Abstract
The purpose of the study was to evaluate the efficacy of an extract of Panax ginseng in patients with fibromyalgia. A randomized, double-blind, controlled clinical trial was carried out over 12 weeks to compare the effects of P. ginseng (100 mg/d) with amitriptyline (25 mg/d) and placebo in 38 patients with fibromyalgia: 13 in Group I (amitriptyline), 13 in Group II (placebo), and 12 in Group III (P. ginseng). Ratings on the Visual Analogue Scale (VAS) revealed a reduction in pain in the P. ginseng group (p < .0001), an improvement in fatigue (p < .0001) and an improvement in sleep (p < .001), with respect to baseline characteristics, but there were no differences between the three groups. With respect to anxiety, improvements occurred in the P. ginseng group compared to baseline (p < .0001); however, amitriptyline treatment resulted in significantly greater improvements (p < .05). P. ginseng reduced the number of tender points and improved patients' quality of life (using the Fibromyalgia Impact Questionnaire - FIQ); however, there were no differences between groups. The beneficial effects experienced by patients for all parameters suggest a need for further studies to be performed on the tolerability and efficacy of this phytotherapic as a complementary therapy for fibromyalgia.
Fibromyalgia; Panax ginseng; Pharmacological Treatment; Complementary Treatment; Phytotherapy
ORIGINAL ARTICLE
Effects of Panax ginseng extract in patients with fibromyalgia: a 12-week, randomized, double-blind, placebo-controlled trial
Alessandra S. BrazI; Liana Clébia S. MoraisI; Ana Patríca PaulaII; Margareth F. F. M. DinizI Reinaldo N. AlmeidaI
ILaboratory of Pharmaceutical Technology, Universidade Federal da Paraíba, Brazil
IIRheumatology Section, Hospital Universitário de Brasília, Faculdade de Ciências da Saúde, Universidade de Brasília, Brazil
Corresponding author Corresponding author: Reinaldo N. Almeida Laboratório de Tecnologia Farmacêutica. Universidade Federal da Paraíba. Caixa Postal 5009 58051-970 João Pessoa, Paraíba, Brazil E-mail: reinaldoan@uol.com.br
ABSTRACT
The purpose of the study was to evaluate the efficacy of an extract of Panax ginseng in patients with fibromyalgia. A randomized, double-blind, controlled clinical trial was carried out over 12 weeks to compare the effects of P. ginseng (100 mg/d) with amitriptyline (25 mg/d) and placebo in 38 patients with fibromyalgia: 13 in Group I (amitriptyline), 13 in Group II (placebo), and 12 in Group III (P. ginseng). Ratings on the Visual Analogue Scale (VAS) revealed a reduction in pain in the P. ginseng group (p < .0001), an improvement in fatigue (p < .0001) and an improvement in sleep (p < .001), with respect to baseline characteristics, but there were no differences between the three groups. With respect to anxiety, improvements occurred in the P. ginseng group compared to baseline (p < .0001); however, amitriptyline treatment resulted in significantly greater improvements (p < .05). P. ginseng reduced the number of tender points and improved patients' quality of life (using the Fibromyalgia Impact Questionnaire - FIQ); however, there were no differences between groups. The beneficial effects experienced by patients for all parameters suggest a need for further studies to be performed on the tolerability and efficacy of this phytotherapic as a complementary therapy for fibromyalgia.
Descriptors: Fibromyalgia; Panax ginseng; Pharmacological Treatment; Complementary Treatment; Phytotherapy.
