Abstracts
INTRODUCTION: The prevalence of depressive disorders in HIV-infected patients ranges from 12% to 66% and is undiagnosed in 50% to 60% of these patients. Depression in HIV-infected individuals may be associated with poor antiretroviral treatment (ART) outcomes, since it may direct influence compliance. OBJECTIVE: To assess the presence of symptoms and risk factors for depression in patients on ART. METHODS: Cross-sectional study. Certified interviewers administered questionnaires and the Beck Depression Inventory (BDI), and participants' self-reported compliance to ART. Clinical and laboratory variables were obtained from clinical records. Patients with BDI > 12 were defined as depressed. RESULTS Out of the 250 patients invited to participate, 246 (98%) consented. Mean age was 41 ± 9.9 years; most were male (63%). Income ranged from 0-14 Brazilian minimum wages. AIDS (CDC stage C) had been diagnosed in 97%, and 81% were in stable immune status. One hundred ninety-one (78%) reported compliance, and 161 (68%) had undetectable viral loads. The prevalence of depressive symptoms was 32% (95% CI 26-40). In multivariate analysis, depressive symptoms were significantly associated with income (prevalence ratio [PR] = 0.85; 95% CI 0.74-0.97; p = 0.02). CONCLUSIONS: Depressive symptoms are frequent in patients on ART, and are associated with low income.
AIDS; CNS depressants; drug side effects; epidemiology; other psychological issues
INTRODUÇÃO:A prevalência de transtornos depressivos em pacientes infectados pelo HIV varia de 12% a 66% e não é diagnosticada em 50% a 60% desses pacientes. A depressão em indivíduos HIV positivo pode se associar a resultados fracos do tratamento antirretroviral (TAR) porque pode influenciar diretamente a aderência ao regime. OBJETIVO: Avaliar a presença de sintomas e de fatores de risco de depressão em pacientes em TAR. MÉTODOS: Estudo em corte transverso. Entrevistadores certificados administraram questionários e o Beck Depression Inventory (BDI), e os participantes fizeram o autorrelato da aderência ao TAR. Variáveis clínicas e laboratoriais foram obtidas dos prontuários clínicos. Os pacientes com escore ao BDI > 12 foram definidos como deprimidos. RESULTADOS: Dos 250 pacientes convidados a participar, 246 (98%) concordaram. A média de idade foi de 41 ± 9,9 anos; a maioria dos pacientes era do sexo masculino (63%). A renda variou de 0-14 salários mínimos brasileiros. A AIDS (estágio C dos CDC) havia sido diagnosticada em 97% e 81% estavam em estado imune estável. Dos pacientes, 191 (78%) relataram aderência e 161 (68%) tinham carga viral não detectável. A prevalência dos sintomas depressivos foi de 32% (IC 95% 26-40). Em análise multivariada, os sintomas depressivos se associaram significativamente à renda (razão de prevalência [RP] = 0,85, IC 95% 0,74-0,97; p = 0,02). CONCLUSÕES: Os sintomas depressivos são frequentes em pacientes em TAR e se associam a uma renda baixa.
AIDS; depressores do SNC; efeitos colaterais de drogas; epidemiologia; outras questões psicológicas
ORIGINAL ARTICLE
Depressive symptoms in HIV-infected patients treated with highly active antiretroviral therapy
Sintomas depressivos em pacientes infectados pelo HIV tratados por terapia antiretroviral altamente ativa
Marysabel Pinto Telis SilveiraI; Marília Cruz GuttierII; Cezar Arthur Tavares PinheiroIII; Tatiana Vanessa Silveira PereiraIV; Ana Laura Sica Cruzeiro,V; Leila Beltrami MoreiraVI
IPharmacy Course, Universidade Federal do Pampa, Uruguaiana, Brazil; Postgraduate Program in Medicine-Medical Sciences, Universidade Federal do Rio Grande do Sul (UFRGS), Brazil
IIPostgraduate Program in Medicine-Medical Sciences, Universidade Federal do Rio Grande do Sul (UFRGS), Brazil
IIISpecialized Service for HIV/AIDS-SAE-Pelotas; Universidade Federal de Pelotas, Brazil
IVPharmacist
VDepartment of Psychology, Life and Health Science Center, Universidade Católica de Pelotas, Brazil
VIDepartment of Pharmacology, Universidade Federal do Rio Grande do Sul, Brazil
Corresponding author Corresponding author: Marysabel Pinto Telis Silveira Rua Dom Pedrito, 842, Laranjal Pelotas, Rio Grande do Sul, Brazil Phone: (+55 53) 30271927, (+55 53) 81176791 E-mail: marysabelfarmacologia@yahoo.com.br
ABSTRACT
INTRODUCTION: The prevalence of depressive disorders in HIV-infected patients ranges from 12% to 66% and is undiagnosed in 50% to 60% of these patients. Depression in HIV-infected individuals may be associated with poor antiretroviral treatment (ART) outcomes, since it may direct influence compliance.
