized in a 4×2 factorial scheme (C. longa levels × inoculation with Salmonella Typhimurium; Table 1). The study included 672 one-day-old male chicks of the CobbTM lineage (sexing performed in the hatchery), which were divided into eight treatment groups with six repetitions and 14 chicks per experimental unit, totaling 48 experimental units per treatment.
Design randomized in a 4×2 factorial scheme (C. longa levels × inoculation by Salmonella Typhimurium)
Feed containing 0, 1, 2, and 3% C. longa was offered to chicks from one to 35 days old (Table 2). Both infected and non-infected chicks were housed separately in similar sheds. The sheds were equipped with feeders, drinkers, and heaters until they were 14 days old. At the time of lodging, the one-day-old chicks were orally inoculated with 0.5 mL of 0.85% buffered saline containing 1.2×104 cfu/mL of Salmonella Typhimurium.
Ingredients, nutritional values (dry matter calculated), and inclusion levels of Curcuma longa in feed of 1 to 35-day-old chicks
The inoculum was prepared using Salmonella Typhimurium isolated from chicks provided by the Laboratory of Bacteriology and typed by the Laboratory of Oswaldo Cruz Foundation-FIOCRUZ (RJ). The isolate was cultivated on xylose-lysine-tergitol-4 (XLT4) agar and incubated at 37 °C for 24 h. Salmonella Typhimurium cells were recovered from the agar and suspended in 0.85% buffered saline solution and adjusted to a concentration of 1.2×104 cfu/mL using the McFarland standard. The concentration was confirmed by quantifying the cfu of serial dilutions plated on XLT4 agar incubated at 37 °C.
Three different experimental feeds were used: pre-starter, starter, and grower. The mashed animal feeds, containing ground maize and soybean meal, were formulated according to the composition and nutritional requirements recommended by Rostagno et al. (2011)Rostagno, H. S.; Albino, L. F. T.; Donzele, J. L.; Gomes, P. C.; Oliveira, R. F.; Lopes, D. C.; Ferreira, A. S.; Barreto, S. L. T. and Euclides, R. F. 2011. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 3.ed. UFV, Viçosa, MG. 252p.. Feed was provided ad libitum throughout the experimental trial. The feed did not contain growth moderators or anti-coccidian agents. The added C. longa powder replaced the starch, which is an inert material.
Dried and sprayed rhizomes of C. longa (as powder) were acquired from Mara Rosa, GO, Brazil (July 2013). Pharmacogenetic evaluation of the botanical material was carried out for quality control. Macroscopic analysis and thin-layer chromatography of the pulverized rhizomes were performed along with a curcumin standard (Merck, Billerica, MA, USA) as described by the Brazilian Pharmacopoeia (Brasil, 2010Brasil. 2010. Farmacopeia Brasileira. 5.ed. Agência Nacional de Vigilância Sanitária, Brasília. 546p.).
Performance was evaluated by determining the average weight, weight gain, feed intake, feed conversion (measured as the total weight of the dead chicks), viability, pH, and intestinal histomorphometry of chicks at 21 and 35 days of age. The average weight was obtained by dividing the total weight of birds by their average number. Weight gain was calculated by the difference between final and initial weight of birds added to the weight of the dead bird and by dividing by the average number of birds. Feed intake was calculated by the ratio between the total feed intake and the average number of birds. Feed conversion ratio was calculated by the feed intake:weight gain ratio, corrected for the total weight of dead birds. Viability was calculated by subtracting mortality from 100 (100 − mortality).
At each time point, chicks were weighed, desensitized, and sacrificed by inner section of the neck vessels, and subsequently necropsied. Fragments of the duodenum were collected from one chick per plot, and the fragments were placed in vials containing 10% buffered formaldehyde to prepare histological sections according to the method of Junqueira and Carneiro (1999)Junqueira, L. C. and Carneiro, J. 1999. Histologia básica. Guanabara, Rio de Janeiro. 427p.. After flushing with hematoxylin and eosin (H&E), samples were subjected to histomorphometric analysis to measure villus height and crypt depth using ImageJ 1.45 (Rasband, 1997Rasband, W. S. 1997-2018. ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA. Available at: <https://imagej.nih.gov/ij/>. Accessed on: Feb. 25, 2018.
https://imagej.nih.gov/ij/...
). Thirty measurements of villus height and 30 sequential measurements of crypt depth were obtained per section and per fragment of each tissue, always from the right to the left of the cut, totaling 180 readings per treatment. The imaging was performed using an optical microscope (DM 4000 B; Leica, Wetzlar, Germany) coupled to a microcomputer.
To test for the presence of Salmonella, swabs were collected from six chicks per treatment. The swabs were transferred to test tubes containing 9 mL of selenite-cystine (SC) broth and incubated at 37 °C for 18-24 h. Aliquots of the incubated broth were transferred to bright green (BG) and XLT4 agar and incubated at 37 °C for 18-24 h. Plates with 3-5 cfu showing the morphological characteristics of Salmonella were transferred to tubes containing triple sugar iron broth and incubated at 37 °C for 18-24 h. Tubes showing Salmonella growth were tested for urease, indole, methyl red, motility, decarboxylase, and lysine. Those showing Salmonella-compatible biochemical reactions were analyzed by using a serological test with Salmonella anti-O-antigen serum (Probac do Brasil, São Paulo, Brazil).
Performance, pH, and intestinal histomorphometry data were subjected to analysis of variance, and the averages were compared by Tukey's test (P = 0.05). When significance was detected, the polynomial regression model was adjusted. To evaluate intestinal colonization by Salmonella Typhimurium, a descriptive test that considered the frequency was used. For the contamination factor, Snedecor's F distribution was used to describe the difference in the effects of the bacterium.