Introduction
Fibromyalgia is a chronic pain syndrome that affects up to 5% of the population worldwide. Clinically, it is characterized by the presence of diffuse pain and hypersensitive points detected through physical examination and generally accompanied by fatigue, sleep and mood disorders.1 Although its pathogenesis has yet to be fully clarified, most of the evidence points to a disorder of central pain modulation, impairments of the descending inhibitory system (serotoninergic, noradrenergic and opioid) and/or hyperactivity of excitatory neurotransmitters such as substance P.2
Treatment of fibromyalgia includes both non-pharmacological therapies and pharmacological interventions. Many patients respond well to exercise,3 and up to 50% of cases respond adequately to treatment with tricyclic antidepressants such as low-dose amitriptyline.4 Various other drugs used in controlled studies have been found to relieve the symptoms of fibromyalgia, including fluoxetine,5 duloxetine,6 milnacipran,7 cyclobenzaprine,8 gabapentin,9 pregabalin10 and tramadol.11 Patients are, however, extremely interested in alternative and complementary medicine for the treatment of this disease.12
Panax ginseng C.A. Meyer is an herb that has been used for hundreds of years in Eastern medicine.13 The principal active components of P. ginseng are the ginsenosides or triterpenoid saponins of ginseng, and approximately 38 types of ginsenosides have been identified.14 Some experimental studies have suggested that ginsenosides act on the central nervous system, particularly in neurodegenerative disorders.15 Ginsenoside Rg3 has been reported to inhibit glutamate receptors (N-methyl-D-aspartate (NMDA) type and non-NMDA), which contribute significantly to the occurrence of brain trauma and convulsions.16 Various studies have demonstrated the antinociceptive effects of P. ginseng in vitro and in vivo.17-19 Shin et al.17 demonstrated a central effect of ginsenosides, but there was no effect on peripheral nociceptors. The ginsenoside Rg3 inhibited calcium channels in the neurons of the dorsal spine region18 and intraperitoneal administration of the extract provoked an analgesic effect, most likely as the result of a nonopioid mechanism.19
No clinical studies have been carried out to evaluate the effect of P. ginseng on chronic pain.
The objective of the present study is to evaluate the therapeutic efficacy of P. ginseng root extract on the control of symptoms in patients with fibromyalgia.
Methods
Subjects
Fifty-two patients with a diagnosis of fibromyalgia, diagnosed according to the criteria defined by the American College of Rheumatology (ACR),1 who were receiving care at the Rheumatology Outpatient Clinic of the Center for Rheumatology, Physical Medicine and Motor Rehabilitation in the city of Santa Rita, Paraíba, Brazil, between February 2008 and May 2009, were selected and invited to participate as volunteers in the study. The study population consisted of women spontaneously attending the clinic or referred by the various units comprising the Santa Rita public healthcare system following an announcement of the study to the physicians in the city. Men were not included in the study because of the lack of controlled scientific studies of the clinical characteristics and severity of fibromyalgia in men.
The following admission criteria were used to screen patients for the study: 1) women aged 21 to 60 years of any ethnic origin with a diagnosis of fibromyalgia (ACR, 1990)1 who were examined at the rheumatology outpatient clinic by a single examiner; and 2) patients whose laboratory tests (complete blood count, erythrocyte sedimentation rate, creatine, aminotransferases and thyroid stimulating hormone) were all within the normal range.
Candidates with the following characteristics were excluded from the study: 1) patients with a diagnosis of untreated inflammatory rheumatic or endocrine disease and/or neurological, renal, infectious or bone disease; patients with glaucoma, urinary retention, coronary heart disease, arrhythmias or congestive heart failure, and pregnant or breastfeeding women; 2) patients who had used tricyclic antidepressants in the previous three months; 3) patients hypersensitive to ginseng or its components, patients with hemorrhages, high estrogen levels or undergoing hormone treatment; and 4) patients taking steroids or antipsychotic drugs contraindicated for use with ginseng. Prior to initiation of the study, the patients who used analgesics, opioids or anti-inflammatory drugs were required to stop using the medication for at least three weeks.
Drugs
The pharmacologically evaluated compounds used in the study were as follows: amitriptyline hydrochloride (25 mg/d), P. ginseng root extract (100 mg/d - 27% of ginsenosides) and placebo (talcum powder) and were prepared in identical form as capsules and provided to the patients in sealed, black bottles. All were supplied by Orient Mix Fitoterápicos do Brasil Ltda. Staff were blind with respect to the medication, and the patients were divided into three groups: Group I (amitriptyline), Group II (placebo) and Group III (P. ginseng).