OBJECTIVE: To assess the presence of symptoms and risk factors for depression in patients on ART.
METHODS: Cross-sectional study. Certified interviewers administered questionnaires and the Beck Depression Inventory (BDI), and participants' self-reported compliance to ART. Clinical and laboratory variables were obtained from clinical records. Patients with BDI > 12 were defined as depressed.
RESULTS Out of the 250 patients invited to participate, 246 (98%) consented. Mean age was 41 ± 9.9 years; most were male (63%). Income ranged from 0-14 Brazilian minimum wages. AIDS (CDC stage C) had been diagnosed in 97%, and 81% were in stable immune status. One hundred ninety-one (78%) reported compliance, and 161 (68%) had undetectable viral loads. The prevalence of depressive symptoms was 32% (95% CI 26-40). In multivariate analysis, depressive symptoms were significantly associated with income (prevalence ratio [PR] = 0.85; 95% CI 0.74-0.97; p = 0.02).
CONCLUSIONS: Depressive symptoms are frequent in patients on ART, and are associated with low income.
Descriptors: AIDS; CNS depressants; drug side effects - other; epidemiology; other psychological issues.
RESUMO
INTRODUÇÃO:A prevalência de transtornos depressivos em pacientes infectados pelo HIV varia de 12% a 66% e não é diagnosticada em 50% a 60% desses pacientes. A depressão em indivíduos HIV positivo pode se associar a resultados fracos do tratamento antirretroviral (TAR) porque pode influenciar diretamente a aderência ao regime.
OBJETIVO: Avaliar a presença de sintomas e de fatores de risco de depressão em pacientes em TAR.
MÉTODOS: Estudo em corte transverso. Entrevistadores certificados administraram questionários e o Beck Depression Inventory (BDI), e os participantes fizeram o autorrelato da aderência ao TAR. Variáveis clínicas e laboratoriais foram obtidas dos prontuários clínicos. Os pacientes com escore ao BDI > 12 foram definidos como deprimidos.
RESULTADOS: Dos 250 pacientes convidados a participar, 246 (98%) concordaram. A média de idade foi de 41 ± 9,9 anos; a maioria dos pacientes era do sexo masculino (63%). A renda variou de 0-14 salários mínimos brasileiros. A AIDS (estágio C dos CDC) havia sido diagnosticada em 97% e 81% estavam em estado imune estável. Dos pacientes, 191 (78%) relataram aderência e 161 (68%) tinham carga viral não detectável. A prevalência dos sintomas depressivos foi de 32% (IC 95% 26-40). Em análise multivariada, os sintomas depressivos se associaram significativamente à renda (razão de prevalência [RP] = 0,85, IC 95% 0,74-0,97; p = 0,02).
CONCLUSÕES: Os sintomas depressivos são frequentes em pacientes em TAR e se associam a uma renda baixa.
Descritores: AIDS; depressores do SNC; efeitos colaterais de drogas - outros; epidemiologia; outras questões psicológicas.
Introduction
The prevalence of depression in HIV-infected patients has been reported to range from 12% to 66%.1-7 Diagnosing and treating these symptoms is critical to improve patients' quality of life; however, 50% to 60% of the cases are not diagnosed.8 Psychological stress and progression of HIV infection have been associated with a lower quality of life.9 Moreover, depression has been associated with poorer adherence to antiretroviral treatment (ART).10
Little has been published on the epidemiology of depression in patients with HIV/AIDS in lower- and middle-income countries. The reported prevalence of depressive symptoms in patients with HIV/AIDS in those countries range from 8% to 60%.11 Despite the variety of instruments used to identify depression, it seems to be a neglected public health issue in developing countries.11
The purpose of this study is to report the prevalence of depressive symptoms and to identify associated risk factors in patients on ART, treated in a specialized HIV/AIDS service in southern Brazil, with a particular emphasis on the association between depression and ART compliance.
Methods
Design
Cross-sectional study.
Setting
Reference center for HIV/AIDS-SAE-Pelotas, in Pelotas, Brazil.
Participants
The study population comprised all patients on ART currently under care at the HIV/AIDS-SAE-Pelotas (N = 800). A sample size of 230 was estimated to measure a prevalence of depressive symptoms of 30 ± 5%, with 95% confidence interval (95%CI).
