ABSTRACT
This research was conducted to determine optimal dietary histidine requirement of grow-out Nile tilapia, Oreochromis niloticus, based on muscle development, expression of muscle-growth-related genes, and blood parameters. Fish (n = 288, initial body weight of 64.17±0.53 g) were fed extruded diets with graded levels of histidine (4.23, 5.44, 7.17, 8.91, and 11.57 g kg−1), containing approximately 289 g kg−1 crude protein and 3565 kcal kg−1 digestible energy. The study followed a completely randomized design with five treatments and four replicates each, for 65 days. There was a quadratic effect of dietary histidine on final body weight, feed conversion, and net protein utilization, and the best values were optimized at 8.09, 7.88, and 7.33 g kg−1, respectively. Feed intake, hepatosomatic index, survival, body composition, and blood parameters of total protein, glucose, triglycerides, cholesterol, hematocrit, and hemoglobin were not affected by dietary treatments. Predominance of hypertrophic growth and higher mRNA levels of myogenin and MyoD were observed in fish fed histidine from 5.44 to 11.57 g kg−1 compared with fish fed histidine at 4.23 g kg−1. The mRNA expression of myostatin was not affected by dietary treatments. The dietary requirement of histidine for grow-out Nile tilapia was determined at 8.09 g kg−1, considering growth performance, muscle development, and gene expression responses.
amino acids; aquaculture; fish nutrition; genomics
Introduction
Many advances in amino acid nutrition have been reached by understanding their availability in feed ingredients and determining their dietary requirements for Nile tilapia, Oreochromis niloticus. These approaches have been valuable in developing well-balanced diets that improve not only growth and feed efficiency, but also fish health and fillet yield.
Fish recruits new muscle fibers throughout their lifetime (Rowlerson and Veggetti, 2001Rowlerson, A. and Veggetti, A. 2001. Cellular mechanisms of post-embryonic muscle growth in aquaculture species. p.103-140. In: Muscle development and growth. Hoar, W. S.; Farrell, A. P. and Johnston, I. A., eds. Academic Press, San Diego. (Fish Physiology v. 18).), and hypertrophic and hyperplasic muscle growth may be influenced by nutrition (Dal Pai et al., 2000Dal Pai, V.; Dal Pai-Silva, M.; Carvalho, E. D.; Fujihraa, C. Y.; Gregório, E. A. and Curi, P. R. 2000. Morphological, histochemical and morphometric study of the myotomal muscle tissue of the Pacu (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei). Anatomia, Histologia, Embryologia 29:283-289. https://doi.org/10.1046/j.1439-0264.2000.00273.x
https://doi.org/10.1046/j.1439-0264.2000...
). Genomics assays have been used to better understand how nutrients can affect muscle-growth-related genes and metabolic responses in fish (Alami-Durante et al., 2010Alami-Durante, H.; Wrutniak-Cabello, C.; Kaushik, S. J. and Médale, F. 2010. Skeletal muscle cellularity and expression of myogenic regulatory factors and myosin heavy chains in rainbow trout (Oncorhynchus mykiss): Effects of changes in dietary plant protein sources and amino acid profiles. Comparative Biochemistry and Physiology Part A: Molelucar & Integrative and Physiology 156:561-568. https://doi.org/10.1016/j.cbpa.2010.04.015
https://doi.org/10.1016/j.cbpa.2010.04.0...
; De Paula et al., 2017De Paula, T. G.; Zanella, B. T. T.; De Almeida Fantinatti, B. E.; De Moraes, L. N.; Da Silva Duran, B. O.; De Oliveira, C. B.; Salomäo, R. A. S.; Da Silva, R. N.; Padovani, C. R.; Dos Santos, V. B.; Mareco, E. A.; Carvalho, R. F. and Dal-Pai-Silva, M. 2017. Food restriction increase the expression of mTORC1 complex genes in the skeletal muscle of juvenile pacu (Piaractus mesopotamicus). PLoS One 12:e0177679. https://doi.org/10.1371/journal.pone.0177679
https://doi.org/10.1371/journal.pone.017...
). Muscle growth processes are regulated by the activation and proliferation of satellite cells and myogenic regulatory factors (MRF), such as MyoD and myogenin, and MyoD regulates myogenic activity in cell differentiation (Watabe, 1999Watabe, S. 1999. Myogenic regulatory factors and muscle differentiation during ontogeny in fish. Journal of Fish Biology 55:1-18. https://doi.org/10.1111/j.1095-8649.1999.tb01042.x
https://doi.org/10.1111/j.1095-8649.1999...
). While MRF are positively associated with fish growth, myostatin negatively regulates myogenesis, inhibiting myoblast cell proliferation and differentiation, and are used as an indicator of negative energy and nutrient balance in fish (Rescan et al., 2001Rescan, P. Y.; Jutel, I. and Rallière, C. 2001. Two myostatin genes are differentially expressed in myotomal muscles of the trout (Oncorhynchus mykiss). Journal of Experimental Biology 204:3523-3529.).
Dietary protein utilization efficiency depends on the content and balance of amino acids in the diet, and a well-balanced diet is important for optimum growth performance. Well-balanced diets also reduce nitrogen excretion and environmental impacts of fish production. Knowledge on the optimum amino acid contents is required for formulating cost-effective diets that improve growth performance and health, and that are environmentally friendly.
Histidine, which is found in high concentration in hemoglobin (Khan and Abidi, 2014Khan, M. A. and Abidi, S. F. 2014. Dietary histidine requirement of Singhi, Heteropneustes fossilis fry (Bloch). Aquaculture Research 45:1341-1354. https://doi.org/10.1111/are.12081
https://doi.org/10.1111/are.12081...
), acts as an antioxidant in the lens, and thereby prevents cataracts in some salmonid species (Waagboø et al., 2010Waagboø, R.; Tröße, C.; Koppe, W.; Fontanillas, R. and Breck, O. 2010. Dietary histidine supplementation prevents cataract development in adult Atlantic salmon, Salmo salar L., in seawater. British Journal of Nutrition 104:1460-1470. https://doi.org/10.1017/S0007114510002485
https://doi.org/10.1017/S000711451000248...
). It plays an important role in protein synthesis (Li et al., 2009Li, P.; Mai, K.; Trushenski, J. and Wu, G. 2009. New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43-53. https://doi.org/10.1007/s00726-008-0171-1
https://doi.org/10.1007/s00726-008-0171-...
), stimulating skeletal muscle growth and muscle-growth-related genes in Nile tilapia juveniles (Michelato et al., 2017Michelato, M.; Zaminhan, M.; Boscolo, W. R.; Nogaroto, V.; Vicari, M.; Artoni, R. F.; Furuya, V. R. B. and Furuya, W. M. 2017. Dietary histidine requirement of Nile tilapia juveniles based on growth performance, expression of muscle-growth-related genes and haematological responses. Aquaculture 467:63-70. https://doi.org/10.1016/j.aquaculture.2016.06.038
https://doi.org/10.1016/j.aquaculture.20...
).
The dietary histidine requirements has been determined for Nile tilapia fingerlings (Santiago and Lovell, 1988Santiago, C. B. and Lovell, R. T. 1988. Amino acid requirements for growth of Nile tilapia. The Journal of Nutrition 118:1540-1546. https://doi.org/10.1093/jn/118.12.1540
https://doi.org/10.1093/jn/118.12.1540...