Study design
The study consisted of a randomized, double-blind, controlled, 12-week clinical trial to compare the treatment of fibromyalgia with amitriptyline, P. ginseng and placebo. The patients were randomized into three groups in a proportion of 1:1:1 to receive one of the three types of medication, which they were instructed to take as a single daily dose, always at 6 p.m. After receiving the capsules on day zero (baseline), the patients returned for follow-up one week later and every three weeks thereafter for a total of six visits (0, 1, 3, 6, 9 and 12 weeks). At each follow-up visit, patients underwent a specialized physical examination. In addition, they were asked whether their symptoms had improved, whether they had remained the same or whether they had intensified, in accordance with a protocol specifically designed for this study.
The study was conducted according to the directives and regulations governing research studies involving human beings, as defined in Resolutions 196/96 and 251/97 of the Brazilian Ministry of Health's National Health Council. The protocol was approved by the Internal Review Board of the Lauro Wanderley Teaching Hospital, Universidade Federal da Paraíba on October 30, 2007, under protocol number 085/05. Patients were informed of the nature and objectives of the study and were admitted only after they had signed an informed consent form in duplicate, thereby confirming agreement to participate in the study and authorizing publication of the results.
Clinical assessment
The Visual Analog Scale (VAS) was used to evaluate pain, fatigue, sleep quality and anxiety and was completed by all patients. It consists of a line extending from 0 to 10 cm on which the patient attributes a value for any given variable at the time of evaluation. Zero represents the absence of that variable and 10 the maximum intensity reported.
During physical examination, a simple count of tender points was made by digitally palpating the region with an approximate pressure of 4 kgf/cm2.20 The sites palpated were those defined by the ACR.1 The test was considered positive when the patients reacted in any of the following three ways after application of digital pressure: 1) involuntary verbal indication of pain; 2) facial expression of pain (confirmed by the investigator's questioning); and/or 3) if the pain was capable of making the patient flinch or remove the investigator's hand.
The quality of life of the patients in this study was evaluated using the Fibromyalgia Impact Questionnaire (FIQ), a disease-specific questionnaire initially proposed by Burckhardt et al.21 for the evaluation of quality of life in patients with fibromyalgia. It was validated for use in the Brazilian population by Marques et al.22 This questionnaire is composed of 10 domains, the first consisting of 10 sub-items or questions and the other nine of only one question each. The first domain contains questions concerning the capacity of the patient to perform certain routine activities. Responses range from 0, always able to perform the activity, to 3, never able to perform the activity. Item two refers to the number of days during which the patient felt well in the previous week and item three to the number of days on which the patient was unable to go to work because of the disease. Possible answers range from 0 to 7 for each item or domain. For domains 4-10, scores range from 0 to 10 in each. These final seven items are designed to collect data on the patient's capacity to work and their perceptions of pain, fatigue, morning stiffness, mood, anxiety and depression. The data from the FIQ are arranged so that no more than 10 points can be scored for any single item. Items 2 and 3 are considered inversely proportional; therefore, the maximum possible score in this questionnaire will generally be 100.
Statistical analysis
Descriptive analyses were performed to obtain the frequency distributions of the nominal variables, as well as the means and standard error of the numerical variables. Comparative analyses between the variables were performed using the Friedman test to evaluate inter- and intragroup differences in variances. Next, Dunn's test was applied to determine whether differences were statistically significant and whether there was a difference between the three treatment groups. The results were analyzed using the Graph Pad Prism software package, version 4.02, and statistical significance was defined as p < .05.
Results
Clinical and demographic data
Fifty-two patients were enrolled in the study, 16 in Group I (amitriptyline), 17 in Group II (placebo) and 19 in Group III (P. ginseng). Nevertheless, only 38 patients completed the entire 12-week follow-up period (13 in Group I, 13 in Group II and 12 in Group III). Of the 14 patients who failed to complete the study, the three in Group I dropped out in the first week of follow-up (two because of side effects), and one returned in the third week. Of the four patients who discontinued in Group II, three dropped out in the first week complaining of various side effects and one abandoned the study because she moved away. In Group III, seven patients dropped out; three reported various side effects, two dropped out in the third week without giving any reason, and two abandoned the study because of lack of time to attend consultations or difficulties in attending the clinic.
The study sample was composed of women 27-58 years of age (mean 43.2 years) who had had the disease for a mean duration of 43.8 months. Of the 38 patients, 19 (50%) were currently employed, while 18 (47.4%) had no paid employment and one was on sick leave. There were no statistically significant differences between the three groups with respect to their baseline epidemiological or clinical characteristics (Table 1).