Results
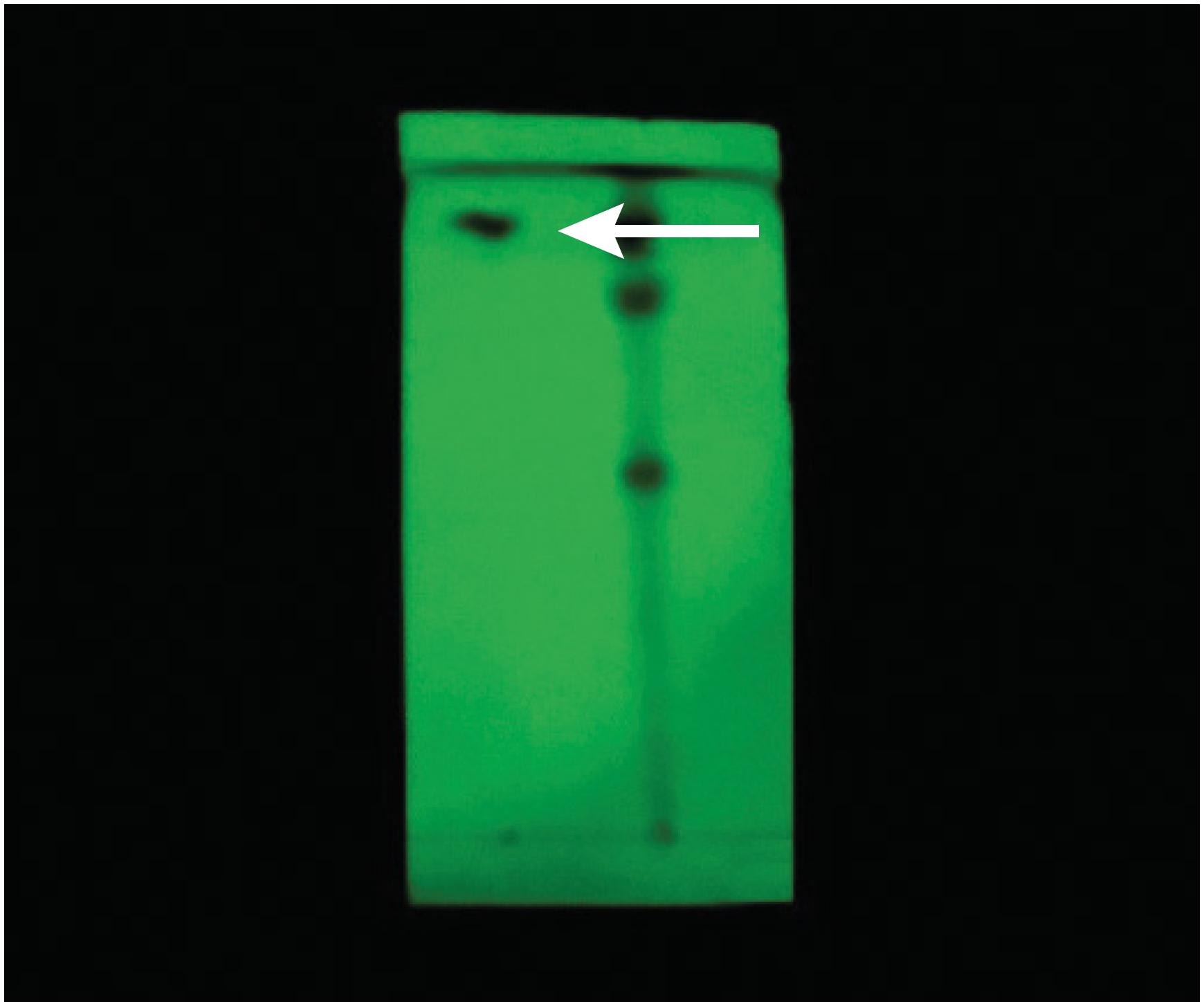
Macroscopic analysis of the pulverized rhizomes of C. longa showed robust yellowish coloration with whitish granulations and its characteristic smell. Illumination of a thin-layer chromatograph of the curcumin standard under ultraviolet light (365 nm), showed green fluorescence (Rf = 0.68) in the upper third of the plate; green fluorescence with the same characteristics as those of the standard was also observed in the chromatograph of C. longa samples (Rf = 0.68; Figure 1).
Thin-layer chromatography of the sprayed rhizomes of Curcuma longa highlighting fluorescent green spot corresponding to curcumin (arrow).
The performance analysis (Table 3) revealed no interaction (P>0.05) between the factors studied (i.e., C. longa levels and Salmonella Typhimurium infection) and final weight, feed intake, and feed conversion on days 1-21. However, supplementation with 3% C. longa resulted in decreased final weight, weight gain, and feed intake compared with the values in the control group (0% C. longa). Regression analysis of C. longa levels in 21-day-old chicks revealed negative linear effects on weight gain (; R2 = 0.1909) and feed intake (; R2 = 0.1912), demonstrating that a higher concentration of C. longa in the feed resulted in decreased weight gain and feed intake. Infection by Salmonella Typhimurium (P<0.05) also affected performance, as inoculation negatively influenced the final weight, weight gain, and feed intake (P<0.05).
Performance variables of chicks inoculated by Salmonella Typhimurium and fed diet with Curcuma longa, up to 21 days old
In 35-day-old chicks (Table 4), no interaction was observed between the studied factors (C. longa levels and Salmonella Typhimurium contamination; P>0.05); however, a difference (P<0.05) was observed between C. longa levels in the feed and final weight, weight gain, and feed conversion. Chicks fed 1% C. longa presented higher final weights and weight gains than chicks fed 2 and 3% C. longa but did not significantly differ from the control. Feed conversion was higher in the group of chicks fed diet supplemented with 1% C. longa than in the groups fed diet supplemented with 0, 2, and 3% C. longa. Similarly, as was observed in the 21-day-old chicks, Salmonella Typhimurium infection in 35-day-old chicks compromised their performance and resulted in lower final weight, weight gain, and feed intake.
Performance variables of chicks contaminated by Salmonella Typhimurium and fed diet with Curcuma longa, up to 35 days old
In the infected poultry groups (Table 5), Salmonella Typhimurium was not recovered from chicks fed diet supplemented with 1% C. longa at any time point (7, 14, 21, or 35 days), indicating that the inoculated bacterium could not colonize their intestines. However, Salmonella Typhimurium was not recovered from any of the inoculated groups after 21 days of age.
