Abstracts
OBJECTIVE: Typical antipsychotic drugs, mainly phenothiazines, have been associated with cataract formation for over forty years. Recently, there has been a concern about atypical antipsychotic drugs' potential for inducing this lenticular pathology. Accordingly, we sought to determine the cataract rate and other ocular side effects in patients on long-term therapy with antipsychotic drugs. METHOD: Eighty outpatients with DSM-IV diagnosis of schizophrenia from two settings who met pre determined inclusion criteria were submitted to an ophthalmological evaluation for ocular abnormalities with emphasis in the lens and cornea. They were divided into two groups: group 1 (n = 52) comprised patients who had been predominantly on typical antipsychotics for at least two years and group 2 (n = 28) patients who had been predominantly on atypical antipsychotics for at least two years. RESULTS: Cataract was found in 26 patients (33%) with predominance of anterior capsular cataract. The cataract rate among patients from group 1 (40%) was higher than among those from group 2 (18%). Visual acuity was reduced in 21 patients (26%). No changes were observed neither in the cornea nor in the retina. CONCLUSIONS: Patients using antipsychotic drugs should be submitted to a periodic ophthalmological evaluation.
Antipsychotic agents; Schizophrenia; Cataract; Risk factors; Polypharmacy
OBJETIVO: Os antipsicóticos típicos, principalmente as fenotiazinas, têm sido associados à formação de catarata há mais de quarenta anos. Nos últimos anos, tem existido um questionamento acerca do potencial dos antipsicóticos atípicos de induzir essa patologia lenticular. Neste estudo, buscamos determinar a taxa de catarata e de outros efeitos oculares adversos em pacientes em uso de antipsicóticos a longo prazo. MÉTODO: Oitenta pacientes tratados ambulatorialmente com diagnóstico de esquizofrenia segundo o DSM-IV, de dois centros, que preencheram os critérios de inclusão pré determinados foram submetidos a uma avaliação oftalmológica para pesquisa de alterações oculares com ênfase no cristalino e na córnea. Eles foram divididos em dois grupos: o grupo 1 (n = 52) era formado por pacientes que tinham usado predominantemente antipsicóticos típicos por pelo menos dois anos e o grupo 2 (n = 28) por pacientes que tinham usado predominantemente antipsicóticos atípicos por pelo menos dois anos. RESULTADOS: Catarata foi encontrada em 26 pacientes (33%) com predomínio de catarata capsular anterior. A taxa de catarata entre os pacientes do grupo 1 (40%) foi maior do que naqueles do grupo 2 (18%). A acuidade visual estava reduzida em 21 pacientes (26%). Não foram encontradas alterações nem na córnea nem na retina. CONCLUSÕES: Pessoas em uso de antipsicóticos devem ser submetidas à avaliação oftalmológica periódica.
Agentes antipsicóticos; Esquizofrenia; Catarata; Fatores de risco; Polimedicação
ORIGINAL ARTICLE
Cataract occurrence in patients treated with antipsychotic drugs
Ocorrência de catarata em pacientes tratados com antipsicóticos
Valéria Barreto Novais e SouzaI; Francisco José Rodrigues de Moura FilhoII; Fábio Gomes de Matos e SouzaI; Camila Farias RochaII; Fernando Antônio Mendes Lopes FurtadoIII; Tiago Bessa Almeida GonçalvesIII; Karla Feitosa Ximenes VasconcelosIII
IMental Health Service, Hospital Universiátio Walter Cantideo (HUWC), Universidade Federal do Ceará (UFC), Fortaleza (CE), Brazil
IISchool of Medicine, Universidade Federal do Ceará (UFC), Fortaleza (CE), Brazil
IIISociedade de Assistência aos Cegos (SAC) Iêda Otoch Baquit, Ophthalmological Unit, Fortaleza (CE), Brazil
Correspondence Correspondence Valéria Barreto Novais e Souza Rua Manoel Jesuíno, 974 - Varjota 60175-270 Fortaleza, CE, Brasil Phone/Fax: (+55 85) 267-3867 E-mail: valbns@yahoo.com.br, drmourafilho@yahoo.com.br
ABSTRACT
OBJECTIVE: Typical antipsychotic drugs, mainly phenothiazines, have been associated with cataract formation for over forty years. Recently, there has been a concern about atypical antipsychotic drugs' potential for inducing this lenticular pathology. Accordingly, we sought to determine the cataract rate and other ocular side effects in patients on long-term therapy with antipsychotic drugs.
