Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder, often associated with other psychiatric comorbidities, functional impairments, and poor long-term outcomes. The objective of this selected review is to describe current advances and challenges in the diagnosis and treatment of ADHD. The disorder is associated with neurobiological underpinnings and is highly heterogeneous in various aspects, such as symptom profiles, cognitive impairments, and neurobiological and genetic features. The efficacy and safety of short-term pharmacological treatments across the life cycle is well studied, but further research investigating long-term treatment, impact of treatment in preschoolers, and non-pharmacological interventions is needed. Future research is also needed to better characterize the neurodevelopmental pathways of the disorder, linking clinical and neurobiological information, less investigated populations, and new interventions.
ADHD; heterogeneity; diagnosis; treatment; neurodevelopment
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder that affects approximately 5% of children and adolescents worldwide.11. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942-8. Although symptoms decline with age (65% of the affected individuals present partial remission), only 15% of children with ADHD show full remission in early adulthood, characterizing a chronic disorder.22. Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619-23.,33. Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159-65. Symptoms include age-inappropriate inattentiveness, hyperactivity, and impulsivity.44. Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJ, Jensen PS, Cantwell DP. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429-33. The disorder is highly burdensome, determining significant functional impairments, such as social and family life problems, low educational attainment and school dropout, low self-esteem, impairment in emotional development, occupational problems, and divorce.44. Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJ, Jensen PS, Cantwell DP. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429-33. Furthermore, it is associated with other psychiatric comorbidities, especially oppositional defiant disorder, anxiety disorder and learning disabilities; it also predicts a diversity of negative outcomes, such as future conduct disorder, antisocial behavior, anxiety and mood disorders, substance abuse, and also physical injuries, traffic accidents, premature pregnancy, sexual transmitted diseases, among others.55. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237-48.,66. Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, et al. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69:1295-303.