Frequency of Salmonella Typhimurium, in cloacal Swab, of inoculated chicks and chicks fed diet supplemented with different levels of Curcuma longa, at 7, 14, 21, and 35 days old
Histomorphometry of the jejunum in 35-day-old chicks (Table 6) revealed no interaction with the concentration of C. longa (P>0.05). Similarly, C. longa supplementation did not influence villus height or crypt depth (P>0.05) at 21 days. However, regression analysis revealed a quadratic effect on villus height (; R2 = 0.7137), demonstrating that supplementation of feed with 2.7% C. longa promoted villus height. Furthermore, regression analysis revealed a significant (P<0.05) linear effect on crypt depth (; R2 = 0.3799), demonstrating increased crypt depth with increasing C. longa supplementation. Salmonella Typhimurium showed significant effects (P<0.05) on villus height and crypt depth in both the control and infected groups of 21- and 35-day-old chicks (Figure 2).
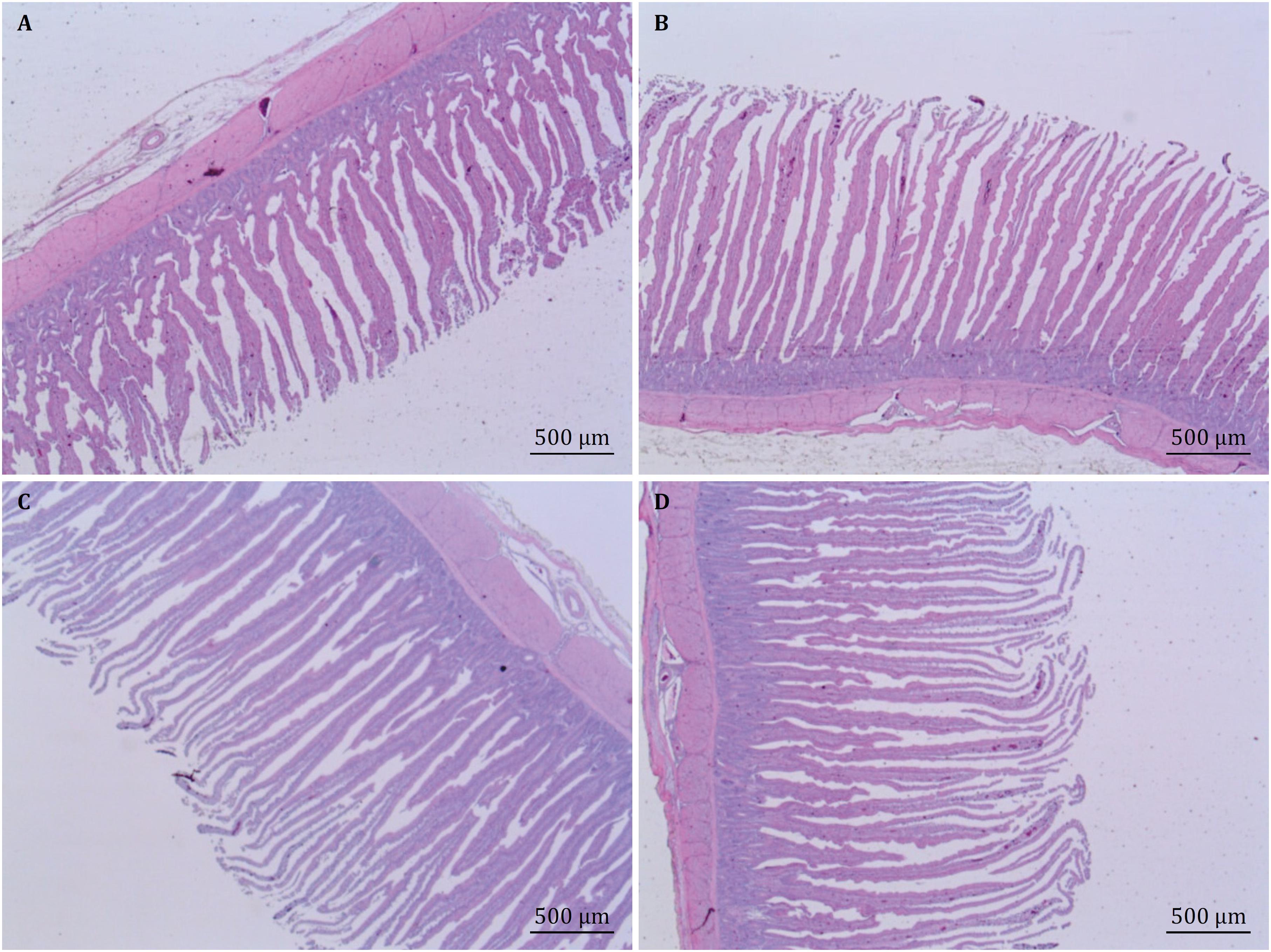
Histological photos (villi height and crypt depth) of the jejunum of 21-day-old chicks uninoculated (A) and inoculated by Salmonella Typhimurium (B), and of 35-day-old chicks uninoculated (C) and inoculated by Salmonella Typhimurium (D), fed diet supplemented with 2% Curcuma longa.
Average of villus height (VH), crypt depth (CD), and villus:crypt ratio of the jejunum of 21- and 35-day-old chicks inoculated by Salmonella Typhimurium and fed diet supplemented with Curcuma longa
Thirty-five-day-old chicks fed 1% C. longa presented significantly greater crypt depth (P<0.05) in the jejunum than the control group, irrespective of Salmonella Typhimurium inoculation. However, C. longa supplementation had no effect (P>0.05) on villus height or villus:crypt ratio.
Histomorphometric analysis of the duodenum did not show a significant effect (P>0.05) of the interaction between C. longa supplementation and Salmonella Typhimurium infection (Table 7) on crypt depth in 21-day-old chicks (Figure 3). However, there was a significant difference (P<0.05) in villus height in the presence of Salmonella Typhimurium, and the values were lower in non-infected chicks.