METHOD: Eighty outpatients with DSM-IV diagnosis of schizophrenia from two settings who met pre determined inclusion criteria were submitted to an ophthalmological evaluation for ocular abnormalities with emphasis in the lens and cornea. They were divided into two groups: group 1 (n = 52) comprised patients who had been predominantly on typical antipsychotics for at least two years and group 2 (n = 28) patients who had been predominantly on atypical antipsychotics for at least two years.
RESULTS: Cataract was found in 26 patients (33%) with predominance of anterior capsular cataract. The cataract rate among patients from group 1 (40%) was higher than among those from group 2 (18%). Visual acuity was reduced in 21 patients (26%). No changes were observed neither in the cornea nor in the retina.
CONCLUSIONS: Patients using antipsychotic drugs should be submitted to a periodic ophthalmological evaluation.
Descriptors: Antipsychotic agents; Schizophrenia; Cataract; Risk factors; Polypharmacy
RESUMO
OBJETIVO: Os antipsicóticos típicos, principalmente as fenotiazinas, têm sido associados à formação de catarata há mais de quarenta anos. Nos últimos anos, tem existido um questionamento acerca do potencial dos antipsicóticos atípicos de induzir essa patologia lenticular. Neste estudo, buscamos determinar a taxa de catarata e de outros efeitos oculares adversos em pacientes em uso de antipsicóticos a longo prazo.
MÉTODO: Oitenta pacientes tratados ambulatorialmente com diagnóstico de esquizofrenia segundo o DSM-IV, de dois centros, que preencheram os critérios de inclusão pré determinados foram submetidos a uma avaliação oftalmológica para pesquisa de alterações oculares com ênfase no cristalino e na córnea. Eles foram divididos em dois grupos: o grupo 1 (n = 52) era formado por pacientes que tinham usado predominantemente antipsicóticos típicos por pelo menos dois anos e o grupo 2 (n = 28) por pacientes que tinham usado predominantemente antipsicóticos atípicos por pelo menos dois anos.
RESULTADOS: Catarata foi encontrada em 26 pacientes (33%) com predomínio de catarata capsular anterior. A taxa de catarata entre os pacientes do grupo 1 (40%) foi maior do que naqueles do grupo 2 (18%). A acuidade visual estava reduzida em 21 pacientes (26%). Não foram encontradas alterações nem na córnea nem na retina.
CONCLUSÕES: Pessoas em uso de antipsicóticos devem ser submetidas à avaliação oftalmológica periódica.
Descritores: Agentes antipsicóticos, Esquizofrenia; Catarata; Fatores de risco; Polimedicação
Introduction
The lens is a biconvex, avascular and transparent body, encased in an elastic capsule, whose purpose is to focus light onto the retina.1 It is formed of roughly 35% water soluble proteins and 65% water.2 Cataract is defined as any clouding or opacification, either congenital or acquired, located in any point of its structure, independently of the visual impairment.1 It is the leading cause of blindness worldwide.3
Cataract formation has several etiologies. The most common are senile, traumatic, induced by diabetes or myotonic dystrophy, congenital, induced by drugs such as amiodarone, corticosteroids and phenothiazines and due to other ocular primary diseases.1,4
Risk factors for this condition have already been described, such as smoking, severe dehydration and alcoholism.5 Epidemiologic studies of geographic correlation have shown an association between hours of exposure to the ultraviolet radiation and the cataract rate without establishing a well-defined causal relationship.4
People with schizophrenia have been documented to be at a higher risk for cataract than the general population.6 Ocular changes were described to be related to the use of phenothiazinic typical antipsychotics, mainly chlorpromazine.7 Other typical antipsychotic drugs, such as thioridazine, were also associated with such ocular changes.8
In the mid-1960s, lenticular opacities induced by phenothiazines were first reported by Greiner and Berry as dense, dark brown lenticular opacities.7 Since then, studies have shown a cataract rate related to therapy with typical antipsychotics, mainly chlorpromazine, which varied from 22% to 80%.9-12 The dosage of chlorpromazine needed for inducing lenticular opacities has varied according to different studies.13-15
Lenticular opacities associated with phenothiazinic typical antipsychotics are due to pigment deposition usually located in the anterior capsule and anterior subcapsular cortex.1,16 These pigments are formed due to a toxic interaction between such drugs and ultraviolet-B light (UV-B) which leads to the production of free-radical metabolites.17,18 In addition, phenothiazines and its metabolites can cause desnaturation of proteins which become cloudy when exposed to solar radiation.11,19 Evidence of the importance of this interaction with the sunlight could be seen in a patient, reported by Deluise, who had been taking chlorpromazine and had one eye partially closed with ptosis due to congenital Marcus-Gunn jaw-wink phenomenon, and developed ocular changes more prominent in the eye with no ptosis.20 Low visual acuity associated with such ocular changes has been described5,20,21 as well as pigmented corneal deposits and pigmentary retinopathy.13,16
The potential of atypical antipsychotics for inducing lenticular changes, as it is known for typical antipsychotics, remains indefinite up to now. No etiologic relationship established between this new group of antipsychotics and cataract formation is known. However, cataract has been documented as an infrequent finding in patients who have been taking some agents of this new generation.22-24 Quetiapine is the most questioned atypical antipsychotic regarding its potential for inducing cataract because it induced this side effect in dogs.25 As the use of atypical antipsychotics has become more and more common, and there is little knowledge on the possible ocular adverse effects of these drugs, it is essential to evaluate patients in long-term treatment with these agents.