All patients who attended the clinic for consultation or in order to receive prescription drugs were invited to join the study. After signing a written informed consent, participants were interviewed. The study was approved by the Scientific Commission of the Universidade Católica de Pelotas and by the Ethics Committee of the Universidade Federal do Rio Grande do Sul (number 2003164).
Trained interviewers administered questionnaires including sociodemographic variables and self-reported adherence, and administered BDI.12 Clinical and laboratory data were obtained from the clinical records.
Main variables of interest
The presence of depressive symptoms was measured by the Beck Depression Inventory (BDI). BDI has 21 items which reflect cognitive, affective, behavioral, and somatic symptoms of depression. Each item is composed of four statements, and the patient is asked to pick the one that best describes how he or she has been feeling for the past week. Statements are scored on a scale value of 0 to 3, according to the intensity of the symptom. Final scores are obtained by adding all points. A cutoff point of 12 on BDI score was used to classify patients with and without depressive symptoms. Intensity of symptoms was classified as mild (12-19), moderate (20-35) or severe (36-41).12
Compliance was evaluated using participants' self-reports on prescribed antiretrovirals taken in the last three days before the interview. A time sheet was used to help patients remember their routine activities (e.g., breakfast, lunch) and to report the medicines in use, doses and time of administration. Individual drug compliance was computed dividing the number of tablets of each antiretroviral drug taken in three days by the number of tablets prescribed in the same days. Rate of regimen compliance was calculated by arithmetic mean for each drug. Participants were considered compliant if they reported taking > 95% of the prescribed doses during the three days prior to the evaluation.13,14
Predictor variables
Sex, age, years on ART, household income (as a multiple of the Brazilian minimal monthly wage of R$ 350.00, which is equivalent to US$ 153.21), employment, receiving social security benefits and living situation. The most recent HIV plasma viral load prior to the interview was identified from clinical records. The lower limit of detection was 50 copies/mL.13 Other clinical data such as: HIV clinical stage as defined by the Centers for Disease Control and Prevention15 and modified according to the criteria adopted in Rio de Janeiro and Caracas;13,15 the CD4 lymphocyte count closest to the interview; the ART regimen; and the duration of ART treatment were collected from clinical records.
Statistical analysis
Data were double-entered into EPI-INFO and analyzed in SPSS for Windows version 16.0 (SAS Institute, Cary, North Carolina, USA). Differences in proportions between patients with or without depressive symptoms were tested by the chi-square test. Poisson regression analyses with depressive symptoms as the outcome variable were carried out to evaluate the individual role of risk factors associated with depressive symptoms. Variables with p < 0.3 in bivariate analysis were included in the multivariable model to estimate the adjusted prevalence ratio for depressive symptoms. Risk factors for non-compliance were evaluated through Poisson regression as well.
Results
Out of 250 patients invited to participate, 246 (98%) consented. Of these, 154 (63%) were male. The mean age was 41 ± 9.9 years (range, 18 to 73 years), and income ranged from 0 to 14 times the minimum wage (median, 1.90; 1 MW = R$ 350.00 or US$ 153.51). AIDS was the most prevalent clinical stage (97%), and 81% had > 200 CD4 cells/mm. One hundred sixty-six (68%) participants had undetectable viral load, and 191 (78%) reported being adherent to treatment. Duration of ART ranged from 4 to 139 months (mean 56.8 ± 34.05), and 55% of participants were using efavirenz (EFV). Table 1 presents sociodemographic characteristics of HIV-infected patients on ART with and without depressive symptoms.
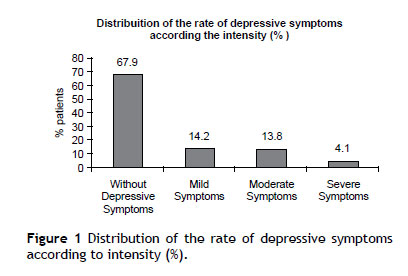
The prevalence of depressive symptoms (BDI score > 12) was 32% (95% CI 26%-40%). Overall, 14% classified as having mild depressive symptoms, 14% as having moderate depressive symptoms and 4% as having severe depressive symptoms (Figure 1). Fatigue (51.2%) and irritability (47.6%) were the most frequently reported symptoms. Predictors of depressive symptoms in HIV-infected patients on ART are presented in Table 2. In multivariate analysis adjusted for sex, age, years of education, income, employment, receiving social security benefits and therapeutic regimen with efavirenz only income remained associated with depressive symptoms (RR = 0.85; 95% CI 0.74-0.97; p = 0.02).