Histological photos (villi height and crypt depth) at the duodenum of 21-day-old chicks uninoculated (A) and inoculated by Salmonella Typhimurium (B), and of 35-day-old chicks uninoculated (C) and inoculated by Salmonella Typhimurium (D), fed diet supplemented with 2% Curcuma longa.
Average of villus height (VH), crypt depth (CD), and villus:crypt ratio of the duodenum of 21- and 35-day-old chicks inoculated by Salmonella Typhimurium and fed diet supplemented with Curcuma longa
Supplementation with C. longa significantly affected villus height (P<0.05). Groups of infected chicks fed feed supplemented with 2% C. longa showed increased villus height (Table 8). In non-infected chicks, supplementation with 2 and 3% C. longa also resulted in higher average villus heights. In 21-day-old infected chicks, regression analysis revealed a quadratic effect on villus height (; R2 = 0.7675), demonstrating that supplementation with 2% C. longa resulted in increased villus height. No significant effect was observed in non-infected chicks.
Values of villus height and villus:crypt ratio in the jejunum of 21-day-old chicks of the control and inoculated with Salmonella Typhimurium and fed diet supplemented with different levels of Curcuma longa
In infected chicks, regression analysis showed a quadratic effect on the relationship between villi and crypts (; R2 = 0.1741; Table 8), and there was a strong relationship between villi and crypts in chicks fed diet supplemented 1.2% C. longa.
For intestinal pH, a significant interaction (P<0.05) was observed between C. longa supplementation and Salmonella Typhimurium infection in all segments of the intestine in 21-day-old chicks (Table 9). Analysis of the effects of the interaction between C. longa supplementation and Salmonella Typhimurium infection on the pH in the duodenum (Table 10) revealed that the pH values of infected chicks without C. longa supplementation were lower than the pH values of the infected chicks fed diet supplemented with 2 and 3% C. longa. In the regression analysis of different levels of C. longa, the infected groups showed a quadratic effect (; R2 = 0.3411), demonstrating that 2.1% C. longa increased the duodenal pH. In addition, non-infected groups fed diet supplemented with 3% C. longa showed a higher pH than groups that received other concentrations of C. longa.
pH values in the intestinal segments of 21-day-old chicks inoculated by Salmonella Typhimurium and fed diet supplemented with Curcuma longa
Values of intestinal pH: duodenum, jejunum, ileum, and colon of 21-day-old chicks in the control and inoculated with Salmonella Typhimurium and fed diet supplemented with different levels of Curcuma longa
Salmonella Typhimurium-infected chicks given feed supplemented with 0 and 1% C. longa showed a lower pH in the duodenum (Table 10). Regression analysis of duodenal pH showed a linear effect (; R2 = 0.3248) for C. longa supplementation, revealing that the pH increased with increasing concentrations of C. longa in the feed.
Among non-infected chicks, the colon pH of chicks in groups fed diet supplemented with 3% C. longa was lower than that of chicks in groups fed diet supplemented with 2% C. longa. Regression analysis showed a significant relationship (P<0.05) between C. longa supplementation and colon pH, with a quadratic effect (; R2 = 0.2318), demonstrating that 1% C. longa supplementation resulted in a higher pH in the colon; no significant effect was observed in non-infected chicks.
In 35-day-old chicks, there was no effect of the interactions between the factors studied (C. longa levels and Salmonella Typhimurium infection). However, the extent of Salmonella Typhimurium infection affected the pH values in the duodenum and ileum, where significantly lower pH values were observed in the Salmonella-infected groups, regardless of C. longa supplementation (Table 11). In the jejunum and ileum, supplementation with 2 and 3% C. longa increased the pH to a higher level than in both the non-supplemented and 1% supplementation groups (P<0.05).
pH values of the intestinal segments of 35-day-old chicks inoculated by Salmonella Typhimurium and fed diet supplemented with Curcuma longa
Discussion
The results of the pharmacogenetic evaluation agreed with the description in the Brazilian Pharmacopoeia (Brasil, 2010Brasil. 2010. Farmacopeia Brasileira. 5.ed. Agência Nacional de Vigilância Sanitária, Brasília. 546p.) for this plant species, indicating that the tested plant species was C. longa.
The performance of 21-day-old chicks decreased in the presence of supplementation with 3% of C. longa and in the absence of Salmonella Typhimurium infection. This result differed from the findings of Abou-Elkhair et al. (2014)Abou-Elkhair, R.; Ahmed, H. A. and Selim, S. 2014. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australasian Journal of Animal Sciences 27:847-854. https://doi.org/10.5713/ajas.2013.13644
https://doi.org/10.5713/ajas.2013.13644...
, who showed no significant difference in body weight and feed intake in a similar experiment, although they used lower concentrations of C. longa in combination with black pepper and oregano seed. Samarasinghe et al. (2003)Samarasinghe, K.; Wenk, C.; Silva, K. F. S. T. and Gunasekera, J. M. D. M. 2003. Turmeric (Curcuma longa) root powder and mannanoligosaccharides as alternatives to antibiotics in broiler chicken diet. Asian-Australasian Journal of Animal Sciences 16:1495-1500. https://doi.org/10.5713/ajas.2003.1495
https://doi.org/10.5713/ajas.2003.1495...
and Al-Jaleel (2012)Al-Jaleel, R. A. 2012. Use of turmeric (Curcuma longa) on the performance and some physiological traits on the broiler diets. The Iraqi Journal of Veterinary Medicine 36:51-57. observed increased weight gain in birds fed diet supplemented with 0.2-0.3% C. longa. According to Al-Jaleel (2012)Al-Jaleel, R. A. 2012. Use of turmeric (Curcuma longa) on the performance and some physiological traits on the broiler diets. The Iraqi Journal of Veterinary Medicine 36:51-57., C. longa supplementation at <2% increased antioxidant activity. Abbas et al. (2010)Abbas, R. Z.; Iqbal, Z.; Khan, M. N.; Zafar, M. A. and Zia, M. A. 2010. Anticoccidial activity of Curcuma longa L. in broilers. Brazilian Archives of Biology and Technology 53:63-67. https://doi.org/10.1590/S1516-89132010000100008
https://doi.org/10.1590/S1516-8913201000...