The aim of this study was to evaluate cataract occurrence and other ocular side effects in patients treated with antipsychotic drugs.
Method
A total of 80 outpatients from two centers, Mental Health Service at the Walter Cantideo University Hospital and São Gerardo Mental Health Hospital, treated with antipsychotics, participated in this naturalistic cross-sectional study.
The inclusion criteria were: having DSM-IV (APA, 1994) diagnosis of schizophrenia, age between 18 to 60 years, and to have been taking antipsychotic drugs (typical, atypical or both) for at least two years. The exclusion criteria were: diabetes, systemic arterial hypertension, ocular diseases previously diagnosed (glaucoma, retinopathies, corneal diseases) and to have taken corticosteroids, amiodarone or to have had any ocular trauma.
This study was approved by The Ethics Committee of the Universidade Federal do Ceará (protocol 143/04) and all participants have signed a consent form agreeing to participate in this study.
All patients were submitted to an ophthalmological evaluation at the (Sociedade de Assistência aos Cegos Iêda Otoch Baquit Ophthalmological Unit). This evaluation consisted of maximum visual acuity exam with optical correction, biomicroscopy of the anterior segment, emphasized in cornea and lens, fundus biomicroscopy and a reexam under mydriasis. It was performed by doctors who had no information regarding the medication in use.
Demographic and clinical data were obtained by direct interview and medical records analyses.
The patients were divided into two groups. Group 1 (n = 52) consisted of patients who either had taken only typical antipsychotics for at least two years or had taken both typical and atypical antipsychotics with more than 2 years of use of typical antipsychotics. Group 2 (n = 28) consisted of patients who either had taken only atypical antipsychotics for at least two years or had taken both typical and atypical antipsychotics, being the use of typical antipsychotics shorter than 2 years.
The data obtained were analyzed with the software Statistical Package for the Social Sciences (SPSS). The data distribution was tested with Kolmogorov-Smirnov test. The variables with a normal distribution were analyzed using parametric tests (ANOVA) and those with significant deviation of normal distribution were analyzed using non-parametric tests (Mann-Whitney U test). Categorical variables were analyzed using chi-square test for homogeneity. The significance level was set at p < 0.05 (two-tailed).
Results
Table 1 describes the demographic and clinical features of the patients from both group 1 and 2.
Group 1 (patients who had taken typical antipsychotics for at least 2 years) showed a different profile from group 2 (patients who had taken predominantly atypical antipsychotics) in the following variables: earlier mental illness onset (F = 4.4; p = 0.038), they used antipsychotic drugs for a longer period of time (F = 24.3; p = 0.000), they used higher doses of antipsychotic drugs (F = 6.3; p = 0.014), they were older (F = 5.9; p = 0.017), they had a higher smoking rate (X2 = 5.9; p = 0.014) and they used more anticholinergic drugs (X2 = 4.4; p = 0.034) and benzodiazepines (X2 = 6.2; p = 0.012). Gender (X2 = 1.3; p = 0.251) and antidepressants use (X2 = 1.5; p = 0.215) did not differ between groups.