; Diógenes et al., 2016Diógenes, A. F.; Fernandes, J. B. K.; Dorigam, J. C. P.; Sakomura, N. K.; Rodrigues, F. H. F.; Lima, B. T. M. and Gonçalves, F. H. 2016. Establishing the optimal essential amino acid ratios in juveniles of Nile tilapia (Oreochromis niloticus) by the deletion method. Aquaculture Nutrition 22:435-443. https://doi.org/10.1111/anu.12262
https://doi.org/10.1111/anu.12262...
; Michelato et al., 2017Michelato, M.; Zaminhan, M.; Boscolo, W. R.; Nogaroto, V.; Vicari, M.; Artoni, R. F.; Furuya, V. R. B. and Furuya, W. M. 2017. Dietary histidine requirement of Nile tilapia juveniles based on growth performance, expression of muscle-growth-related genes and haematological responses. Aquaculture 467:63-70. https://doi.org/10.1016/j.aquaculture.2016.06.038
https://doi.org/10.1016/j.aquaculture.20...
). However, the dietary histidine requirement for grow-out Nile tilapia remains poorly documented. Thus, reliable data about histidine requirements are required to elaborate well-balanced and low-cost diet for this fish species. Therefore, the objective of this research was to determine the dietary histidine requirement of grow-out Nile tilapia, based on growth performance, muscle development, expression of muscle growth-related genes, and blood parameters as criteria responses.
Material and Methods
This research was conducted according to the local ethical and animal welfare guidelines in Toledo, Paraná, Brazil (24°43'12" S, 53°44'6" W), approved under case no. 05/14.
Five diets containing graded levels of L-histidine were formulated. Increased L-histidine supplementation levels was compensated by decreasing equal amounts of L-alanine (Table 1). Diets were elaborated to contain approximately 289 g kg−1 crude protein and 3565 kcal kg−1 digestible energy, while meeting the dietary amino acid requirements of Nile tilapia (Furuya, 2010Furuya, W. M. 2010. Tabelas brasileiras para a nutrição de tilápias. GFM, Toledo.; NRC, 2011NRC - National Research Council. 2011. Nutrient requirements of fish and shrimp. National Academy Press, Washington, DC.), except for histidine. The histidine levels based on laboratorial analysis were 4.23, 5.44, 7.17, 8.91, and 11.57 g kg−1. The digestible energy contents of the experimental diets were estimated from data previously established for Nile tilapia (Gonçalves et al., 2009Gonçalves, G. S.; Pezzato, L. E.; Barros, M. M.; Rocha, D. F.; Kleeman, G. K. and Santa Rosa, M. J. 2009. Energia e nutrientes digestíveis de alimentos para a tilápia do Nilo. Boletim do Instituto de Pesca 35:201-213.; Guimarães et al., 2012Guimarães, I. G.; Pezzato, L. E.; Barros, M. M. and Fernandes, R. N. 2012. Apparent nutrient digestibility and mineral availability of protein-rich ingredients in extruded diets for Nile tilapia. Revista Brasileira de Zootecnia 41:1801-1808. https://doi.org/10.1590/S1516-35982012000800001
https://doi.org/10.1590/S1516-3598201200...
) (Table 2). The proximate composition and amino acids content of the experimental diets were confirmed by laboratorial analysis (Table 2).
Diets were ground into 800-µm mesh, prior to mixing all ingredients in an “V” mixer (MA200; Marconi, Piracicaba, SP, Brazil), extruded in a single screen experimental feed mill (Extec, Ribeirão Preto, SP, Brazil) to produce 3-mm diameter pellets, and dried for 24 h in ventilated oven at 55 oC.
Two hundred and eighty-eight masculinized Nile tilapia (initial body weight 64.17 g) were randomly distributed into 24 250-L tanks at 12 fish per tank. Each tank was equipped with a continuous water flow-through system (1.5 L min−1). Five treatment diets were randomly assigned to 24 tanks at four replicates per diet. A constant and natural photoperiod of 12 h light:12 h dark was kept during the experimental trial. Fish were hand-fed four days per week at 8.00, 11.00, 14.00, and 17.00 h until apparent satiety, for 65 days. Water quality parameters were monitored daily during the feeding trial and the parameter values (mean±standard error of the mean) were as follow: water temperature, 28.00±0.20 °C ; pH, 7.00±0.10; and dissolved oxygen, 6.00±0.20 mg L−1.
At the beginning of the experiment, sixteen fish were fasted for 24 h and randomly sampled for whole-body composition. Fish were euthanized with an overdose of 100 mg L−1 of benzocaine of prior to sampling. At the end of the feeding trial, fish were fasted for 24 h, counted, and weighed. Six fish from each tank were collected for whole-body nutrient composition and retention assays.
Body weight gain, feed intake, feed conversion ratio, net protein utilization, hepatosomatic index, and survival rate were assessed. For body weight gain calculation, fish were weighed at the beginning and end of the experimental trial. Feed intake was recorded daily, on a dry matter basis. Feed conversion ratio was determined by dividing feed intake by body weight gain. Net protein utilization was assessed by dividing the mean body protein retained by protein intake. Hepatosomatic index was calculated by dividing the mean liver weight by body weight. Survival rate was assessed considering the number of fish that died during the experimental trial.
Three fish per aquarium were collected to determine hepatosomatic index parameter. Proximate composition of diets and whole-body fish were determined according to standard method (AOAC, 1990AOAC - Association of Official Analytical Chemists. 1990. Official methods of analysis. AOAC, Washington, DC.). Moisture was analyzed by oven-drying at 105 °C until constant weight; crude lipid analyses were performed by the ether-extraction method using a Soxhlet extractor (Tecnal, TE-044, Piracicaba, SP, Brazil). Crude protein (Nx6.25) followed the Kjeldahl method (Tecnal, MA-036, Piracicaba, SP, Brazil) after acid hydrolysis. Ash analysis was obtained by combustion in a muffle furnace at 550 °C overnight (Tecnal, 2000B, Belo Horizonte, MG, Brazil). Amino acid profiles of the diets were determined by High-Performance Liquid Chromatograph (HPLC) (Hitachi, Tokyo, Japan) after acid hydrolysis (Rayner, 1985Rayner, C. J. 1985. Protein hydrolysis of animal feeds for amino acid content. Journal of Agriculture and Food Chemistry 33:722-725. https://doi.org/10.1021/jf00064a039
https://doi.org/10.1021/jf00064a039...
). Tryptophan was analyzed after alkaline hydrolysis of the sample with lithium hydroxide.
At the end of the feeding trial, three fish from each aquarium were randomly collected to process the morphometric analysis of white skeletal muscle (n = 12 fish per treatment). Samples of muscle tissue were collected from the epaxial region, frozen in liquid nitrogen, and stored in freezer at −80 °C. Histological sections of white skeletal muscle (5-7 µm) were performed using −20 °C cryostat (CM1950, Leica Microsystems, Berlin, Germany) and stained with hematoxylin and eosin (HE) following standard methodology (Bancroft and Steven, 1990Bancroft, J. D. and Steven, A. 1990. Theory and practice of histological techniques. Churchill Livingstone, New York.).