used 1, 2, and 3% C. longa as an antimicrobial agent and observed increased feed intake, weight gain, and anti-coccidian activity in birds fed diet supplemented with 3% C. longa. Abdel-Rahman et al. (2014)Abdel-Rahman, H. A.; Fathallah, S. I.; Helal, M. A.; Nafea, A. A. and Zahran, I. S. 2014. Effect of turmeric (Curcuma longa), fenugreek (Trigonella foenum-graecum L.) and/or bioflavonoid supplementation to the broiler chicks diet and drinking water on the growth performance and intestinal morphometeric parameters. Global Veterinaria 12:627-635. observed a greater final weight of birds fed diet supplemented with a mixture of herbs, including Curcuma powder and fenugreek (Trigonella foenum-graecum), when compared with the control group.
In this study, feed intake decreased during the study period in all trials, resulting in lower weight gains and final weights of the chicks given feed supplemented with 3% C. longa. This reduction in feed intake can be attributed to the organoleptic characteristics of C. longa, such as its strong odor, present in the feed (Péret-Almeida et al., 2008Péret-Almeida, L.; Naghetini, C. C.; Nunan, E. A.; Junqueira, R. G. and Glória, M. B. A. 2008. Atividade antimicrobiana in vitro do rizoma em pó, dos pigmentos curcuminóides e dos óleos essenciais da Curcuma longa L. Ciência e Agrotecnologia 32:875-881. https://doi.org/10.1590/S1413-70542008000300026
https://doi.org/10.1590/S1413-7054200800...
; Sueth-Santiago et al., 2015Sueth-Santiago, V.; Mendes-Silva, G. P.; Decoté-Ricardo, D. and Lima, M. E. F. 2015. Curcumina, o pó dourado do açafrão-da-terra: introspecções sobre química e atividades biológicas. Química Nova 38:538-552. https://doi.org/10.5935/0100-4042.20150035
https://doi.org/10.5935/0100-4042.201500...
). Moreover, the excretions from chicks in the groups given feed supplemented with higher concentrations of C. longa exhibited a more yellowish hue. These findings agree with those of Wahlstron and Blennow (1978)Wahlstron, B. and Blennow, G. A. 1978. A study on the fate of curcumim in the rat. Acta Pharmacolica et Toxicologica 43:86-92. https://doi.org/10.1111/j.1600-0773.1978.tb02240.x
https://doi.org/10.1111/j.1600-0773.1978...
, who showed that mice administered C. longa at a dose of 1 g kg−1 live weight eliminated 75% of the curcumin in feces. Additionally, Lao et al. (2006)Lao, C. D.; Ruffin M. T.; Normolle, D.; Heath, D. D.; Murray, S. I.; Bailey, J. M.; Boggs, M. E.; Crowell, J.; Rock, C. L. and Brenner, D. E. 2006. Dose escalation of a curcuminoid formulation. BMC Complementary and Alternative Medicine 6:10.https://doi.org/10.1186/1472-6882-6-10
https://doi.org/10.1186/1472-6882-6-10...
observed more yellow stools in humans administered 12 g/day of C. longa in food.
Contamination with Salmonella Typhimurium on the first day of life led to poor performance. These results suggest that the inoculated bacteria affected the gastrointestinal tract by promoting an imbalance in the microbiota and injuring the mucous membranes, reducing digestion and feed absorption. This confirms the results of Borsoi et al. (2011)Borsoi, A.; Santos, L. R.; Rodrigues, L. B.; Moraes, H. L. S.; Salle, C. T. P. and Nascimento, V. P. 2011. Behavior of Salmonella Heildelberg and Salmonella Enteritidis strains following broiler chick inoculation: Evaluation of cecal morphometry, liver and cecum bacterial counts and fecal excretion patterns. Brazilian Journal of Microbiology 42:266-273. https://doi.org/10.1590/S1517-83822011000100034
https://doi.org/10.1590/S1517-8382201100...
, who reported reduced intestinal absorption of nutrients in the presence of enteropathogens.
Feed conversion by 35-day-old chicks was improved when 1% C. longa was included in the feed, regardless of Salmonella Typhimurium infection. In contrast, Emadi and Kermanshahi (2006)Emadi, M. and Kermanshahi, H. 2006. Effect of turmeric rhizome powder on performance and carcass characteristics of broiler chickens. International Journal of Poultry Science 5:1069-1072. https://doi.org/10.3923/ijps.2006.1069.1072
https://doi.org/10.3923/ijps.2006.1069.1...
and Botelho (2014)Botelho, L. F. R. 2014. Açafrão (Curcuma longa) em rações para frangos de corte contendo sorgo em substituição ao milho. Dissertação (M.Sc.). Universidade Estadual de Montes Claros, Janaúba. did not observe any beneficial effects of Curcuma on performance at supplementation levels of 0.25, 0.50, 0.75% and 0.5, 1.0, 1.5, 2.0%, respectively. This variation in the effects of C. longa on the performance of chicks could be explained by variability in the quantity of phytochemicals in the plant, as well as other factors, such as the age and developmental stage of the plant, time of harvest, temperature, water availability, UV radiation, soil nutrients, altitude, and atmospheric composition. These factors can directly influence the relative proportions of these compounds in the plant.