ADHD is a cause of significant economic costs to society. A recent systematic literature review suggests that the annual incremental costs of the disorder in the U.S. are at least US$ 143 billion.77. Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51:990-1002 e2. Despite that, the disorder is still poorly recognized and treated, and there is a lack of public policies developed to address this condition. In the U.S., less than two-thirds of adolescents with ADHD had ever received some kind of treatment.88. Merikangas KR, He JP, Burstein M, Swendsen J, Avenevoli S, Case B, et al. Service utilization for lifetime mental disorders in U.S. adolescents: results of the National Comorbidity Survey-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2011;50:32-45. This situation is even more dramatic in low- and middle-income countries. For example, in a community sample of two cities in Brazil that ascertained approximately 10,000 children, 43% of children with ADHD had been previously referred to mental health services, but only 23% had access to some kind of treatment (A Graeff-Martins, personal communication).
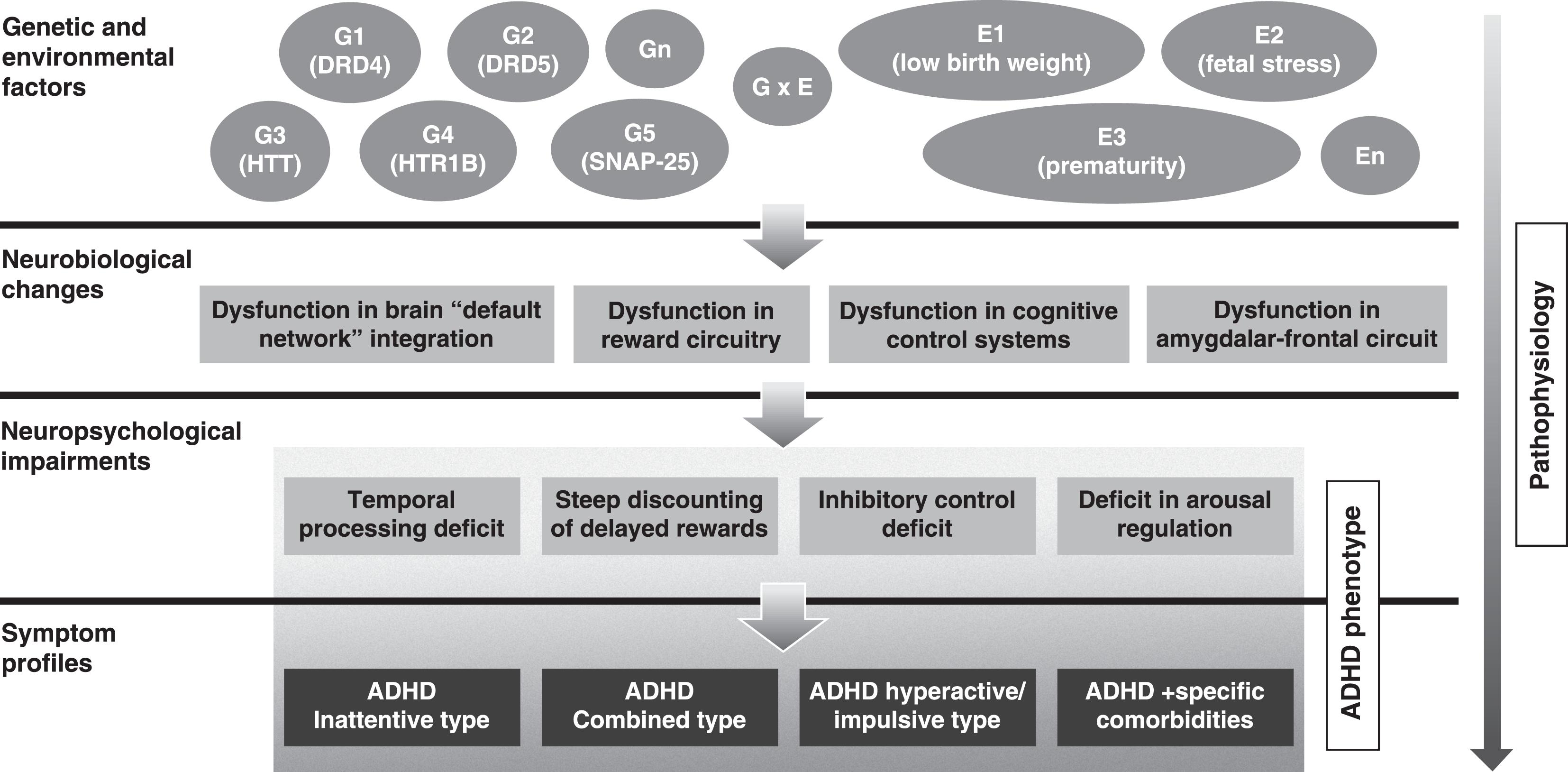
The etiology of ADHD has not been completely elucidated.99. Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013;54:3-16. Studies have identified a robust genetic contribution to the disorder, with pooled heritability rate of 76%.1010. Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215-20. Current data indicate that multiple common genetic variants of small effect - including genes of dopaminergic, noradrenergic, serotoninergic and other systems - and possible rare variants, are implicated in the etiology of ADHD.99. Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013;54:3-16. Environmental variables are also known to play a role in the disease etiology. Low birth weight, prematurity, and intra-utero exposure to tobacco are among the environmental factors most strongly linked to the development of the disorder.99. Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013;54:3-16. It is likely that multiple etiological pathways involving different genetic variants and exposures in complex interaction underlie specific neuropsychological characteristics and clinical symptoms of the disorder (Figure 1). The complexity of the pathways leading to ADHD results in remarkable heterogeneity in many aspects of the disorder, which might be limiting the ability to elucidate the disease etiology.1111. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010 27;6:155-79.
Schematic representation of ADHD pathophysiology. The figure shows the path to ADHD phenotype and the various levels where heterogeneity may occur. Genetic (G1-Gn) and environmental factors (E1-En), and complex gene-environment interactions (GxE) lead to various neurobiological changes, which in turn lead to different neuropsychological impairments and symptom profiles. ADHD = attention-deficit/hyperactivity disorder
Various characteristics of ADHD have been summarized by previous systematic reviews.11. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942-8.,33. Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159-65.,99. Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013;54:3-16.,1212. Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038-55.,1313. Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275-89. The objective of this selected review of the literature is to describe current advances and challenges in the conceptualization, diagnosis, and treatment of the disorder.