Efficacy of P. ginseng using VAS
Pain: Reductions in mean pain scores were found in the group that used P. ginseng (F(5) = 8.8; p < .0001), and Dunn's test showed that these differences were present from the sixth week on when compared to pretreatment scores. There were reductions of 31.7% in mean pain scores at the sixth week (p < .001), of 35.6% at the ninth week and of 39.4% at the 12th week (p < .001) of follow-up compared to baseline values. In the amitriptyline group, reductions were also found in mean pain scores (F(5) = 8.8; p < .001). Post-tests indicated that these reductions occurred from the third week on (p < .05), with reductions in pain of 25.4% at the 3rd week, 30.1% at the 6th week (p < .01), 37.2% at the 9th week (p < .001) and 44.6% at the 12th week of treatment (p < .001). Statistically significant differences also occurred in the group of women who received placebo (F(5) = 8.7; p < .001) at the ninth and twelfth weeks of evaluation, with reductions of 35.7% (p < .01) and 48% (p < .001), respectively (Figure 1). After applying Dunn's test for multiple comparisons of mean improvement in the scores of the three groups, no statistically significant differences were found between the groups (F(2) = .2; p > .05).
Fatigue: There were statistically significant reductions in the mean scores for fatigue in the group that received P. ginseng (F(5) = 8.9; p < .001). Dunn's test indicated statistically significant differences in comparison with pretreatment values at the third week (25.9% of improvement; p < .05), sixth week (24.7%; p < .05); ninth week (39.3%; p < .001) and 12th week of follow-up (46.5%; p < .001). Significant differences were also found in the amitriptyline group (F(5) = 5.9; p < .001), with significant reductions in fatigue recorded at week 6 (30.8%; p < .01), 9 (34.5%; p < .01) and 12 (38.7%; p < .001) compared to baseline values. Statistically significant differences were also found in the group of women who received placebo (F(5) = 16.7; p < .001). Post-tests showed an improvement at the 3rd week (24.2% improvement; p < .01), 6th week (28.3%; p < .001), 9th week (40.3%; p < .001) and 12th week of evaluation (51.5%; p < .001) (Figure 2). After correction for multiple comparisons, there were no statistically significant differences in improvement between the mean scores of the three groups (F(2) = .9; p > .05).
Sleep quality: In evaluations of sleep quality, statistically significant reductions were found in the mean sleep scores of the patients treated with P. ginseng (F(5) = 5.7; p < .001). These differences were also found at weeks 6 (p < .01), 9 (p < .01) and 12 (p < .01), with an improvement in sleep quality of 42%, 44.3% and 44.3%, respectively, when compared to baseline data. In the patients treated with amitriptyline, improvements were found in the mean scores for sleep quality (F(5) = 5.8; p < .001) in the first week of treatment (43.3%; p < .05) and at weeks 3 (45.7%; p < .01), 6 (53.3%; p < .001), 9 (55.9%; p < .001) and 12 (54.6%; p < .001). Improvements in patients who received placebo were also statistically significant (F(5) = 19.9; p < .001), as shown at weeks 1 (21.6% of improvement; p < .05), 3 (29.5%; p < .001), 6 (32.9%; p < .001), 9 (49.9%; p < .001) and 12 (61.3%; p < .001) compared to baseline data (Figure 3). After correction for multiple comparisons, there were no statistically significant differences between the mean improvement scores in the three groups (F(2) = 2.6; p > .05).