In chicks given feed supplemented with 1% Curcuma, Salmonella was not recovered at any stage (7, 14, 21, and 35 days), despite inoculation with Salmonella Typhimurium on the first day of life. This suggests that feed containing 1% Curcuma prevented intestinal colonization by the inoculated bacterium. Thus, 1% supplementation showed antimicrobial activity, promoting alterations in the microbiota and acting against the inoculated bacterium (Lorenzi and Matos, 2002Lorenzi, H. and Matos, F. J. de A. 2002. Plantas medicinais no Brasil/Nativas e exóticas. Instituto Plantarum, Nova Odessa. 512p.). Additionally, chicks may have eliminated Salmonella intermittently and in low numbers, thus limiting their isolation (Barancelli et al., 2012Barancelli, G. V.; Martin, J. G. P. and Ermani, P. 2012. Salmonella em ovos: relação entre produção e consumo seguro. Segurança Alimentar e Nutricional 19:73-82.). Attia et al. (2017)Attia, Y. A.; Al-Harthi, M. A. and Hassan, S. S. 2017. Turmeric (Curcuma longa Linn.) as a phytogenic growth promoter alternative for antibiotic and comparable to mannan oligosaccharides for broiler chicks. Revista Mexicana de Ciencias Pecuarias 8:11-21. https://doi.org/10.22319/rmcp.v8i1.4309
https://doi.org/10.22319/rmcp.v8i1.4309...
also obtained significant results using 1% of C. longa demonstrating better performance feed as a phytogenic feed additive without negative effects on the productive and economic traits of broilers.
Salmonella Typhimurium was not recovered from any infected 21-day-old chick, which can be explained by the elimination of this bacterium accompanied by a gradual change in the intestinal microbiota and the development of intestinal lymphoid tissue, which occurs naturally with advancing age to increase resistance to microbial attack. Similarly, Beal et al. (2004)Beal, R. K.; Wigley, P.; Powers, C.; Hulme, S. D.; Barrow, P. A. and Smith, A. L. 2004. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Veterinary Immunology and Immunopathology 100:151-164. https://doi.org/10.1016/j.vetimm.2004.04.005
https://doi.org/10.1016/j.vetimm.2004.04...
infected birds with Salmonella Typhimurium in the first, third, and sixth weeks of life and observed that the rates of persistent infection were lower in older infected birds. The resistance of the birds and the invasive capacity of the serovar also influence colonization by and excretion of Salmonella (Barrow, 2000Barrow, P. A. 2000. The paratyphoid salmonellae. Review Science Technology 19:351-375.; Andrade et al., 2007Andrade, M. A.; Mesquista, A. J.; Stringhini, J. H.; Chaves, L. S.; Mattos, M. S.; Oliveira, A. S. C. and Moraes, D. M. C. 2007. Excreção fecal de Salmonella Enteritidis em duas linhagens de frangos de corte. Ciência Animal Brasileira 8:757-765.).
Intestinal histomorphometry showed that supplementation with 1% Curcuma increased crypt depth in the duodenum, indicating an attempt to recover the structure of the villi. These results agree with those of Abdel-Rahman et al. (2014)Abdel-Rahman, H. A.; Fathallah, S. I.; Helal, M. A.; Nafea, A. A. and Zahran, I. S. 2014. Effect of turmeric (Curcuma longa), fenugreek (Trigonella foenum-graecum L.) and/or bioflavonoid supplementation to the broiler chicks diet and drinking water on the growth performance and intestinal morphometeric parameters. Global Veterinaria 12:627-635., who also observed greater crypt depth in birds fed diet supplemented with a mixture of herbs composed of C. longa powder and Trigonella foenum-graecum.
Villus height and villus:crypt ratio are markers of mucosal integrity and intestinal function. As observed in this study, by 21 days, the pathogen had compromised the intestinal epithelium of the duodenum, resulting in increased villus height and a decreased villus:crypt ratio. Higher villi indicate greater digestive capacity; however, the weak relationship between these evaluations suggest that cellular proliferation had occurred to restore the villi that were destroyed. Similar results were observed by Viola et al. (2008)Viola, E. S.; Vieira, S. L.; Torres, C. A.; Freitas, D. M. and Berres, J. 2008. Desempenho de frangos de corte sob suplementação com ácidos lático, fórmico, acético e fosfórico no alimento ou na água. Revista Brasileira de Zootecnia 37:296-302. https://doi.org/10.1590/S1516-35982008000200016
https://doi.org/10.1590/S1516-3598200800...
and Santos (2010)Santos, G. C. 2010. Alternativas ao uso de promotores químicos de crescimento sobre o desempenho e características de carcaça de frangos de corte. Dissertação (M.Sc.). Universidade Federal do Vale do Jequitinhonha e Mucuri, Diamantina., who reported that a lower villus:crypt ratio indicates the presence of destroyed villi and increased cellular proliferation in the crypts, resulting from an attempt to restore the damaged intestinal epithelium and overcome microbiological challenge in the intestine.
In non-infected chicks fed diet supplemented with 2 and 3% C. longa, trophic action was observed in the intestinal mucous of the duodenum, influencing cellular proliferation and increasing villus height to repair the damaged structure. Among the concentrations evaluated, the villi were highest in the Salmonella Typhimurium-infected group with 3% C. longa supplementation.
The level of C. longa supplementation as well as the presence of the pathogen altered the pH of the duodenum. The increased pH in the groups receiving C. longa supplementation facilitated bacterial proliferation to increase the diversity of the intestinal microbiota, particularly bacteria that are beneficial to the host. Huang et al. (2008)Huang, D. S.; Li, D. F.; Xing, J. J.; Ma, Y. X.; Li, Z. J. and Lv, S. Q. 2008. Effects of feed particle size and feed form on survival of Salmonella Typhimurium in the alimentary tract and cecal S. Typhimurium reduction in growing broilers. Poultry Science 85:831-836. https://doi.org/10.1093/ps/85.5.831
https://doi.org/10.1093/ps/85.5.831...
reported that the low pH values in Salmonella-infected chicks could be due to increased intestinal fermentation by Salmonella, which produces volatile fatty acids. A high dose of Curcuma (3%) likely increased the prevalence of acidophilus bacteria (i.e., Lactobacillus) in this segment of the intestine. This prediction agrees with the results of Lu et al. (2003)Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J. J. and Le, M. D. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Applied and Environmental Microbiology 69:6816-6824. https://doi.org/10.1128/AEM.69.11.6816-6824.2003
https://doi.org/10.1128/AEM.69.11.6816-6...