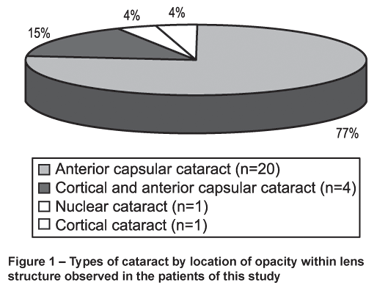
Lenticular opacities were found in 26 (33%) patients with absolute predominance of anterior capsular cataract (n = 20) (77%) - Figure 1. A dot-like pattern was found in 17 (65%) of these patients, whereas a stellate pattern was found in 9 (35%) (Figure 2a and 2b). In group 1 (n = 52), twenty-one patients (40%) had lenticular opacities, whereas in group 2 (n = 28) this finding was seen in only five patients (18%). The difference between the two groups reached statistical significance (F = 4.3; p = 0.041).
No pigment deposition was found neither in the cornea nor in the retina of these patients. As for visual acuity, twenty-one patients (26%) had their best-corrected visual acuity reduced, and 91% of these patients had only a slight reduction (VAcc 20/25 to 20/60).
The typical antipsychotics used by patients were: chlorpromazine, levomeprazine, haloperidol, thioridazine, fluphenazine, sulpiride, zuclopentixol and pimozid. Chlorpromazine and levomeprazine were the most frequently used typical antipsychotics (Figure 3). The atypical antipsychotics were: olanzapine, risperidone, ziprasidone, aripiprazole, clozapine and quetiapine. Olanzapine was the most frequently used atypical antipsychotic (Figure 4). Over half of the patients (n = 44) were also concomitantly on at least one drug of one or more of the following groups: anticholinergic drugs, benzodiazepines and antidepressants.
Discussion
In this study we found a rate of 33% of lenticular opacities in people with schizophrenia treated with antipsychotic drugs. This finding corroborates previous ones by McCarty et al. who reported a cataract rate of 26% in schizophrenic people, which is much higher than in the general population (< 1%).6 In fact, chlorpromazine and other phenothiazinic antipsychotic drugs have been associated with cataract formation.6,15,25,26 A factor that may have contributed to such a high rate in our study is the geographic location of Fortaleza, which is in an equatorial region with strong insolation (2.800 hours/year)27 since there is an association between cataract occurrence and solar exposure,28,29 and the interaction between phenothiazines and the sunlight plays an essential role in cataract formation induced by these drugs.17,18
A higher rate of cataract was observed in group 1, which used typical antipsychotic drugs at higher dosages for a longer duration when compared to group 2. This finding can be explained by the high rate of use of phenothiazines (70% of the typical antipsychotics used were phenothiazines). The literature has associated lens opacities with cumulative dosages and duration of exposure.15,30,31 Some studies showed that chlorpromazine at 800 mg/day for 2 years is oculotoxic,15 while in others, chlorpromazine at 200 mg/day over 2 years caused pigmentary deposits in the lens.14 In addition, smoking has also been associated with cataract formation and the majority of the smoking patients belong to group 1. As a consequence, group 1 patients have been exposed to at least two risk factors.32
Patients from group 2 predominantly used atypical antipsychotics, which have not been associated with cataract formation. Cataract is an infrequent side effect of therapy with olanzapine, ziprasidone and quetiapine with rates lower than in the general population.22-24 All five patients from this group who presented lenticular opacities had taken only atypical antipsychotics. Three of these patients had used olanzapine, one had used risperidone and another had used risperidone, olanzapine and ziprasidone. In fact, the manufacturers of olanzapine and ziprasidone mention cataract as an infrequent adverse effect of these drugs with no known casual connection.33,34
Ocular changes during quetiapine therapy were observed in humans and a periodic ophthalmic evaluation is formally recommended for patients taking this drug.25,35 In our study, just one patient had taken quetiapine at 50 mg/day for 1 year. This 35-year-old male patient had cortical cataract, with no clouding of anterior capsule, and he had taken phenothiazines, levomeprazine and fluphenazine for 23 years. In addition, he was used to smoking 20 cigarettes a day, so being exposed to several risk factors for this lenticular pathology. Nasrallah at al. reported lenticular opacities in 15 patients who had taken quetiapine, at a rate of 0.005%, which is much lower than in the general population.23 Valibhai et al. reported a case of a patient who had no lenticular opacities on the examination of the lens right before the onset of treatment with quetiapine and 15 months after the use of this drug cataract has been formed.36
The possible effect of other drugs on the lens cannot be neglected mainly for being a cross-sectional study and over half of patients were also concomitantly on other drugs, such as prometazin (n = 28), diazepan (n = 12), nitrazepan (n = 5), alprazolan (n = 4), clonazepan (n = 3), amitriptiline (n = 7), fluoxetine (n = 3), sertraline (n = 2) and imipramine (n = 1). Some studies have suggested a possible link between cataract formation and drugs such as tricyclic antidepressants and benzodiazepines.16,37,38 Thus patients from group 1 who had a rate of use of benzodiazepines higher than those form group 2 could have been exposed to one additional contributing factor.