The smallest diameter (n = 200 muscle fibers) from each fish was measured (Dubowitz and Brooke, 1973Dubowitz, V. and Brooke, M. H. 1973. Muscle biopsy: A modern approach. W. B. Saunders Company Ltd, London.) and distributed into three diameter classes (<20, 20-50, or >50 µm) according to previously established methodology (Almeida et al., 2008Almeida, F. L. A.; Carvalho, R. F.; Pinhal, D.; Padovani, C. R.; Martins, C. and Dal Pai-Silva, M. 2008. Differential expression of myogenic regulatory factor MyoD in pacu skeletal muscle (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei) during juvenile and adult growth phases. Micron 39:1306-1311. https://doi.org/10.1016/j.micron.2008.02.011
https://doi.org/10.1016/j.micron.2008.02...
). All images were captured by high-resolution camera (Q Color 3, Olympus, Melville, NY, USA) coupled to a light microscope (Olympus BX40, Melville, NY, USA). Morphometric measurements were realized using Image Pro Plus® 4.5 image analysis software (Media Cybernetics, Silver Spring, MD, USA). Mean values of muscle fiber diameter were determined, and the frequency of occurrence was expressed as number of fiber per diameter class relative to the total number of measured fibers. After anesthesia with benzocaine (100 mg L−1), blood samples were collected from the caudal vein of fish (n = 3 fish per aquarium), using a sterile syringe (1.0 mL) rinsed with an anticoagulant solution (3% ethylene dimethyl tetra amine – EDTA). All fish were fasted for 24 h prior to sampling.
Hemoglobin was determined by the cyanmethemoglobin colorimetric method (Collier, 1944Collier, H. B. 1944. The standardization of blood haemoglobin determinations. Canadiam Medical Association Journal 50:550-552.), by using a commercial kit (Gold Analisa Diagnóstica®, Belo Horizonte, MG, Brazil). The hematocrit percentage was determined by the microhematocrit method (Goldenfarb et al., 1971Goldenfarb, P. B.; Bowyer, F. P.; Hall, E. and Brosius, E. 1971. Reproducibility in the hematology laboratory: the microhematocrit determinations. American Journal of Clinical Pathology 56:35-39. https://doi.org/10.1093/ajcp/56.1.35
https://doi.org/10.1093/ajcp/56.1.35...
). The total plasma proteins were measured by using the standard method (Jain, 1986Jain, N. C. 1986. Schalm’s veterinary hematology. 4th ed. Lea and Febiger, Philadelphia.). Blood biochemical analysis were performed using a partial blood aliquot (1 mL) placed in non-heparinized tubes and centrifuged at 3000 g for 5 min (Quimis, Diadema, SP, Brazil). After centrifugation, the plasma was used to determine glucose, triglycerides, and total cholesterol using a commercial kit (Gold Analisa Diagnóstica®, Belo Horizonte, MG, Brazil).
At the end of feeding, total RNA was extracted from white skeletal muscle tissue from three fish per aquarium) using TRIzol® (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. Samples of mRNA from the same fish used for white skeletal muscle morphometric analysis were collected. The cDNA synthesis was performed from total RNA (2 µg) using the First Strand Synthesis Kit (GE Healthcare Bio-Sciences, Piscataway, NJ, USA), as recommended by the manufacturer’s protocol. Primer pairs for MyoD, myogenin, myostatin, and 18S ribosomal RNA were designed with reference to cDNA nucleotide sequence from Oreochromis niloticus, available in GenBank (http://www.ncbi.nlm.nih.gov/pubmed/nucleotide) (Table 3).
The quantitative real time-polymerase chain reaction (qRT-PCR) was carried out in a thermocycler (Stratagene MxPro3005P, La Jolla, CA, USA), using SYBR Green PCR Master Mix Kit (Stratagene, La Jolla, CA, USA). A total of 20 ng of cDNA template and 10 µM of each primer in a 20 µL of total volume qRT-PCR program consisted of denaturation step at 95°C for 10 min, followed by 40 cycles of 15 s denaturation at 95°C, 30 s annealing at 60°C, 30 s extension at 72°C, ended with a dissociation curve. Analysis of qRT-PCR was performed in duplicate for each sample and the threshold cycle (Ct) values obtained by amplification were measured and a relative change in the expression level of one specific gene was presented as 2-ΔΔCt (Livak and Schmittgen, 2001Livak, K. J. and Schmittgen, T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402-408.). The gene expression was normalized relative to a housekeeping gene 18S ribosomal RNA – 18SrRNA.
Data were tested for normality analysis with the Shapiro-Wilk test (P>0.05) and transformed when necessary. The study was conducted as a completely randomized design according to the following general model:
in which Yij represents the observation, β0 represents the equation constant, β1Xi represents the linear regression coefficient, β2X2i represents the quadratic regression coefficient, and εij represents the residual error.
Morphometric data of muscle fibers and gene expression showed non-normal distribution and were analyzed by a nonparametric Kruskal-Wallis test (P<0.05) complemented with Dunn’s multiple comparison test (P<0.05). All data were analyzed using SAS software (Statistical Analysis System, version 9.3).
Results
The feeding trial produced no unexpected results. The lower mortality was unrelated to experimental treatments. No external pathological signs were observed in fish from any groups. The increase in inclusion levels of histidine resulted in quadratic effect on body weight gain (y = 0.555 + 43.451X – 2.684X2, R2 = 0.798) (Table 4), and the maximum body weight gains were estimated in fish fed histidine at 8.09 g kg−1 (Figure 1). Similarly, a quadratic effect of histidine on feed conversion ratio (y = 1.868 – 0.205X + 0.013X2, R2 = 0.663) and net protein utilization (y = 23.545 + 6.782X – 0.462X2, R2 = 0.513) were observed, and the best results were obtained in fish fed histidine at 7.88 and 7.33 g kg−1, respectively. Feed intake, hepatosomatic index, and survival rate were not significantly (P>0.05) affected by the graded histidine levels tested (Table 4).
Dietary histidine level to optimize body weight gain, estimated by the quadratic equation for grow-out Nile tilapia.
Whole body moisture, crude protein, crude lipids, and ash were not affected by dietary treatments (Table 5). Similarly, blood glucose, triglycerides, cholesterol, total plasma protein, hematocrit, and hemoglobin parameters were not influenced by dietary histidine levels (Table 6).
Muscles were characterized by fibers with different diameters giving a typic mosaic appearance. Fish fed histidine at 4.23 g kg−1 had more (P<0.05) muscle fibers of <20 µm diameter than those fed histidine at 5.44 to 11.57 g kg−1. However, dietary histidine supplementation had no effect (P>0.05) on the abundance of fibers in the 20-50 µm and >50 µm diameter size classes (Table 7).