Therapeutic regimen containing EFV was independently associated with adherence to ART (Table 3).
Discussion
The study showed that 32% of patients had depressive symptoms as measured by BDI and their presence was associated with low income. Prior studies have shown prevalence of depression among HIV-infected patients between 12 to 66%.1-7 Such variation may be explained by the different instruments used to diagnose depression and by other methodological differences, such as the cutoff point used. Different BDI cut points have been used; even in their original paper, Beck et al.16 suggested that the choice of an adequate cutoff point depended on sample characteristics and study goals. We used 12 as a cutoff, which allowed us to identify the largest number of patients with symptoms of mild depression or worse.12
EFV is a drug that has been associated with a number of neuropsychological side effects and mood disorders.17-19 In a randomized clinical trial, Lochet et al.19 found that neuropsychiatric adverse reactions associated with EFV occurred mainly during the first month of therapy and often persisted thereafter. Gutierrez et al.20 studied predictive factors of neuropsychiatric adverse events associated with EFV and found that the only risk factor associated with central nervous system toxicity, after adjusting for body weight and hepatitis C virus co-infection, was EFV plasma concentration.Considering depressive symptoms, some authors have found no association18,21 while others found a high prevalence of depression associated with EFV use.19,22 In the present study, over half of studied patients were taking an ART regimen containing EFV. No association with depressive symptoms was found, although a statistically non-significant relative risk reduction of 28% for depressive symptoms among patients not using EFV was identified. Despite the conflicting results in the literature, this finding is important for the evaluation of patients already in treatment, since depression may worsen adherence23 and contribute to treatment failure.
Participants were considered adherent if they reported taking > 95% of the prescribed doses during the three days prior to the evaluation.13,14 Those patients under therapeutics regimen including efavirenz were 27% more likely to be compliant than patients not taking efavirenz regimen. This might be explained by the fewer tablets needed or lower frequency of doses in therapeutics regimen including efavirenz.
The study has some limitations to be considered. The cross-sectional design did not allow us to establish a causal relationship between the potential risk factors and the presence of depressive symptoms. Antidepressant drug use was not investigated and may have led to underestimation of depressive symptoms prevalence. Treatment length varied greatly, which may have contributed to absence of association between depression and clinical stage, since depression is more prevalent immediately after the AIDS diagnosis.5 Another limitation is the fact that the study was conducted only with HIV-infected patients on ART, being most of them stage C (AIDS).
In conclusion, it was found that almost one in three patients on ART has depressive symptoms and participants presenting symptoms of depression are mostly low-income patients. It is suggested that clinicians carefully assess the possibility of depressive symptoms in their patients on ART, and especially those of lower income. Future studies with prospective design, starting at the moment of HIV diagnosis, should be able to more clearly explore the causal and temporal relationship between depression, compliance and ART.
Acknowledgments
We thank the staff and patients of the Specialized Service for HIV/AIDS-SAE-Pelotas, Universidade Federal de Pelotas , Pelotas, Brazil. We are also grateful to the students of the Pharmacy Course at, Universidade Católica de Pelotas who have worked on this project and to the faculty of the University of California, San Francisco, the Universidade Federal da Bahia , and the Center for the Study of AIDS of Rio Grande do Sul in Porto Alegre, Brazil (CEARGS) for their assistance.
Name of the department and institution to which the study should be attributed: Pharmacy Course, Universidade Federal do Pampa , Uruguaiana, Brazil and Postgraduate Program in Medicine-Medical Sciences, Universidade Federal do Rio Grande do Sul , Porto Alegre, Brazil.
Received on June 29, 2011; accepted on October 12, 2011
- 1. Gibbie T, Mijch A, Ellen S, Hoy J, Hutchison C, Wright E, Chua P, Judd F. Depression and neurocognitive performance in individuals with HIV/AIDS: 2-year follow-up. Hiv Med. 2006;7:112-21.
- 2. Judd F, Komiti A, Chua P, Mijch A, Hoy J, Grech P, Street A, Lloyd J, Williams. Nature of depression in patients with HIV/AIDS. Aust N Z J Psychiatry. 2005;39:826-32.
- 3. Judd FK, Cockram AM, Komiti A, Mijch AM, Hoy J, Bell R. Depressive symptoms reduced in individuals with HIV/AIDS treated with highly active antiretroviral therapy: a longitudinal study. Aust N Z J Psychiatry. 2000;34:1015-21.