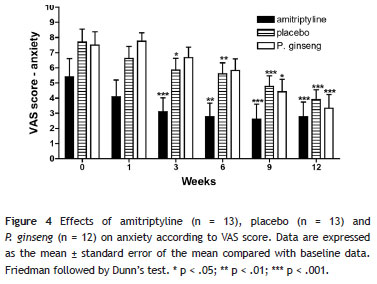
Anxiety: There were reductions in mean anxiety scores in the group of women who received P. ginseng (F(5) = 8.1; p < .001), as shown in the 9th week (44% improvement; p < .05) and in the 12th week of treatment (a 55.6% reduction in anxiety; p < .001). In the patients who were given amitriptyline, significant improvements were found in anxiety (F(5) = 7.0; p < .001), as shown at weeks 3 (43.7% improvement; p < .001), 6 (49.3%; p < .01), 9 (52.0%; p < .001) and 12 (49.3%; p < .001). In the placebo group, differences were also found in mean anxiety scores (F(5) = 11.7; p < .001), with a significant improvement occurring at weeks 3 (23.9%; p < .05), 6 (26.9%; p < .01), 9 (37.97%; p < .001) and 12 (49.0%; p < .001) compared to baseline values (Figure 4). After correction for multiple comparisons between the mean scores of the three groups (at six weeks of the study), there was a statistically significant improvement in anxiety in the amitriptyline group compared to the placebo and P. ginseng groups (F(2) = 5.6; p < .05): amitriptyline versus placebo (difference in means: -2.3; p < .05); amitriptyline versus P. ginseng (-2.5; p < .05) and placebo versus P. ginseng (-.2; p > .05).
Efficacy of P. ginseng using tender point count
A simple tender point count was performed in the three groups, with improvements found in the mean scores of all three groups compared to baseline (Figure 5). In the patients who received P. ginseng, there was a reduction in the number of tender points (F(5) = 14.1; p < .001). These improvements were detected with Dunn's test at weeks 3 (21.1% improvement; p < .01), 6 (31.9%; p < .001), 9 (33.5%; p < .001) and 12 (40.5%; p < .001). Statistically significant improvements also occurred in the group that received amitriptyline (F(5) = 11.1; p < .001), as shown at the 3rd week (33.7% improvement; p < .001), 6th week (30.6%; p < .01), 9th week (37.7%; p < .001) and 12th week (41.7%; p < .001). Statistically significant improvements were also found in the placebo group (F(5) = 12.8; p < .001) at weeks 6 (21.5% improvement; p < .05), 9 (29%; p < .001) and 12 (45%; p < .001). After correction for multiple comparisons of the mean number of tender points between the three groups, there were no statistically significant differences (F(2) = .3; p > .05).
Efficacy of P. ginseng on quality of life using the FIQ
Statistically significant improvements in quality of life were found in all three groups (Figure 6). In the patients using P. ginseng, significant reductions occurred in mean total FIQ scores (F(5) = 6.3; p < .001) at weeks 9 (38.2% improvement; p < .01) and 12 (48.3%; p < .001). In the amitriptyline group, there were statistically significant improvements in mean total FIQ scores (F(5) = 3.0; p < .05) at weeks 9 (39.3%; p < .05) and 12 (40.9%; p < .05). In the patients who received placebo, improvements were found in mean total FIQ scores (F(5) = 3.8; p < .01) at week 12 (38.7%; p < .01). After correction for multiple comparisons of the mean FIQ scores, there were no statistically significant differences between the three groups (F(2) = .3; p > .05).
Discussion
In this randomized, double-blind, 12-week trial, P. ginseng C.A. Meyer was used to treat fibromyalgia. This phytotherapic is commercially available worldwide, and in Oriental medicine, it is primarily indicated for fatigue and weakness. Recently, P. ginseng has become well-known in Europe and in the United States for its effects on multiple organs and systems, particularly in the control of organic homeostasis.23 Nevertheless, although the substance is popularly indicated for the treatment of chronic pain and fatigue, no studies have yet been conducted on the use of P. ginseng for the treatment of fibromyalgia.
The analgesic effects of P. ginseng have yet to be evaluated in clinical studies. Its antinociceptive effects have been demonstrated in previous preclinical studies.17 The inhibitory effects of ginsenosides on substance P-induced pain and the capacity of this substance to inhibit calcium channels in dorsal spinal neurons have been confirmed.18 The effects of P. ginseng on pain (evaluated by the VAS) were not different from the effects achieved with the other therapies used in this study.
Based upon these data, the authors believe that the positive effects on pain obtained with P. ginseng may be associated with one or both of the aforementioned pharmacological effects. The effects of amitriptyline in the patients in the present sample occurred from the 3rd week on, which was expected because this drug has been used successfully at doses of 12.5 to 50 mg/d to treat chronic neuropathic pain and fibromyalgia.8,24
The VAS evaluation of fatigue revealed an improvement in the patients in the study compared to baseline data, beginning in the 3rd week of therapy with P. ginseng; however, there was no difference between the groups.