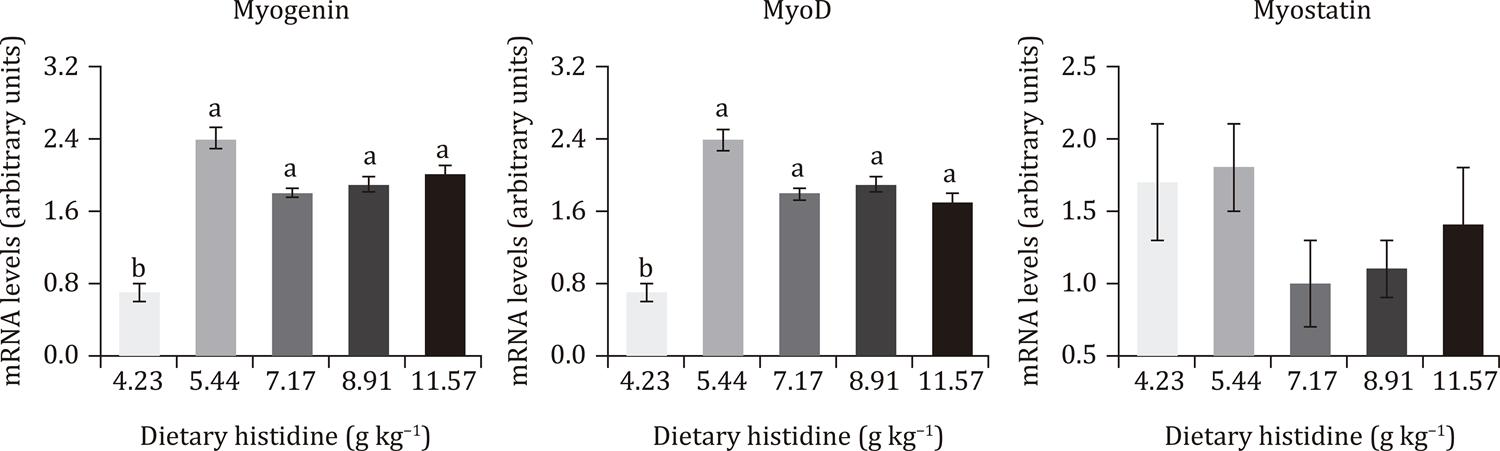
Fish fed histidine at 5.44 to 11.57 g kg−1 showed higher mRNA expression of MyoD and myogenin than those fed at 4.23 g kg−1. However, mRNA expression of MyoD and myogenin did no differ between fish fed histidine from 5.44 to 11.57 g kg−1. The mRNA expression of myostatin was not influenced by treatments (Figure 2).
Real-time PCR quantification of myogenin, MyoD, and myostatin performed on the white skeletal muscle tissue of grow-out Nile tilapia fed graded levels of histidine.
Data are means of four aquaria of 12 Nile tilapia at the age of 100-165 days. Different lowercase letters indicate differences between treatments by Dunn’s multiple comparison test (P<0.05).
Discussion
In this study, the dietary histidine requirement was established at 8.09 g kg−1 based on body weight gain and was found to be higher than previously established for Nile tilapia fingerlings fed pelleted purified diets (Santiago and Lovell, 1988Santiago, C. B. and Lovell, R. T. 1988. Amino acid requirements for growth of Nile tilapia. The Journal of Nutrition 118:1540-1546. https://doi.org/10.1093/jn/118.12.1540
https://doi.org/10.1093/jn/118.12.1540...
), and Nile tilapia fingerlings fed extruded practical diets (Michelato et al., 2017Michelato, M.; Zaminhan, M.; Boscolo, W. R.; Nogaroto, V.; Vicari, M.; Artoni, R. F.; Furuya, V. R. B. and Furuya, W. M. 2017. Dietary histidine requirement of Nile tilapia juveniles based on growth performance, expression of muscle-growth-related genes and haematological responses. Aquaculture 467:63-70. https://doi.org/10.1016/j.aquaculture.2016.06.038
https://doi.org/10.1016/j.aquaculture.20...
). Differences on growth response to histidine supplementation may be related to diet type and composition, particularly related to amino acids content, balance, and availability. Amino acids content and availability are markedly variable between feed ingredients (Guimarães et al., 2008Guimarães, I. G.; Pezzato, L. E. and Barros, M. M. 2008. Amino acid availability and protein digestibility of several protein sources for Nile tilapia, Oreochromis niloticus. Aquaculture Nutrition 14:396-404. https://doi.org/10.1111/j.1365-2095.2007.00540.x
https://doi.org/10.1111/j.1365-2095.2007...
), and amino acids balance is widely known to affect global protein utilization (NRC, 2011NRC - National Research Council. 2011. Nutrient requirements of fish and shrimp. National Academy Press, Washington, DC.). Thus, determining the dietary histidine requirement by analyzing the availability of amino acids should be considered to improve accuracy to determine the dietary requirements of amino acids.
Many recent studies have evaluated the dietary requirements of amino acids such as leucine (Gan et al., 2016Gan, L.; Zhou, L. L.; Li, X. X. and Yue, Y. R. 2016. Dietary leucine requirement of juvenile Nile tilapia Oreochromis niloticus. Aquaculture Nutrition 22:1040-1046. https://doi.org/10.1111/anu.12353
https://doi.org/10.1111/anu.12353...
), lysine (Michelato et al., 2016Michelato, M.; Vidal, L. V. O.; Xavier, T. O.; Moura, L. B.; Almeida, F. L. A.; Pedrosa, V. B.; Furuya, V. R. B. and Furuya, W. M. 2016. Dietary lysine requirement to enhance muscle development and fillet yield of finishing Nile tilapia. Aquaculture 457:124-130. https://doi.org/10.1016/j.aquaculture.2016.02.022
https://doi.org/10.1016/j.aquaculture.20...
), tryptophan (Zaminhan et al., 2017Zaminhan, M.; Boscolo, W. R.; Neu, D. H.; Feiden, A.; Furuya, V. R. B. and Furuya, W. M. 2017. Dietary tryptophan requirements of juvenile Nile tilapia fed corn-soybean meal-based diets. Animal Feed Science and Technolology 227:62-67. https://doi.org/10.1016/j.anifeedsci.2017.03.010
https://doi.org/10.1016/j.anifeedsci.201...
), and valine (Xiao et al., 2018Xiao, W.; Li, D. Y.; Zhu, J. L.; Zou, Z. Y.; Yue, Y. R. and Yang, H. 2018. Dietary valine requirement of juvenile Nile tilapia, Oreochromis niloticus. Aquaculture Nutrition 24:315-323. https://doi.org/10.1111/anu.12562
https://doi.org/10.1111/anu.12562...
); the revised dietary requirement figures provided by these studies are higher than those previously established for Nile tilapia. This trend is expected because genetic improvements have been successfully applied to improve the growth rate of Nile tilapia (Bentsen et al., 2017Bentsen, H. B.; Gjerde, B.; Eknath, A. E.; De Vera, M. S. P.; Velasco, R. R.; Danting, J. C.; Dionisio, E. E.; Longalong, F. M.; Reyes, R. A.; Abella, T. A.; Tayamen, M. M. and Ponzoni, R. W. 2017. Genetic improvement of farmed tilapias: Response to five generations of selection for increased body weight at harvest in Oreochromis niloticus and the further impact of the project. Aquaculture 468:206-217. https://doi.org/10.1016/j.aquaculture.2016.10.018
https://doi.org/10.1016/j.aquaculture.20...
). In addition, appropriate fish management, combined with advances in feed evaluation, formulation, and processing, has markedly contributed to global Nile tilapia production.