, who reported that several factors might alter the pH and intestinal microbiota, such as age, antimicrobial supplementation, and infection by pathogenic microbes.
Conclusions
The inclusion of 1% C. longa in chicken feed improves performance, preserves intestinal integrity, and inhibits intestinal colonization by Salmonella Typhimurium when inoculated on the first day of life. Chicks provided feed containing 3% C. longa have worse performance, with reduced feed intake, irrespective of bacterial infection.
Acknowledgments
We acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- Abbas, R. Z.; Iqbal, Z.; Khan, M. N.; Zafar, M. A. and Zia, M. A. 2010. Anticoccidial activity of Curcuma longa L. in broilers. Brazilian Archives of Biology and Technology 53:63-67. https://doi.org/10.1590/S1516-89132010000100008
» https://doi.org/10.1590/S1516-89132010000100008 - Abdel-Rahman, H. A.; Fathallah, S. I.; Helal, M. A.; Nafea, A. A. and Zahran, I. S. 2014. Effect of turmeric (Curcuma longa), fenugreek (Trigonella foenum-graecum L.) and/or bioflavonoid supplementation to the broiler chicks diet and drinking water on the growth performance and intestinal morphometeric parameters. Global Veterinaria 12:627-635.
- Abou-Elkhair, R.; Ahmed, H. A. and Selim, S. 2014. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australasian Journal of Animal Sciences 27:847-854. https://doi.org/10.5713/ajas.2013.13644
» https://doi.org/10.5713/ajas.2013.13644 - Al-Jaleel, R. A. 2012. Use of turmeric (Curcuma longa) on the performance and some physiological traits on the broiler diets. The Iraqi Journal of Veterinary Medicine 36:51-57.
- Andrade, M. A.; Mesquista, A. J.; Stringhini, J. H.; Chaves, L. S.; Mattos, M. S.; Oliveira, A. S. C. and Moraes, D. M. C. 2007. Excreção fecal de Salmonella Enteritidis em duas linhagens de frangos de corte. Ciência Animal Brasileira 8:757-765.
- Attia, Y. A.; Al-Harthi, M. A. and Hassan, S. S. 2017. Turmeric (Curcuma longa Linn.) as a phytogenic growth promoter alternative for antibiotic and comparable to mannan oligosaccharides for broiler chicks. Revista Mexicana de Ciencias Pecuarias 8:11-21. https://doi.org/10.22319/rmcp.v8i1.4309
» https://doi.org/10.22319/rmcp.v8i1.4309 - Barancelli, G. V.; Martin, J. G. P. and Ermani, P. 2012. Salmonella em ovos: relação entre produção e consumo seguro. Segurança Alimentar e Nutricional 19:73-82.
- Barrow, P. A. 2000. The paratyphoid salmonellae Review Science Technology 19:351-375.
- Beal, R. K.; Wigley, P.; Powers, C.; Hulme, S. D.; Barrow, P. A. and Smith, A. L. 2004. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Veterinary Immunology and Immunopathology 100:151-164. https://doi.org/10.1016/j.vetimm.2004.04.005
» https://doi.org/10.1016/j.vetimm.2004.04.005 - Borsoi, A.; Santos, L. R.; Rodrigues, L. B.; Moraes, H. L. S.; Salle, C. T. P. and Nascimento, V. P. 2011. Behavior of Salmonella Heildelberg and Salmonella Enteritidis strains following broiler chick inoculation: Evaluation of cecal morphometry, liver and cecum bacterial counts and fecal excretion patterns. Brazilian Journal of Microbiology 42:266-273. https://doi.org/10.1590/S1517-83822011000100034
» https://doi.org/10.1590/S1517-83822011000100034 - Botelho, L. F. R. 2014. Açafrão (Curcuma longa) em rações para frangos de corte contendo sorgo em substituição ao milho. Dissertação (M.Sc.). Universidade Estadual de Montes Claros, Janaúba.
- Brasil. Ministério da Agricultura Pecuária e Abastecimento. 1998. Portaria 448, de 10 de setembro de 1998. Proibe a fabricação, importação, a comercialização e o emprego de preparações farmacêuticas de uso veterinário, de rações e de aditivos alimentares contendo cloranfenicol, furazolidona e nitrofurazona, em animais cujos produtos sejam destinados à alimentação humana. Diário Oficial da União, Brasília, DF.
- Brasil. 2010. Farmacopeia Brasileira. 5.ed. Agência Nacional de Vigilância Sanitária, Brasília. 546p.
- Chambers, J. R. and Gong, J. 2011. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Research International 44:3149-3159. https://doi.org/10.1016/j.foodres.2011.08.017
» https://doi.org/10.1016/j.foodres.2011.08.017 - Eevuri, T. R. and Putturu, R. 2013. Use of certain herbal preparations in broiler feeds - A review. Vet World 6:172-179. https://doi.org/10.5455/vetworld.2013.172-179
» https://doi.org/10.5455/vetworld.2013.172-179 - El-Hakim, A. S. A.; Cherian, G. and Ali, M. N. 2009. Use of organic acid, herbs and their combination to improve the utilization commercial low protein broiler diets. International Journal of Poultry Science 8:14-20. https://doi.org/10.3923/ijps.2009.14.20
» https://doi.org/10.3923/ijps.2009.14.20 - Emadi, M. and Kermanshahi, H. 2006. Effect of turmeric rhizome powder on performance and carcass characteristics of broiler chickens. International Journal of Poultry Science 5:1069-1072. https://doi.org/10.3923/ijps.2006.1069.1072
» https://doi.org/10.3923/ijps.2006.1069.1072 - Huang, D. S.; Li, D. F.; Xing, J. J.; Ma, Y. X.; Li, Z. J. and Lv, S. Q. 2008. Effects of feed particle size and feed form on survival of Salmonella Typhimurium in the alimentary tract and cecal S Typhimurium reduction in growing broilers. Poultry Science 85:831-836. https://doi.org/10.1093/ps/85.5.831
» https://doi.org/10.1093/ps/85.5.831 - Hur, J.; Jawale, C. and Lee, J. H. 2012. Antimicrobial resistance of Salmonella isolated from food animals: A review. Food Research International 45:819-830. https://doi.org/10.1016/j.foodres.2011.05.014
» https://doi.org/10.1016/j.foodres.2011.05.014 - Huyghebaert, G.; Ducatelle, R. and Van Immerseel, F. 2011. An update on alternatives to antimicrobial growth promoters for broilers. The Veterinary Journal 187:182-188. https://doi.org/10.1016/j.tvjl.2010.03.003
» https://doi.org/10.1016/j.tvjl.2010.03.003 - Junqueira, L. C. and Carneiro, J. 1999. Histologia básica. Guanabara, Rio de Janeiro. 427p.