Schizophrenia itself does not appear to be associated with an increased risk of cataract, and studies have shown that the use of a general antipsychotic drug is not associated with the occurrence of cataract but long-term chlorpromazine and other phenothiazines use is related to cataract formation.39
Conclusions
Higher cataract rate was found in the group of patients predominantly on typical antipsychotics when compared to the group on atypical antipsychotics.
It corroborates the previous recommendations that patients who have been taking phenothiazines should be submitted to a periodic ophthalmic evaluation.
There is little information on the potential for inducing cataract of the new generation antipsychotic drugs, being this side effect infrequently observed in patients who have been using olanzapine, ziprasidone and quetiapine. We support the view that patients on long-term atypical antipsycotic agents therapy, mainly quetiapine, should also be submitted to an ophthalmic examination once the literature did not show yet very well-defined evidences that all these agents are really free of this side effect.
Prospective studies are very important to replicate these findings using a non-naturalistic design that controls the drugs per dose and duration of intake.
Acknowledgements
The authors wish to thank Dr. Marluce Alves de Oliveira, M.D from São Gerardo Mental Health Hospital who arranged for patients from this hospital to participate in our study.
Disclosures

Submitted: February 17, 2008
Accepted: May 27, 2008
- 1. Kanski JJ. Clinical Ophthalmology 5th ed. Oxford (UK): Butterworth-Heinemann; 2006.
- 2. Spalton DJ, Hitchings RA, Hunter PA. Atlas Colorido de Clinica Oftalmológica 2nd ed. São Paulo: Manole; 1998.
- 3. Brian G, Taylor H. Cataract Blindness - challenges for the 21st century. Bull. W.H.O 2001;79(3):249-56.
- 4. Yanoff. Ophthalmology 2st ed. Mosby International Ltd; 2004.
- 5. Harding JJ, Heyningen RV. Epidemiology and risk factors for cataract. Eye 1987;1(Pt 5):537-41.
- 6. McCarty CA, Wood CA, Fu CL, Livingston PM, Mackersey S, Stanislavsky Y, Taylor HR. Schizophrenia, psychotropic medications and cataract. Ophthalmology 1999;106(4):683-7.
- 7. Greiner A, Berry K. Skin pigmentation and corneal and lens opacities with prolonged chlorpromazine therapy. Can Med Assoc J 1964;90:663-5.
- 8. Siddall J. Ocular complications related to phenothiazines. Dis Nerv Syst 1968;29(3):10-3.
- 9. Barsa JA, Newton JC, Saunders JC. Lenticular and corneal opacities during phenothiazine therapy. JAMA 1965;193:10-2.
- 10. Smith D, Pantelis C, McGrath J, Tangas C, Copolov D. Ocular abnormalities in chronic schizophrenia: clinical implications. Aust N Z J Psychiatry 1997;31(2):252-6.
- 11. Siddall J. The ocular toxic findings with prolonged and high dosage chlorpromazine intake. Arch Ophthalmol 1965;74(4):461-4.
- 12. Paranhos FR. Study of the incidence of stellar cataract in patients using chlorpromazine. Arch Bras Oftalmol 1991;54(2):63-8.
- 13. Siddall JR. Ocular toxic changes associated with chlorpromazine and thioridazine. Can J Ophthalmol 1966;1(3):190-8.
- 14. Bock R, Swain J. Ophthalmological findings in patients on long-term chlorpromazine therapy. Am J Ophthalmol 1963;56:808-10.
- 15. Satanove A. Pigmentation due to phenothiazine in high and prolonged dosage. JAMA 1965;191(4):263-8.
- 16. Isaac NE, Walker AM, Jick H, Gorman M. Exposure to phenothiazine drugs and risk of cataract. Arch Ophthalmol 1991;109(2):256-60.