Dietary histidine at 11.63 g kg−1 was found to optimize body weight gain in this research. This value is higher than previously established by Santiago and Lovell (1988)Santiago, C. B. and Lovell, R. T. 1988. Amino acid requirements for growth of Nile tilapia. The Journal of Nutrition 118:1540-1546. https://doi.org/10.1093/jn/118.12.1540
https://doi.org/10.1093/jn/118.12.1540...
and should be updated for Nile tilapia nutrition. To date, the optimum histidine requirement found in this study is close to the level used in commercial feeds currently used for Nile tilapia in Brazil (Montanhini Neto and Ostrensky, 2015Montanhini Neto, R. and Ostrensky, A. 2015. Evaluation of commercial feeds intended for the Brazilian production of Nile tilapia (Oreochromis niloticus L.): Nutritional and environmental implications. Aquaculture Nutrition 21:311-320. https://doi.org/10.1111/anu.12154
https://doi.org/10.1111/anu.12154...
).
Histidine has been described as a limiting amino acid for salmonids; furthermore, histidine is known to prevent cataracts, acting as a potent antioxidant in the lens (Remø et al., 2017Remø, S. C.; Hevrøy, E. M.; Breck, O.; Olsvik, P. A. and WaagbØ, R. 2017. Lens metabolomic profiling as a tool to understand cataractogenesis in Atlantic salmon and rainbow trout reared at optimum and high temperature. PLoS One 12:e0175491. https://doi.org/10.1371/journal.pone.0175491
https://doi.org/10.1371/journal.pone.017...
). However, by the end of this study, using visual assessment, cataract was not detected. Cataract occurrence is fish is more frequent in cold-warm species than in warm-water species (Khan and Abidi, 2014Khan, M. A. and Abidi, S. F. 2014. Dietary histidine requirement of Singhi, Heteropneustes fossilis fry (Bloch). Aquaculture Research 45:1341-1354. https://doi.org/10.1111/are.12081
https://doi.org/10.1111/are.12081...
). Consistent with the findings of this study, cataract was not observed in African catfish (Clarias gariepinus; Khan and Abidi, 2009Khan, M. A. and Abidi, S. F. 2009. Optimum histidine requirement of fry African catfish, Clarias gariepinus (Burchell). Aquaculture Research 40:1000-1010. https://doi.org/10.1111/j.1365-2109.2009.02164.x
https://doi.org/10.1111/j.1365-2109.2009...
) and Catla (Catla catla; Zehra and Khan, 2016Zehra, S. and Khan, M. A. 2016. Dietary histidine requirement of fingerling Catla Catla (Hamilton) based on growth, protein gain, histidine gain, RNA/DNA ratio, haematological indices and carcass composition. Aquaculture Research 47:1028-1039. https://doi.org/10.1111/are.12558
https://doi.org/10.1111/are.12558...
) fed histidine-deficient diets.
As observed in this study, excess histidine induces amino acid catabolism reducing dietary protein utilization in fish (Aragão et al., 2004Aragão, C.; Conceição, L. E. C.; Martins, D.; Rønnestad, I.; Gomes, E. and Dinis, M. T. 2004. A balanced dietary amino acid profile improves amino acid retention in post-larval Senegalese sole (Solea senegalensis). Aquaculture 233:293-304. https://doi.org/10.1016/j.aquaculture.2003.08.007
https://doi.org/10.1016/j.aquaculture.20...
). Reduced dietary protein in response to excess supplemental histidine has been described in Indian major carp (Cirrhinus cirrhosis; Ahmed and Khan, 2005Ahmed, I. and Khan, M. A. 2005. Dietary histidine requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquaculture Nutrition 11:359-366. https://doi.org/10.1111/j.1365-2095.2005.00358.x
https://doi.org/10.1111/j.1365-2095.2005...
) and in Singhi (Heteropneustes fossilis; Khan and Abidi, 2014Khan, M. A. and Abidi, S. F. 2014. Dietary histidine requirement of Singhi, Heteropneustes fossilis fry (Bloch). Aquaculture Research 45:1341-1354. https://doi.org/10.1111/are.12081
https://doi.org/10.1111/are.12081...
).
In this work, hepatosomatic index of fish was not affected by dietary histidine levels. In contrast, Farhat and Khan (2013)Farhat and Khan, M. A. 2013. Effects of varying levels of dietary L-histidine on growth, feed conversion, protein gain, histidine retention, hematological and body composition in fingerling stinging catfish Heteropneustes fossilis (Bloch). Aquaculture 404-405:130-138. https://doi.org/10.1016/j.aquaculture.2013.04.020
https://doi.org/10.1016/j.aquaculture.20...
and Zehra and Khan (2016)Zehra, S. and Khan, M. A. 2016. Dietary histidine requirement of fingerling Catla Catla (Hamilton) based on growth, protein gain, histidine gain, RNA/DNA ratio, haematological indices and carcass composition. Aquaculture Research 47:1028-1039. https://doi.org/10.1111/are.12558
https://doi.org/10.1111/are.12558...
found that histidine dosage affected hepatosomatic index in stinging catfish (Heteropneustes fossilis) and Catla. Hepatosomatic index, as an indicator of condition and nutritional status of fish, reflects the accumulation of visceral fat caused by imbalanced diets (Michelato et al., 2017Michelato, M.; Zaminhan, M.; Boscolo, W. R.; Nogaroto, V.; Vicari, M.; Artoni, R. F.; Furuya, V. R. B. and Furuya, W. M. 2017. Dietary histidine requirement of Nile tilapia juveniles based on growth performance, expression of muscle-growth-related genes and haematological responses. Aquaculture 467:63-70. https://doi.org/10.1016/j.aquaculture.2016.06.038
https://doi.org/10.1016/j.aquaculture.20...
). Liver weight is affected by many factors and is highly variable. However, this is a not reliable indicator of fish health and condition (Chellappa et al., 1995Chellappa, S.; Huntingforf, F. A.; Strang, R. H. C. and Thomson, R. Y. 1995. Condition factor and hepatosomatic index as estimates of energy status in male three‐spined stickleback. Journal of Fish Biology 47:775-787. https://doi.org/10.1111/j.1095-8649.1995.tb06002.x
https://doi.org/10.1111/j.1095-8649.1995...
).
Reduced feed intake has been documented in fish fed amino acid-deficient diets; this has been demonstrated in grass carp (Ctenopharyngodon Idella; Gao et al., 2016Gao, Y. J.; Liu, Y. J.; Chen, X. Q.; Yang, H. J.; Li, X. F. and Tian, L. X. 2016. Effects of graded levels of histidine on growth performance, digested enzymes activities, erythrocyte osmotic fragility and hypoxia-tolerance of juvenile grass carp Ctenopharyngodon idella. Aquaculture 452:388-394. https://doi.org/10.1016/j.aquaculture.2015.11.019
https://doi.org/10.1016/j.aquaculture.20...
). However, in this study, fish feed intake was not affected by dietary treatments. Similar results were described for Singhi (Khan and Abidi, 2014Khan, M. A. and Abidi, S. F. 2014. Dietary histidine requirement of Singhi, Heteropneustes fossilis fry (Bloch). Aquaculture Research 45:1341-1354. https://doi.org/10.1111/are.12081
https://doi.org/10.1111/are.12081...
) and Japanese flounder (Paralichthys olivaceus) juveniles (Han et al., 2013Han, Y.; Koshio, S.; Ishikawa, M. and Yokoyama, S. 2013. Interactive effects of dietary arginine and histidine on the performances of Japanese flounder Paralichthys olivaceus juveniles. Aquaculture 414-415:173-182. https://doi.org/10.1016/j.aquaculture.2013.07.001
https://doi.org/10.1016/j.aquaculture.20...