Diagnosis
ADHD as a neurodevelopmental disorder
Accumulating evidence supports the notion that ADHD is better understood as a neurodevelopmental disorder characterized by a maturational delay. In the last years, the frequent description of an immature child or adolescent made by parents and teachers has found support in neuroimaging studies.1414. Kieling C, Goncalves RR, Tannock R, Castellanos FX. Neurobiology of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2008;17:285-307, viii. A National Institute of Mental Health longitudinal study provided unique developmental insights into the neurobiology of ADHD. In a landmark report published in 2007, Shaw et al.1515. Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649-54. studied the cortical thickness of 223 patients who had ADHD and 223 typically developing controls. Their results indicated that, rather than a deviation from typical development, ADHD is characterized by a marked delay in reaching peak thickness in many regions and particularly in the prefrontal cortex (median age 10.5 and 7.5 years for the ADHD and control groups, respectively).1515. Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649-54. More recently, the same group extended this finding, suggesting a mirror phenomenon in terms of measures of cortical surface area: among children with ADHD, the median age by which 50% of right prefrontal cortical vertices attained peak area was 14.6 years - in comparison to 12.7 years in the healthy control group.1616. Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72:191-7.
The developmental aspect of ADHD must be taken into account when characterizing clinical presentation and elaborating new diagnostic criteria. The diagnosis of ADHD is established clinically, based on criteria defined by diagnostic classification systems such as the DSM and ICD. Similar to other psychiatric conditions, there is no ancillary test with sufficient predictive power for the diagnosis of ADHD. Core features of the disorder are developmentally inappropriate symptoms of inattention, hyperactivity, and impulsivity (Table 1). Notwithstanding the emphasis regarding the need for a developmental perspective in the assessment of ADHD, limited evidence-based data is available on specific manifestations of the disorder at each period of life.
The validity of ADHD among preschoolers has been an area of particular controversy in the literature. Although there is increasing evidence that ADHD constitutes a valid diagnosis before the age of 6,1717. Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-22. challenges in the diagnostic procedures include, for example, the impossibility of observation in multiple settings for those children not attending preschool - and subsequent lack of information about pervasiveness. Several studies have shown that currently available criteria reliably identify ADHD in children as young as 2 years old and that these individuals have clinically significant impairment across all relationships and settings.1818. Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: continuity from ages 3 to 6. Am J Psychiatry. 2012;169:1157-64.
Research has also documented the validity of ADHD diagnosis among older adolescents and young adults. Despite the observed age-dependent decline in ADHD symptoms, a substantial proportion of individuals continue to present clinically relevant symptoms as they enter into adulthood. Reduction of hyperactive/impulsive symptoms is more significant than that of inattentive symptoms (remission in 70 vs. 40% of individuals, respectively).1919. Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816-8. Among the challenges in characterizing ADHD in older individuals, there is the inappropriateness of the description of symptoms (especially hyperactivity/impulsivity symptoms) to capture developmental specific clinical manifestation, and difficulties in assessing retrospectively the presence of symptoms in childhood.2020. Matte B, Rohde LA, Grevet EH. ADHD in adults: a concept in evolution. Atten Defic Hyperact Disord. 2012;4:53-62. In addition, the clinical picture in adults might be specifically characterized by symptoms related to executive dysfunctions and emotional impulsivity. More importantly, adults might present substantial impairment even with lower number of symptoms in any of the two dimensions (inattention and/or hyperactivity/impulsivity). Thus, a lower symptom threshold for the diagnosis in adults was proposed for the new version of the DSM - DSM-5.2020. Matte B, Rohde LA, Grevet EH. ADHD in adults: a concept in evolution. Atten Defic Hyperact Disord. 2012;4:53-62.
Other challenges in ADHD diagnosis
In addition to frequent developmentally inappropriate symptoms (at least 6/9 in at least one of the dimensions), there are other requisites for the diagnosis of ADHD according to the DSM-IV-TR. One of the most fragile criteria for the diagnosis of ADHD is the requirement of symptoms causing impairment before the age of 7 years (criterion B). This criterion has been retained in DSM editions despite the lack of empirical support, and a systematic review of the literature found 31 studies questioning its validity, especially to older individuals, for whom there can be problems in terms of retrospective recall.2121. Kieling C, Kieling RR, Rohde LA, Frick PJ, Moffitt T, Nigg JT, et al. The age at onset of attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:14-6. Applying this evidence-based approach, the forthcoming DSM-5 will reformulate the criterion, expanding the maximum age at onset to 12 years. This reaffirms ADHD as a disorder of childhood onset, both minimizing false negatives and causing minimal increase in prospectively assessed prevalence rates.2222. Polanczyk G, Caspi A, Houts R, Kollins SH, Rohde LA, Moffitt TE. Implications of extending the ADHD age-of-onset criterion to age 12: results from a prospectively studied birth cohort. J Am Acad Child Adolesc Psychiatry. 2010;49:210-16.