The present study is the first to evaluate the effects of P. ginseng on fatigue in patients with fibromyalgia. In the group receiving amitriptyline, contrary to its effects on pain, the drug only affected fatigue significantly after six weeks of therapy (i.e., the improvement in fatigue found in the P. ginseng group, although not significantly different from the improvement achieved by the other two groups, occurred earlier than the improvement with the standard drug used for the treatment of this disease). Our data support conclusions reached in a meta-analysis conducted by Häuser et al.4 In this meta-analysis, strong evidence was found against the use of amitriptyline and duloxetine for the treatment of fatigue in fibromyalgia.
There were no differences between the three groups in sleep quality. There are no studies in the literature evaluating the effects of P. ginseng on sleep. It is known that tricyclic antidepressants tend to improve the quality and depth of sleep in patients with fibromyalgia.24 These findings were confirmed in the present study in which an improvement in sleep occurred earlier in the amitriptyline group.
Anxiety was defined prior to application of the instrument as a state of mild uneasiness or apprehension. More specific evaluation methods for anxiety and/or depression, such as the Hospital Anxiety and Depression Scale or the Beck Depression Inventory, were not used. In this study, P. ginseng effectively reduced anxiety by the 9th week of therapy (VAS), whereas an improvement was observed in both the placebo and amitriptyline groups by the 3rd week.
A randomized, controlled, double-blind study was conducted in which the effects of the phytotherapeutic substance Hypericum perfuratum were compared with the effects of amitriptyline for the treatment of fibromyalgia. As evaluated according to the VAS and FIQ, significant improvements occurred in both groups when compared with baseline data.25 In agreement with the findings of the study conducted with H. perfuratum, treatment with P. ginseng resulted in an improvement in anxiety compared to pretreatment values; however, when the three groups were compared (ANOVA on six weeks of the study), amitriptyline was found to be more effective than the other treatments. Contrary to the present study, in the previous clinical trial, there was no placebo group and the Beck Depression Inventory was used.
Another important pain evaluation instrument used to assess patients with fibromyalgia is the tender point count. In the present study, there was a reduction in tender point count compared to baseline values in all three groups. A study similar to the present trial evaluated Chorella pyrenoidosa (green algae) in patients with fibromyalgia and also used the tender point count as one of the pain assessment instruments. These authors reported a significant reduction in the number of tender points in the patients treated with C. pyrenoidosa compared to the placebo group.26
The importance of the tender point count in the diagnosis of fibromyalgia remains a subject of debate in the literature.20 Moreover, the authors agree with Harth and Nelson27 that abandoning the tender point count may mean a return to the diagnostic dilemma prevalent prior to the establishment of the ACR criteria.
The quality of life and functional capacity of patients with fibromyalgia have been evaluated using various questionnaires that allow a more objective evaluation of subjective symptoms such as pain, anxiety, depression and general well-being, among others. Burckhardt et al.21 proposed and tested the use of an instrument for evaluating quality of life that was developed specifically for use in patients with fibromyalgia, the FIQ. According to these authors, the maximum possible score in patients with fibromyalgia is 100, and the mean score found in patients with fibromyalgia is approximately 50. Patients whose quality of life is strongly affected generally score > 70. The patients in the present study had mean baseline scores of 54, 52 and 55 for the amitriptyline, placebo and P. ginseng groups, respectively.
The FIQ was tested and validated in Brazil by Marques et al.22 who considered it valid and reliable for the measurement of the functional capacity and health status of Brazilian fibromyalgia patients. We used this instrument in our study because the questionnaire was specifically designed for use in fibromyalgia and had already been used in various studies of the disease.