- 4. Judd FK, Mijch AM. Depressive symptons in patients with HIV infection. Aust N Z J Psychiatry. 1996;30:104-09.
- 5. Lyketsos CG, Hoover DR, Guccione M, Dew MA, Wesch J, Bing EG, Treisman GJ. Depressive symptoms over the course of HIV infection before AIDS. Soc Psychiatry Psychiatr Epidemiol. 1996a;31:212-19.
- 6. Mello VA, Malbergier A. Depression in women infected with HIV. Rev Bras Psiquiatr. 2006;28(1):10-7.
- 7. Williams JB, Rabkin JG, Remien RH, Gorman JM, Ehrhardt AA. Multidisciplinary baseline assessment of homosexual men with and without human immunodeficiency virus infection II. Standardized clinical assessment of current and lifetime psychopathology. Arch Gen Psychiatry. 2001;48(2):124-30.
- 8. Malbergier A. Aids e Psiquiatria - um Guia para os Profissionais de Saúde. Rio de Janeiro: Revinter; 2000.
- 9. Leserman J, Petitto JM, Golden RN, Gaynes BN, Gu HB, Perkins DO, Silva SG, Folds JD, Evans DL. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. Am J Psychiatry. 2000;157:1221-8.
- 10. Macintyre RC, Holzemer WL, Philippek M. Complementary and alternative medicine and HIV/AIDS. Part II: Select literature review. J Assoc Nurses AIDS Care. 1997;8:25-38.
- 11. Fleischer NL, Fernald LC, Hubbard AE. Depressive Symptoms in Low-Income Women in Rural Mexico. Epidemiology. 2007;18:678-85.
- 12. Cunha JA. Manual da versão em português das escalas de Beck. Casa do Psicólogo Livraria e Editora Ltda; 2001.
- 13. Ministério da Saúde do Brasil. Recomendações para terapia anti-retroviral em adultos e adolescentes infectados pelo HIV-2006. Retrieved July 19, 2007, from: //www.aids.gov.br/assistencia/concenso_brasileiro2007.doc
- 14. Silveira MPT, Pinheiro CAT, Guttier MC, Pereira TVS, Moreira LB. Description of pharmaceutical care to assess their effectiveness on adherence to antirretroviral therapy - A randomized clinical trial. JMMS. 2010;1(5):171-177. Available online: http://www.interesjournals.org/JMMS
- 15. Centers for Disease Control And Prevention Report of the NIH panel to define principles of therapy of HIV infection and guideliness for the use of antiretroviral agents in HIV-infected adults and adolescents TM. US. Department of Health and Human Services. Atlanta: 2000.
- 16. Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review: Baltimore 1998 In: Castanha AR, Coutinho MPL, Saldanha AAW, Ribeiro CG. Psychosocial repercussions of depression in the context of AIDS. Psicologia: ciência e profissão. 2006;26(1):70-81.
- 17. Boly L, Cafaro V, Dyner T. Depressive symptoms predict increased incidence of nuropsychiatric side effects in patients treated with efavirenz. J Acquir Immune Defic Syndr. 2006;42(4):514-5.
- 18. Clifford DB, Evans S, Yang YJ, Acosta EP, Goodkin K, Tashima K, et al . Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Int Med. 2005;143(10):714-21.
- 19. Lochet P, Peyrière H, Lotthé A, Mauboussim JM, Delmas B, Reynes J. Long-term assessment of neuropsychiatric adverse reactions associated with efavirenz. HIV Med. 2003;4:62-6.
- 20. Gutierrez F, Navarro A, Padilha S, Anton R, Masia M, Borrás J, Martín-Hidalgo A. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis. 2005;41:1648-53.
- 21. Fumaz CR, Muñoz-Moreno JA, Moltó J, Negredo E, Ferrer MJ, Sirera G, Pérez-Alvarez N, Gómez G, Burger D, Clotet B. Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr. 2005;38(5):560-5.
- 22. Spire B, Carrieri P, Garzot MA, L'Henaff M, Obadia Y. Factors associated with efavirenz discontinuation in a large community-based sample of patients. AIDS Care. 2004;16(5):558-64.
- 23. Starace S, Ammassari A, Trotta MP, De Longis P, Izzo C, Scalzini A d'Arminio Monforte A, Wu AW, Antinori A; AdICoNA Study Group; NeuroICoNA Study Group. Depression is a risk factor forsuboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002:31(Suppl.3):136-9.
Corresponding author:
Publication Dates
-
Publication in this collection
19 June 2012 -
Date of issue
June 2012
History
-
Received
29 June 2011 -
Accepted
12 Oct 2011