The requirement of some impairment from the symptoms in two or more settings (criterion C) is intended to avoid including situational-specific problems (e.g., only at home). Interestingly, the low concordance between parental and teacher reports (even when focusing on symptoms at school) suggests that, to capture pervasiveness, information should be gathered with both sources.2323. Sayal K, Goodman R. Do parental reports of child hyperkinetic disorder symptoms at school predict teacher ratings? Eur Child Adolesc Psychiatry. 2009;18:336-44. The diagnosis of ADHD is currently excluded in the presence of disorders such as schizophrenia (criterion E). Another change announced for the DSM-5 is the removal of the impossibility of diagnosing ADHD and autism spectrum disorders, as there is sufficient evidence to justify the comorbid diagnosis.2424. Rohde LA. Is there a need to reformulate attention deficit hyperactivity disorder criteria in future nosologic classifications? Child Adolesc Psychiatr Clin N Am. 2008;17:405-20, x.
Data converge to confirm that ADHD is a disorder with developmental features that are associated with neurobiological underpinnings. Future research - preferably using longitudinal designs - is needed to better characterize the neurodevelopmental pathways of the disorder, linking both clinical and neurobiological information.
ADHD as a heterogeneous disorder
It is noteworthy that children with ADHD vary significantly from each other. ADHD, as other psychiatric disorders, is a highly heterogeneous disorder in respect to various aspects, such as symptom profiles, neuropsychological profiles, and neurobiological and genetic features (Figure 1).
One aspect of ADHD heterogeneity is related to its clinical presentation. Diagnosis of mental disorders, according to diagnostic manuals, may be assigned from different combinations of criteria listed under the same disorder.2525. American Psychiatric Association. Diagnostic and statistical manual of mental disorders - DSM-IV-TR¯. 4th ed. Arlington: American Psychiatric Publishing; 2000. In the case of ADHD, six symptoms are required for an individual to meet diagnostic criteria. Because the criteria are subdivided into symptom domains (inattention and hyperactivity/impulsivity), it is possible that two individuals diagnosed with ADHD do not have the same group of symptoms. The classification of ADHD diagnosis into types (predominantly inattentive, hyperactive-impulsive, and combined types) is an attempt to deal with the heterogeneity of clinical presentations. Even so, two individuals with identical ADHD subtypes might be similar in as few as three symptoms. In a community sample of 189 individuals with ADHD, a total of 173 combinations of symptomatic profiles were found. Furthermore, it was detected a median agreement between symptoms of 0.61, with 30% of the sample showing an agreement lower than half of the symptoms (G Salum, personal communication). This indicates the limited ability of the current clinical diagnostic criteria in defining homogeneous populations, which may be one reason why the field has not yet been successful in finding biological markers of ADHD.1111. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010 27;6:155-79. Conversely, a recent comprehensive meta-analyses did not detect significant differences in several assessed areas comparing subjects with different ADHD types (main comparisons between predominantly inattentive and combined types), arguing that these phenotype differences might not be so relevant.2626. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490-9. Based on these findings and on the low developmental stability of ADHD types, the ADHD working group for the DSM-5 recently proposed the downgrade of the types to current presentation.
Another facet of ADHD heterogeneity is neuropsychological heterogeneity. ADHD has been shown to be associated with various neuropsychological impairments. Studies have found that, on average, individuals with ADHD, compared to controls, have worse performance in executive functions, such as: inhibition, working memory, memory span, processing speed, arousal, temporal information processing, response variability; and have also impairments in motivational processes.27. Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336-46. 28. Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183-213. 29. Nikolas MA, Nigg JT. Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology. 2013;27:107-20. 30. Toplak ME, Rucklidge JJ, Hetherington R, John SC, Tannock R. Time perception deficits in attention-deficit/ hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. J Child Psychol Psychiatry. 2003;44:888-903. 31. Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Inhibitory deficits in attention-deficit/hyperactivity disorder are independent of basic processing efficiency and IQ. J Neural Transm. 2008;115:261-8. 32. Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, Alderson RM. Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): a ubiquitous core symptom or manifestation of working memory deficits? J Abnorm Child Psychol. 2009;37:521-34. 27-3333. Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry 2006;67:21-6. However, the findings of neuropsychological impairments are only of moderate effect sizes and not all individuals with the disorder have these dysfunctions.2727. Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336-46.,3434. Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57:1224-30. Sonuga-Barke et al.3535. Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345-55. evaluated three neuropsychological domains: inhibitory control, delay aversion, and temporal processing, and found that ADHD children had poorer performance on all domains. However, only 71% of the individuals displayed some deficit, and 70% of those showed just one dysfunction.3535. Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345-55. The results suggest that the three domains are independent components of ADHD and support a triple pathway model for ADHD. Fair et al.3636. Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109:6769-74. recently conducted another study illustrating the neuropsychological heterogeneity in ADHD. They identified distinct neuropsychological subgroups within ADHD children and also within typically developing children.3636. Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109:6769-74. The findings support that ADHD heterogeneity, as well as heterogeneity in typical population, must be considered when trying to elucidate ADHD etiology.