).
Whole body protein of fish used in this research was not affected by dietary histidine supplementation, consistent with findings for Japanese flounder (Han et al., 2013Han, Y.; Koshio, S.; Ishikawa, M. and Yokoyama, S. 2013. Interactive effects of dietary arginine and histidine on the performances of Japanese flounder Paralichthys olivaceus juveniles. Aquaculture 414-415:173-182. https://doi.org/10.1016/j.aquaculture.2013.07.001
https://doi.org/10.1016/j.aquaculture.20...
) and grass carp (Gao et al., 2016Gao, Y. J.; Liu, Y. J.; Chen, X. Q.; Yang, H. J.; Li, X. F. and Tian, L. X. 2016. Effects of graded levels of histidine on growth performance, digested enzymes activities, erythrocyte osmotic fragility and hypoxia-tolerance of juvenile grass carp Ctenopharyngodon idella. Aquaculture 452:388-394. https://doi.org/10.1016/j.aquaculture.2015.11.019
https://doi.org/10.1016/j.aquaculture.20...
), but in contrast to findings observed in Catla fed supplemental histidine (Zehra and Khan, 2016Zehra, S. and Khan, M. A. 2016. Dietary histidine requirement of fingerling Catla Catla (Hamilton) based on growth, protein gain, histidine gain, RNA/DNA ratio, haematological indices and carcass composition. Aquaculture Research 47:1028-1039. https://doi.org/10.1111/are.12558
https://doi.org/10.1111/are.12558...
), because histidine positively stimulates protein synthesis and, consequently, leads to increased body weight in fish. This positive effect has been observed in blunt snout bream (Megalobrama amblycephala; Wilson-Arop et al., 2018Wilson-Arop, O. M.; Liang, H.; Ge, X.; Ren, M.; Habte-Tsion, H. M. and Ji, K. 2018. Dietary histidine requirement of juvenile blunt snout bream (Megalobrama amblycephala). Aquaculture Nutrition 24:1122-1132. https://doi.org/10.1111/anu.12651
https://doi.org/10.1111/anu.12651...
).
The presence of the narrowest muscle fibers (<20 µm diameter) indicated that hyperplasic growth was still occurring in skeletal muscle of fish used in this research. However, predominance of the widest fibers (>50 µm of diameter) confirmed the occurrence of active hypertrophic growth in many fish species (Rowlerson and Veggetti, 2001Rowlerson, A. and Veggetti, A. 2001. Cellular mechanisms of post-embryonic muscle growth in aquaculture species. p.103-140. In: Muscle development and growth. Hoar, W. S.; Farrell, A. P. and Johnston, I. A., eds. Academic Press, San Diego. (Fish Physiology v. 18).), including Nile tilapia juveniles (Michelato et al., 2007). Similarly, pacu (Piaractus mesopotamicus) juveniles have been shown to have more of the narrowest (<20 µm of diameter) than the widest (>50 µm diameter) muscle fibers; the opposite pattern was observed in adult pacu (Almeida et al., 2008)Almeida, F. L. A.; Carvalho, R. F.; Pinhal, D.; Padovani, C. R.; Martins, C. and Dal Pai-Silva, M. 2008. Differential expression of myogenic regulatory factor MyoD in pacu skeletal muscle (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei) during juvenile and adult growth phases. Micron 39:1306-1311. https://doi.org/10.1016/j.micron.2008.02.011
https://doi.org/10.1016/j.micron.2008.02...
.
High mRNA myostatin levels are associated with the inhibition of myoblasts and satellite cell proliferation in a quiescent and undifferentiated state, and are negatively associated with muscle growth (Watabe, 1999Watabe, S. 1999. Myogenic regulatory factors and muscle differentiation during ontogeny in fish. Journal of Fish Biology 55:1-18. https://doi.org/10.1111/j.1095-8649.1999.tb01042.x
https://doi.org/10.1111/j.1095-8649.1999...
). In the present work, dietary histidine did not affect (P>0.05) mRNA levels of myostatin.
Blood biochemical and hematological parameters of fish were not affected by histidine levels in this study. All biochemical and hematological parameters evaluated fell within the reference intervals for cultured tilapia (Hrubec et al., 2000Hrubec, T. C.; Cardinale, J. L. and Smith, S. A. 2000. Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis Hybrid). Veterinary Clinical Pathology 29:7-12. https://doi.org/10.1111/j.1939-165X.2000.tb00389.x
https://doi.org/10.1111/j.1939-165X.2000...
). In accordance, no effects on triglycerides, glucose, hematocrit, and hemoglobin were described in Japanese flounder juveniles fed diets containing graded levels of histidine (Han et al., 2013Han, Y.; Koshio, S.; Ishikawa, M. and Yokoyama, S. 2013. Interactive effects of dietary arginine and histidine on the performances of Japanese flounder Paralichthys olivaceus juveniles. Aquaculture 414-415:173-182. https://doi.org/10.1016/j.aquaculture.2013.07.001
https://doi.org/10.1016/j.aquaculture.20...
). Histidine is highly concentrated in hemoglobin protein (Khan and Abidi, 2014Khan, M. A. and Abidi, S. F. 2014. Dietary histidine requirement of Singhi, Heteropneustes fossilis fry (Bloch). Aquaculture Research 45:1341-1354. https://doi.org/10.1111/are.12081
https://doi.org/10.1111/are.12081...
). No anemia was observed in fish analyzed in this study, in contrast with the lower hematocrit and hemoglobin described in Catla fed a histidine-deficient diet (Zehra and Khan, 2016Zehra, S. and Khan, M. A. 2016. Dietary histidine requirement of fingerling Catla Catla (Hamilton) based on growth, protein gain, histidine gain, RNA/DNA ratio, haematological indices and carcass composition. Aquaculture Research 47:1028-1039. https://doi.org/10.1111/are.12558
https://doi.org/10.1111/are.12558...
).
In summary, the dietary requirements of grout-out Nile tilapia were estimated at 8.09, 7.88, and 7.33 g kg−1 to optimize body weight gains, feed conversion ratio, and net protein utilization, respectively. In addition, fish fed histidine from 5.44 to 11.57 g kg−1 demonstrated predominance of hypertrophic muscle-growth, in line with higher mRNA expression of myogenin and MyoD. Histidine may be a limiting amino acid in plant-based diets and the dietary requirements determined in the current study may be considered to formulate diets for grow-out Nile tilapia.
Conclusions
The dietary requirement of histidine for grow-out Nile tilapia was found to be 8.09 g kg−1 based on growth performance, muscle development, and gene expression responses.
Acknowledgments
The experiment was financially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Financing Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors also wish to thank Ajinomoto do Brazil – Animal Nutrition Division – for donation of amino acids and analysis.