Coleman et al.28 performed a meta-analysis of the use of P. ginseng to improve quality of life. They evaluated nine clinical trials, eight of which were placebo-controlled. Of these, the doses of P. ginseng used varied from 80 to 400 mg/d. The different studies ranged from 2 to 9 months in duration and evaluated quality of life in various sample populations: type II diabetes mellitus, menopausal women, patients with memory impairment and healthy volunteers. No significant differences in responses were found between the different doses used, either when P. ginseng was prescribed alone or together with vitamins and/or mineral salts. According to Coleman, the majority of studies of the effect of P. ginseng on quality of life failed to show any improvement in final scores with the use of this plant substance; however, the possibility that improvement in some parameters of quality of life may have been related to its use cannot be discarded.28 Comparisons of these data with those from the present study are difficult because the evaluation instruments used were different and there are no other placebo-controlled clinical trials on the use of P. ginseng in patients with fibromyalgia.
In a study to compare the effects of H. perfuratum with those of amitriptyline for the treatment of fibromyalgia, significant improvements in quality of life compared to baseline were found in patients in both the treatment and control groups.25 In the present study, total FIQ scores improved in the P. ginseng group as well as in the placebo and amitriptyline groups. The percentage of patients who improved with P. ginseng was higher than the percentages in the placebo and amitriptyline groups at the 12th week of treatment, although this difference was not significant.
An important question raised by the authors concerns the therapeutic response obtained in the placebo. In recent years, scientific interest has grown in understanding and determining the true placebo effect, principally in relation to the development of new drugs and/or active components. In 2008, a study published the findings of an evaluation of the positive and negative effects of placebo on the opioid and dopaminergic pathways (receptors D2 and D3) in men and women following a stressful situation, as measured by positron emission tomography. Activation of the opioid and dopaminergic pathways were observed in various areas of the brain, principally in the nucleus accumbens in this study.29
In fibromyalgia, alterations in the pain-inhibiting descending pathways, including the serotoninergic, noradrenergic and possibly dopaminergic and opioid pathways, have been observed.5 Tramadol, an opioid analgesic, has been shown to exert a beneficial effect in patients with fibromyalgia,11 and a D2 and D3 dopamine receptor agonist has shown promising results in the treatment of this condition.30 Likewise, the possibility of the participation of a placebo in these pathways cannot be discarded in view of the therapeutic response observed in this study sample. Therefore, the response observed in the placebo group in the present study may have occurred as a function of various other factors, including the sample size, the relief of symptoms after receiving attention, adequate diagnosis and treatment, a good doctor-patient relationship and the positive expectation of the patients with respect to a new treatment.
Based on the data obtained in this study, it is possible to conclude that the P. ginseng root extract effectively promoted an improvement in pain, fatigue and sleep quality (as evaluated by the VAS) compared to baseline data. It was less effective than amitriptyline in improving patients' anxiety, but it effectively reduced the number of tender points and improved quality of life compared to baseline values. No significant differences were found between this group and the groups using the other two therapeutic interventions.
Although baseline values of several variables were not different, especially in the group that received the P. ginseng, a possible interference of the evolution of symptoms during the study may also be considered. There were no statistically significant differences between the three groups with respect to the baseline epidemiological or clinical characteristics of the patients who concluded the study.
This study represents the first step in the evaluation of the effect of P. ginseng in this group of patients. The extract was well-tolerated; however, further studies need to be carried out with larger sample sizes, with samples that include both male and female patients and with progressive doses to permit a better evaluation of the tolerability and efficacy of this treatment. Following further evaluation, P. ginseng may represent an option for the treatment of acute and/or chronic pain and may constitute a future therapeutic option for patients with fibromyalgia.
Acknowledgments
The authors are grateful to the Laboratório Orient Mix Fitoterápicos do Brasil for supplying the drugs used in this study.
Received on June 29, 2011; accepted on May 19, 2012
- 1. Wolfe F, Smythe HA, Yunus MB, Bennet RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160-72.
- 2. Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain Activity associated with Slow Temporal Summation of C fiber Evoked Pain in Fibromyalgia Patients and Healthy Controls. Eur J Pain. 2008;12:1078-89.
- 3. Rooks DS. Talking to patients with fibromyalgia about physical activity and exercise. Curr Opin Rheumatol. 2008;20:208-12.
- 4. Häuser W, Bernardy K, Üçeyler N, Sommer C. Treatment of fibromyalgia syndrome with antidepressant. A meta-analysis. JAMA. 2009;301:198-209.