Taking into account heterogeneity in neuropsychological and symptom profiles, theoretical models of ADHD have proposed that dysfunctions in multiple pathways may be involved in the disorder, leading to specific impairments.3535. Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345-55.,3737. Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785-806.,3838. Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231-8. Nigg & Casey3737. Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785-806. proposed that ADHD may arise from: a) dysfunctions in brain cognitive control systems, b) over-reactivity in nucleus accumbens circuit, or c) under-reactivity in amygdalar-frontal circuit; each leading to related but distinct etiological phenotypes, which are, respectively: a) primary inattentive, disorganized, and ineffective behaviors; b) impulsive, overactive behaviors; and c) impulsive conduct problems, antisociality. Sonuga-Barke, who had previously proposed a dual pathway model (i.e. delay aversion and executive dysfunction pathways),3838. Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231-8.,3939. Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29-36. recently suggested that three dissociable pathways might be involved in ADHD etiology.3535. Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345-55. The neuroimaging literature has shown that ADHD is related to alterations in several brain systems, including systems involved in attention, cognitive control, emotional, sensorimotor, and reward processes.1212. Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038-55.,). Most of those studies have focused on single process hypotheses and have not addressed the multiple pathways model. Further evaluation of how distinct neuropsychological or behavioral phenotypes relate to differential neural pathways may be particularly relevant to elucidate heterogeneity of ADHD neural substrates.
ADHD heterogeneity is also evident at the genetic level. Genome-wide association studies are inconclusive, but candidate genes studies have found evidence for the association of various genes with elevated risk for ADHD.4848. Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, et al. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:884-97.,4949. Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51-90. This may suggest that each genetic marker accounts for only a small proportion of heritability, indicating that ADHD arises from a complex interaction of genetic susceptibility and environmental conditions.5050. Nigg J, Nikolas M, Burt SA. Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:863-73.,5151. Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33:159-80. Numerous possibilities of gene combinations added to countless possible interactions with environmental conditions generate pronounced heterogeneity from the genetic point of view.
Therefore, there is compelling evidence that the heterogeneity of ADHD might be one reason for the diversity of findings and lack of replication. It has become crucial that research concerning all aspects of ADHD, such as neurobiology, diagnosis, and treatment, take heterogeneity into account and employ new approaches to deal with this issue.
Treatment
Efficacy of interventions
The recommendations for ADHD treatment indicate multimodal approaches including pharmacotherapy and psychosocial interventions as the most effective. First-line medications for the treatment of ADHD are stimulants (methylphenidate, mixed amphetamine salts, and amphetamine derivatives - lisdexamfetamine and dextroamphetamine). Second-line medications are atomoxetine, tricyclic antidepressants, bupropion, and alpha-agonists.
Meta-analyses and systematic reviews show moderate to large effect sizes of stimulants in short-term reduction of symptoms.52. Van der Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clin Psychol Rev. 2008;28:783-800. 53. Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19:353-64. 54. Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165:1475-88. 55. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4. 52-5656. Klassen A, Miller A, Raina P, Lee SK, Olsen L. Attention-deficit hyperactivity disorder in children and youth: a quantitative systematic review of the efficacy of different management strategies. Can J Psychiatry. 1999;44:1007-16. Most of the studies comparing short and long-acting methylphenidate compounds found no significant differences in effect sizes.5555. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4.,5757. Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T. 2009;34:678-94. However, a meta-analysis looking at medications for adults with ADHD found that the clinical response for short-acting stimulants was greater than for long-acting stimulants.5858. Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl). 2008;197:1-11.