References
- Ahmed, I. and Khan, M. A. 2005. Dietary histidine requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquaculture Nutrition 11:359-366. https://doi.org/10.1111/j.1365-2095.2005.00358.x
» https://doi.org/10.1111/j.1365-2095.2005.00358.x - Alami-Durante, H.; Wrutniak-Cabello, C.; Kaushik, S. J. and Médale, F. 2010. Skeletal muscle cellularity and expression of myogenic regulatory factors and myosin heavy chains in rainbow trout (Oncorhynchus mykiss): Effects of changes in dietary plant protein sources and amino acid profiles. Comparative Biochemistry and Physiology Part A: Molelucar & Integrative and Physiology 156:561-568. https://doi.org/10.1016/j.cbpa.2010.04.015
» https://doi.org/10.1016/j.cbpa.2010.04.015 - Almeida, F. L. A.; Carvalho, R. F.; Pinhal, D.; Padovani, C. R.; Martins, C. and Dal Pai-Silva, M. 2008. Differential expression of myogenic regulatory factor MyoD in pacu skeletal muscle (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei) during juvenile and adult growth phases. Micron 39:1306-1311. https://doi.org/10.1016/j.micron.2008.02.011
» https://doi.org/10.1016/j.micron.2008.02.011 - AOAC - Association of Official Analytical Chemists. 1990. Official methods of analysis. AOAC, Washington, DC.
- Aragão, C.; Conceição, L. E. C.; Martins, D.; Rønnestad, I.; Gomes, E. and Dinis, M. T. 2004. A balanced dietary amino acid profile improves amino acid retention in post-larval Senegalese sole (Solea senegalensis). Aquaculture 233:293-304. https://doi.org/10.1016/j.aquaculture.2003.08.007
» https://doi.org/10.1016/j.aquaculture.2003.08.007 - Bancroft, J. D. and Steven, A. 1990. Theory and practice of histological techniques. Churchill Livingstone, New York.
- Bentsen, H. B.; Gjerde, B.; Eknath, A. E.; De Vera, M. S. P.; Velasco, R. R.; Danting, J. C.; Dionisio, E. E.; Longalong, F. M.; Reyes, R. A.; Abella, T. A.; Tayamen, M. M. and Ponzoni, R. W. 2017. Genetic improvement of farmed tilapias: Response to five generations of selection for increased body weight at harvest in Oreochromis niloticus and the further impact of the project. Aquaculture 468:206-217. https://doi.org/10.1016/j.aquaculture.2016.10.018
» https://doi.org/10.1016/j.aquaculture.2016.10.018 - Chellappa, S.; Huntingforf, F. A.; Strang, R. H. C. and Thomson, R. Y. 1995. Condition factor and hepatosomatic index as estimates of energy status in male three‐spined stickleback. Journal of Fish Biology 47:775-787. https://doi.org/10.1111/j.1095-8649.1995.tb06002.x
» https://doi.org/10.1111/j.1095-8649.1995.tb06002.x - Collier, H. B. 1944. The standardization of blood haemoglobin determinations. Canadiam Medical Association Journal 50:550-552.
- Dal Pai, V.; Dal Pai-Silva, M.; Carvalho, E. D.; Fujihraa, C. Y.; Gregório, E. A. and Curi, P. R. 2000. Morphological, histochemical and morphometric study of the myotomal muscle tissue of the Pacu (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei). Anatomia, Histologia, Embryologia 29:283-289. https://doi.org/10.1046/j.1439-0264.2000.00273.x
» https://doi.org/10.1046/j.1439-0264.2000.00273.x - De Paula, T. G.; Zanella, B. T. T.; De Almeida Fantinatti, B. E.; De Moraes, L. N.; Da Silva Duran, B. O.; De Oliveira, C. B.; Salomäo, R. A. S.; Da Silva, R. N.; Padovani, C. R.; Dos Santos, V. B.; Mareco, E. A.; Carvalho, R. F. and Dal-Pai-Silva, M. 2017. Food restriction increase the expression of mTORC1 complex genes in the skeletal muscle of juvenile pacu (Piaractus mesopotamicus). PLoS One 12:e0177679. https://doi.org/10.1371/journal.pone.0177679
» https://doi.org/10.1371/journal.pone.0177679 - Diógenes, A. F.; Fernandes, J. B. K.; Dorigam, J. C. P.; Sakomura, N. K.; Rodrigues, F. H. F.; Lima, B. T. M. and Gonçalves, F. H. 2016. Establishing the optimal essential amino acid ratios in juveniles of Nile tilapia (Oreochromis niloticus) by the deletion method. Aquaculture Nutrition 22:435-443. https://doi.org/10.1111/anu.12262
» https://doi.org/10.1111/anu.12262 - Dubowitz, V. and Brooke, M. H. 1973. Muscle biopsy: A modern approach. W. B. Saunders Company Ltd, London.
- Farhat and Khan, M. A. 2013. Effects of varying levels of dietary L-histidine on growth, feed conversion, protein gain, histidine retention, hematological and body composition in fingerling stinging catfish Heteropneustes fossilis (Bloch). Aquaculture 404-405:130-138. https://doi.org/10.1016/j.aquaculture.2013.04.020
» https://doi.org/10.1016/j.aquaculture.2013.04.020 - Furuya, W. M. 2010. Tabelas brasileiras para a nutrição de tilápias. GFM, Toledo.
- Gan, L.; Zhou, L. L.; Li, X. X. and Yue, Y. R. 2016. Dietary leucine requirement of juvenile Nile tilapia Oreochromis niloticus. Aquaculture Nutrition 22:1040-1046. https://doi.org/10.1111/anu.12353
» https://doi.org/10.1111/anu.12353 - Gao, Y. J.; Liu, Y. J.; Chen, X. Q.; Yang, H. J.; Li, X. F. and Tian, L. X. 2016. Effects of graded levels of histidine on growth performance, digested enzymes activities, erythrocyte osmotic fragility and hypoxia-tolerance of juvenile grass carp Ctenopharyngodon idella. Aquaculture 452:388-394. https://doi.org/10.1016/j.aquaculture.2015.11.019
» https://doi.org/10.1016/j.aquaculture.2015.11.019 - Goldenfarb, P. B.; Bowyer, F. P.; Hall, E. and Brosius, E. 1971. Reproducibility in the hematology laboratory: the microhematocrit determinations. American Journal of Clinical Pathology 56:35-39. https://doi.org/10.1093/ajcp/56.1.35
» https://doi.org/10.1093/ajcp/56.1.35 - Gonçalves, G. S.; Pezzato, L. E.; Barros, M. M.; Rocha, D. F.; Kleeman, G. K. and Santa Rosa, M. J. 2009. Energia e nutrientes digestíveis de alimentos para a tilápia do Nilo. Boletim do Instituto de Pesca 35:201-213.
- Guimarães, I. G.; Pezzato, L. E. and Barros, M. M. 2008. Amino acid availability and protein digestibility of several protein sources for Nile tilapia, Oreochromis niloticus. Aquaculture Nutrition 14:396-404. https://doi.org/10.1111/j.1365-2095.2007.00540.x
» https://doi.org/10.1111/j.1365-2095.2007.00540.x - Guimarães, I. G.; Pezzato, L. E.; Barros, M. M. and Fernandes, R. N. 2012. Apparent nutrient digestibility and mineral availability of protein-rich ingredients in extruded diets for Nile tilapia. Revista Brasileira de Zootecnia 41:1801-1808. https://doi.org/10.1590/S1516-35982012000800001
» https://doi.org/10.1590/S1516-35982012000800001 - Han, Y.; Koshio, S.; Ishikawa, M. and Yokoyama, S. 2013. Interactive effects of dietary arginine and histidine on the performances of Japanese flounder Paralichthys olivaceus juveniles. Aquaculture 414-415:173-182. https://doi.org/10.1016/j.aquaculture.2013.07.001
» https://doi.org/10.1016/j.aquaculture.2013.07.001 - Hrubec, T. C.; Cardinale, J. L. and Smith, S. A. 2000. Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis Hybrid). Veterinary Clinical Pathology 29:7-12. https://doi.org/10.1111/j.1939-165X.2000.tb00389.x
» https://doi.org/10.1111/j.1939-165X.2000.tb00389.x - Jain, N. C. 1986. Schalm’s veterinary hematology. 4th ed. Lea and Febiger, Philadelphia.