- 17. Forrest IS, Forrest FM, Berger M. Free radicals as metabolites of drugs derived from phenothiazines. Biochem Biophys Acta 1958;29(2):441.
- 18. Thaler JS, Curinga R, Kiracofe G. Relation of graded ocular anterior chamber pigmentation to phenothiazine intake in schizophrenicsquantification procedures. Am J Optom Physiol Opt 1985;62(9):600-4
- 19. Howard RO, McDonald CJ, Dunn B, Creasey WA. Experimental chlorpromazine cataracts. Invest Ophthalmol 1969;8(4):413-21.
- 20. Deluise V, Flynn J. Asymmetrical anterior segment changes induced by chlorpromazine. Ann Ophthalmol 1981;8:953-5.
- 21. Forrest FM, Snow HL. Prognosis of eye complications caused by phenothiazines. Dis Nerv Syst 1968;29(3): Suppl:26-8.
- 22. Shahzad S, Suleman MI, Shahab H, Mazour I, Kaur A, Rudzinskiy P, Lippmann S. Cataract occurrence with antipsychotic drugs. Psychosomatics 2002;43(5):354-9.
- 23. Nasrallah HA, Dev V, Rak I, Raniwalla J. Safety update with quetiapine and lenticular examinations: experience with 300,000 patients. In: Abstracts of the 38th Annual Meeting of the American College of Neuropsychopharmacology. Nashville, Tenn, ACNP; 1999.
- 24. Laties AM, Dev VJ, Geller W, Rak I, Brecher M, Nasrallah H. Safety update on lenticular opacities: benign experience with 620,000 US patient exposures to quetiapine. In: Abstracts of the 39th Annual Meeting of the American College of Neuropsychopharmacology. Nashville, Tenn, ACNP; 2000.
- 25. Seroquel Package Insert. Wilmington, Del, Zeneca Pharmaceuticals; 2001.
- 26. Garner LL, Wang RI, Hieb E. Eye changes following phenothiazine administration. Wis Med J 1974;73(9):S119-21.
-
27Banco do Nordeste. Produtos e atrativos turísticos-parte 1. Available from: Http://www.bnb.gov.br/content/aplicacao/prodetur/downloads/docs/pdits_ce_vol_ii_ diagn_10_produt_e_atrat_01.pdf > File downloaded on 07/25/07.
- 28. Zigman S, Datiles M, Torczynski E. Sunlight and human cataracts. Invest Ophtalmol Vis Sci 1979;18(5):462-7.
- 29. Hiller R, Giacometti L, Yuen K. Sunlight and cataract: an epidemiologic investigation. Am J Epidemiol 1977;105(5):450-9.
- 30. Barnes GJ, Cameron ME. Skin and eye changes with chlorpromazine therapy. Med J Aust 1966;1(12):478-81.
- 31. Rasmussen K, Kirk L, Faurbye A. Depositis in the lens and cornea of the eye during long-term chlorpromazine medication. Acta Psychioatr Scand 1976;53(1):1-6.
- 32. Christen WG, Manson JE, Seddon JM, Glynn RJ, Buring JE, Rosner B, Hennekens CH. A prospective study of cigarette smoking and risk of cataract in men. JAMA 1992;268(8):989-93.
- 33. Zyprexa Package Insert. Indianapolis, Eli Lilly and Co, 2001.
- 34. Geodon, in Physicians' Desk Reference, 56th ed. Montvale, NJ, Medical Economics Co, 2002. p. 2688-92.
- 35. Seroquel Professional Information Brochure. Wilmington, Del, Zeneca Pharmaceuticals, 1997.
- 36. Valibhai F, Phan NB, Still DJ, True J. Cataracts and quetiapine. Am J Psychiatry 2001;158(6):966.
- 37. Collman GW, Shore DL, Shy CM, Checkoway H, Luria AS. Sunlight and other risk factors for cataracts: an epidemiologic study. Am J Public Health 1988;78(11):1459-62.
- 38. [No authors listed] Epidemiology of cataract. Lancet 1982;1(8286):1392-3.
- 39. Ruigómez A, García Rodríguez LA, Dev VJ, Arellano F, Raniwala J. Are schizophrenia or antipsychotic drugs a risk factor for cataracts? Epidemiology 2000;11:620-3
Correspondence
Publication Dates
-
Publication in this collection
25 Sept 2008 -
Date of issue
Sept 2008
History
-
Accepted
27 May 2008 -
Received
17 Feb 2008