- Khan, R. U.; Naz, S.; Javdani, M.; Nikousefat, Z.; Selvaggi, M.; Tufarelli, V. and Laudadio, V. 2012. The use of Turmeric (Curcuma longa) in poultry feed. World's Poultry Science Journal 68:97-103. https://doi.org/10.1017/S0043933912000104
» https://doi.org/10.1017/S0043933912000104 - Lan, Y.; Verstegen, M. W. A.; Tamminga, S. and Williams, B. A. 2005. The role of the commensal gut microbial community in broiler chickens. World's Poultry Science Journal 61:95-104. https://doi.org/10.1079/WPS200445
» https://doi.org/10.1079/WPS200445 - Lao, C. D.; Ruffin M. T.; Normolle, D.; Heath, D. D.; Murray, S. I.; Bailey, J. M.; Boggs, M. E.; Crowell, J.; Rock, C. L. and Brenner, D. E. 2006. Dose escalation of a curcuminoid formulation. BMC Complementary and Alternative Medicine 6:10.https://doi.org/10.1186/1472-6882-6-10
» https://doi.org/10.1186/1472-6882-6-10 - Lorenzi, H. and Matos, F. J. de A. 2002. Plantas medicinais no Brasil/Nativas e exóticas. Instituto Plantarum, Nova Odessa. 512p.
- Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J. J. and Le, M. D. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Applied and Environmental Microbiology 69:6816-6824. https://doi.org/10.1128/AEM.69.11.6816-6824.2003
» https://doi.org/10.1128/AEM.69.11.6816-6824.2003 - Nunes, A. D.; Vaz, A. C. N.; Raspantini, L. E.; Silva, E. M. and Albuquerque, R. 2009. Desempenho e morfologia intestinal de frangos de corte alimentados com rações contendo aditivos alternativos a antimicrobianos. Brazilian Journal of Veterinary and Animal Science 46:500-506.
- Péret-Almeida, L.; Naghetini, C. C.; Nunan, E. A.; Junqueira, R. G. and Glória, M. B. A. 2008. Atividade antimicrobiana in vitro do rizoma em pó, dos pigmentos curcuminóides e dos óleos essenciais da Curcuma longa L. Ciência e Agrotecnologia 32:875-881. https://doi.org/10.1590/S1413-70542008000300026
» https://doi.org/10.1590/S1413-70542008000300026 - Pickler, L.; Hayashi, R. M.; Lourenço, M. C.; Miglino, L. B.; Caroni, L. F.; Beirão, B. C. B.; Silva, A. V. F. and Santin, E. 2012. Avaliação microbiológica, histológica e imunológica de frangos de corte desafiados com Salmonella Enteritidis e Minnesota e tratados com ácidos orgânicos. Pesquisa Veterinária Brasileira 32:27-36. https://doi.org/10.1590/S0100-736X2012000100006
» https://doi.org/10.1590/S0100-736X2012000100006 - Rasband, W. S. 1997-2018. ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA. Available at: <https://imagej.nih.gov/ij/>. Accessed on: Feb. 25, 2018.
» https://imagej.nih.gov/ij/ - Rostagno, H. S.; Albino, L. F. T.; Donzele, J. L.; Gomes, P. C.; Oliveira, R. F.; Lopes, D. C.; Ferreira, A. S.; Barreto, S. L. T. and Euclides, R. F. 2011. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 3.ed. UFV, Viçosa, MG. 252p.
- Samarasinghe, K.; Wenk, C.; Silva, K. F. S. T. and Gunasekera, J. M. D. M. 2003. Turmeric (Curcuma longa) root powder and mannanoligosaccharides as alternatives to antibiotics in broiler chicken diet. Asian-Australasian Journal of Animal Sciences 16:1495-1500. https://doi.org/10.5713/ajas.2003.1495
» https://doi.org/10.5713/ajas.2003.1495 - Santos, G. C. 2010. Alternativas ao uso de promotores químicos de crescimento sobre o desempenho e características de carcaça de frangos de corte. Dissertação (M.Sc.). Universidade Federal do Vale do Jequitinhonha e Mucuri, Diamantina.
- Sueth-Santiago, V.; Mendes-Silva, G. P.; Decoté-Ricardo, D. and Lima, M. E. F. 2015. Curcumina, o pó dourado do açafrão-da-terra: introspecções sobre química e atividades biológicas. Química Nova 38:538-552. https://doi.org/10.5935/0100-4042.20150035
» https://doi.org/10.5935/0100-4042.20150035 - Viola, E. S.; Vieira, S. L.; Torres, C. A.; Freitas, D. M. and Berres, J. 2008. Desempenho de frangos de corte sob suplementação com ácidos lático, fórmico, acético e fosfórico no alimento ou na água. Revista Brasileira de Zootecnia 37:296-302. https://doi.org/10.1590/S1516-35982008000200016
» https://doi.org/10.1590/S1516-35982008000200016 - Wahlstron, B. and Blennow, G. A. 1978. A study on the fate of curcumim in the rat. Acta Pharmacolica et Toxicologica 43:86-92. https://doi.org/10.1111/j.1600-0773.1978.tb02240.x
» https://doi.org/10.1111/j.1600-0773.1978.tb02240.x
Publication Dates
-
Publication in this collection
04 July 2019 -
Date of issue
2019
History
-
Received
13 Sept 2018 -
Accepted
24 Jan 2019