- 5. Arnold LM, Hess EV, Hudson JI, Welge JA, Berno SE, Keck PEJR. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am J Med. 2002;112:191-7.
- 6. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;Suppl2:S63-74.
- 7. Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W, Palmer RH. The efficacy and safety of milnacipran for treatment of fibromyalgia. A randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:398-409.
- 8. Carette S, Bell MJ, Reynoulds WJ, Haraqui B, Mccain GA, Bykerk VP, et al. Comparison of amitriptyline, cyclobenzaprine and placebo in the treatment of fibromyalgia. A randomized double-blind clinical trial. Arthritis Rheum. 1994;37:32-40.
- 9. Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336-44.
- 10. Mease PJ, Russell IJ, Arnold LM, Florian H, Young JPJR, Martin SA, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol. 2008;35:502-14.
- 11. Bennet RM, Schein J, kosinski MR, Hewitt DJ, Jordan DM, Rosenthal NR. Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Rheum. 2005;53:519-27.
- 12. Holdcraft LC, Assefi N, Buchwald D. Complementary and alternative medicine in fibromyalgia and related syndromes. Best Pract Res Clin Rheumatol. 2003;17:667-83.
- 13. Kiefer D, Pantuso T. Panax ginseng Am Fam Physician. 2003;68:1539-42.
- 14. Choi S, Kim T, Shin Y, Lee C, Park M, Lee H, Song J. Effects of a polyacetylene from Panax ginseng on Na(+) currents in rat dorsal root ganglion neurons. Brain Res. 2008;29:75-83.
- 15. Kim Y, Kim S, Markelonis G, Oh T. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. Neurosci Res. 1998;54:123.
- 16. Radad K, Gille G, Liu L, Rausch W. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175-86.
- 17. Shin Y, Jung O, Nah J, Nam K, Kim C, Nah S. Ginsenosides that produce differential antinociception in mice. Gen Pharmacol. 1999;32:653-9.
- 18. Rhim H, Kim H, Lee D, Oh T, Nah S. Ginseng and ginsenoside Rg3, a newly identified active ingredient of ginseng, modulate Ca2+ channel currents in rat sensory neurons. Eur J Pharmacol. 2002;436:151-8.
- 19. Ramarao P, Bhargava H. Antagonism of the acute pharmacological actions of morphine by Panax ginseng extract. Gen Pharmacol. 1990;21:877-80.
- 20.Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ. 1994;309:696-9.
- 21. Burckhardt CS, Clarck SR, Bennet RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728-33.
- 22. Marques AP, Santos AMB, Assumpção A, Matsutani LA, Lage LV, Pereira CAB. Validação da versão brasileira do Fibromyalgia impact questionaire (FIQ). Rev Bras Reumatol. 2006;46:24-31.
- 23. Choi KT. Botanical characteristics, pharmacological effects and medicinal components of korean Panax ginseng C.A. Meyer. Acta Pharmacol Sin. 2008;29:1109-18.
- 24. Godfrey RG. A guide to the understanding and use of tricyclic antidepressant in the overall management of fibromyalgia and other chronic pain syndromes. Arch Intern Med. 1996;156:1047-52.
- 25. Kuhara M, Alves A, Feldman D: A 12 week, randomized, controlled trial of Hypericum perfuratum and amitryptiline for the treatment of fibromyalgia. Arthritis Rheum. 2004;50(Suppl):490-1.
- 26. Merchant RE, Carmack CA, Wise CM. Nutritional Supplementation with Chlorella pyrenoidosa for patients with fibromyalgia syndrome: a pilot study. Phytother Res. 2000;14:167-73.
- 27. Harth M, Nelson WR: The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol. 2007;35:915-21.
- 28. Coleman CI, Hebert JH, Reddy P. The effects of Panax ginseng on quality of life. J Clin Pharm Ther. 2003;28:5-15.
- 29. Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220-31.
- 30. Holman A, Myers R. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthitis Rheum. 2005;5:2495-505.
Corresponding author:
Publication Dates
-
Publication in this collection
04 Apr 2013 -
Date of issue
Mar 2013
History
-
Received
29 June 2011 -
Accepted
19 May 2012