- Khan, M. A. and Abidi, S. F. 2009. Optimum histidine requirement of fry African catfish, Clarias gariepinus (Burchell). Aquaculture Research 40:1000-1010. https://doi.org/10.1111/j.1365-2109.2009.02164.x
» https://doi.org/10.1111/j.1365-2109.2009.02164.x - Khan, M. A. and Abidi, S. F. 2014. Dietary histidine requirement of Singhi, Heteropneustes fossilis fry (Bloch). Aquaculture Research 45:1341-1354. https://doi.org/10.1111/are.12081
» https://doi.org/10.1111/are.12081 - Li, P.; Mai, K.; Trushenski, J. and Wu, G. 2009. New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43-53. https://doi.org/10.1007/s00726-008-0171-1
» https://doi.org/10.1007/s00726-008-0171-1 - Livak, K. J. and Schmittgen, T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402-408.
- Michelato, M.; Vidal, L. V. O.; Xavier, T. O.; Moura, L. B.; Almeida, F. L. A.; Pedrosa, V. B.; Furuya, V. R. B. and Furuya, W. M. 2016. Dietary lysine requirement to enhance muscle development and fillet yield of finishing Nile tilapia. Aquaculture 457:124-130. https://doi.org/10.1016/j.aquaculture.2016.02.022
» https://doi.org/10.1016/j.aquaculture.2016.02.022 - Michelato, M.; Zaminhan, M.; Boscolo, W. R.; Nogaroto, V.; Vicari, M.; Artoni, R. F.; Furuya, V. R. B. and Furuya, W. M. 2017. Dietary histidine requirement of Nile tilapia juveniles based on growth performance, expression of muscle-growth-related genes and haematological responses. Aquaculture 467:63-70. https://doi.org/10.1016/j.aquaculture.2016.06.038
» https://doi.org/10.1016/j.aquaculture.2016.06.038 - Montanhini Neto, R. and Ostrensky, A. 2015. Evaluation of commercial feeds intended for the Brazilian production of Nile tilapia (Oreochromis niloticus L.): Nutritional and environmental implications. Aquaculture Nutrition 21:311-320. https://doi.org/10.1111/anu.12154
» https://doi.org/10.1111/anu.12154 - NRC - National Research Council. 2011. Nutrient requirements of fish and shrimp. National Academy Press, Washington, DC.
- Rayner, C. J. 1985. Protein hydrolysis of animal feeds for amino acid content. Journal of Agriculture and Food Chemistry 33:722-725. https://doi.org/10.1021/jf00064a039
» https://doi.org/10.1021/jf00064a039 - Remø, S. C.; Hevrøy, E. M.; Breck, O.; Olsvik, P. A. and WaagbØ, R. 2017. Lens metabolomic profiling as a tool to understand cataractogenesis in Atlantic salmon and rainbow trout reared at optimum and high temperature. PLoS One 12:e0175491. https://doi.org/10.1371/journal.pone.0175491
» https://doi.org/10.1371/journal.pone.0175491 - Rescan, P. Y.; Jutel, I. and Rallière, C. 2001. Two myostatin genes are differentially expressed in myotomal muscles of the trout (Oncorhynchus mykiss). Journal of Experimental Biology 204:3523-3529.
- Rostagno, H. S.; Albino, L. F. T.; Hannas, M. I.; Donzele, J. L.; Sakomura, N. K.; Perazzo, F. G.; Saraiva, A.; Teixeira, M. L.; Rodrigues, P. B.; Oliveira, R. F.; Barreto, S. L. T. and Brito, C. O. 2017. Tabelas brasileiras para aves e suínos: Composição de alimentos e exigências nutricionais. 4.ed. UFV, Viçosa, MG.
- Rowlerson, A. and Veggetti, A. 2001. Cellular mechanisms of post-embryonic muscle growth in aquaculture species. p.103-140. In: Muscle development and growth. Hoar, W. S.; Farrell, A. P. and Johnston, I. A., eds. Academic Press, San Diego. (Fish Physiology v. 18).
- Santiago, C. B. and Lovell, R. T. 1988. Amino acid requirements for growth of Nile tilapia. The Journal of Nutrition 118:1540-1546. https://doi.org/10.1093/jn/118.12.1540
» https://doi.org/10.1093/jn/118.12.1540 - Waagboø, R.; Tröße, C.; Koppe, W.; Fontanillas, R. and Breck, O. 2010. Dietary histidine supplementation prevents cataract development in adult Atlantic salmon, Salmo salar L., in seawater. British Journal of Nutrition 104:1460-1470. https://doi.org/10.1017/S0007114510002485
» https://doi.org/10.1017/S0007114510002485 - Watabe, S. 1999. Myogenic regulatory factors and muscle differentiation during ontogeny in fish. Journal of Fish Biology 55:1-18. https://doi.org/10.1111/j.1095-8649.1999.tb01042.x
» https://doi.org/10.1111/j.1095-8649.1999.tb01042.x - Wilson-Arop, O. M.; Liang, H.; Ge, X.; Ren, M.; Habte-Tsion, H. M. and Ji, K. 2018. Dietary histidine requirement of juvenile blunt snout bream (Megalobrama amblycephala). Aquaculture Nutrition 24:1122-1132. https://doi.org/10.1111/anu.12651
» https://doi.org/10.1111/anu.12651 - Xiao, W.; Li, D. Y.; Zhu, J. L.; Zou, Z. Y.; Yue, Y. R. and Yang, H. 2018. Dietary valine requirement of juvenile Nile tilapia, Oreochromis niloticus. Aquaculture Nutrition 24:315-323. https://doi.org/10.1111/anu.12562
» https://doi.org/10.1111/anu.12562 - Zaminhan, M.; Boscolo, W. R.; Neu, D. H.; Feiden, A.; Furuya, V. R. B. and Furuya, W. M. 2017. Dietary tryptophan requirements of juvenile Nile tilapia fed corn-soybean meal-based diets. Animal Feed Science and Technolology 227:62-67. https://doi.org/10.1016/j.anifeedsci.2017.03.010
» https://doi.org/10.1016/j.anifeedsci.2017.03.010 - Zehra, S. and Khan, M. A. 2016. Dietary histidine requirement of fingerling Catla Catla (Hamilton) based on growth, protein gain, histidine gain, RNA/DNA ratio, haematological indices and carcass composition. Aquaculture Research 47:1028-1039. https://doi.org/10.1111/are.12558
» https://doi.org/10.1111/are.12558
Publication Dates
-
Publication in this collection
03 Apr 2020 -
Date of issue
2020
History
-
Received
29 Aug 2018 -
Accepted
22 Oct 2019