Non-stimulants are considered second-line medications in cases of treatment failure, intolerance or contraindication to stimulants and may be an option in specific cases of comorbidity.5959. Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921. There is good level of evidence provided by meta-analysis of efficacy for atomoxetine.5757. Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T. 2009;34:678-94.,6060. Cheng JY, Chen RY, Ko JS, Ng EM. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology (Berl). 2007;194:197-209.,6161. Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, Wyk GW. Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis. J Atten Disord. 2011;15:674-83. Extended-release clonidine62. Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:1551-9. 63. Kollins SH, Jain R, Brams M, Segal S, Findling RL, Wigal SB, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127:e1406-13. 62-6464. Jain R, Segal S, Kollins SH, Khayrallah M. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:171-179. and extended-release guanfacine6565. Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-84.,6666. Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155-65. are also efficacious options for the treatment of ADHD, alone or in combination with stimulants. Other pharmacologic alternatives evaluated for the treatment of ADHD are modafinil, bupropion,5757. Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T. 2009;34:678-94. and tricyclic antidepressants,6767. Spencer T, Biederman J, Wilens T, Harding M, O'Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry 1996;35:409-32. but all of them have showed weaker evidence of efficacy.
Behavioral therapy for ADHD aims to modify the physical and social environment in order to modify behavior. For preschoolers, parent training is the recommended behavioral intervention. This modality of treatment aims to help parents stop inefficacious patterns of interaction with their child by reinforcing child's prosocial behavior and to extinguish unwanted behaviors.6868. Lee PC, Niew WI, Yang HJ, Chen VC, Lin KC. A meta-analysis of behavioral parent training for children with attention deficit hyperactivity disorder. Res Dev Disabil. 2012;33:2040-49.,6969. Antshel KM, Barkley R. Psychosocial interventions in attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2008;17:421-37, x.
A recent meta-analysis examined 40 studies addressing the efficacy of behavior parent training for parents of children of ADHD.6868. Lee PC, Niew WI, Yang HJ, Chen VC, Lin KC. A meta-analysis of behavioral parent training for children with attention deficit hyperactivity disorder. Res Dev Disabil. 2012;33:2040-49. A moderate effect size was found regarding the improvement of child behavior and parenting behavior (both measured objectively), and parental perception of parenting. However, these results were observed only immediately after treatment. In a long term (up to 3 years), results remained significant, but with lower magnitude (small effect size). Improvement was more limited for children with associated oppositional defiant disorder or other behavioral problems. Another meta-analysis summarized randomized controlled trials that assessed the efficacy of the following non-pharmacological treatment modalities: restricted elimination diets, artificial food color exclusions, free fatty acid supplementation, cognitive training, neurofeedback, and behavioral interventions.1313. Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275-89. Given the restriction to high-quality trials, six to 15 studies were included for each modality. Authors conducted two sets of analysis: the first considered ADHD assessment by raters closest to the therapeutic setting, and the second considered only probably blinded assessment of the outcomes by the individual studies. According to the first set of analysis, all six modalities of intervention produced significant effects. According to the second set of analysis, only free fatty acid supplementation and artificial food color exclusion produced significant effects, and the effects of the other four modalities were non-significant.1313. Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275-89.
Long-term efficacy and effectiveness
The short-term efficacy of ADHD treatment is well documented, but fewer studies, with variable results, have evaluated long-term efficacy and effectiveness. Poor adherence and difficulties in retention during follow-up are probably some of the reasons for this gap in the literature. However, since ADHD is a lifelong condition, it is fundamental to determine the long-term outcomes of the different treatment modalities. The Multimodal Treatment Study of Children with ADHD (MTA)7070. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073-86. is a large trial that compared four treatment modalities (behavioral intervention, medication, combined treatment, or routine community) in respect to several outcomes during 14 months (controlled phase) and the subsequent 8 years (open phase). Results indicated that, after 14 months of follow-up, medication alone or combined with behavior intervention had better results on improving ADHD and oppositional defiant disorder symptoms, compared to behavior intervention and community care.7070. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073-86. Combined behavioral intervention and medication was no better than medication management, but allowed the use of lower stimulant doses. Secondary analysis looked at rates of success defined by a cutoff on the SNAP-IV score at the end of the treatment.7171. Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168-79. Results from the secondary analysis found increased success rates for combined treatment and medication management and confirmed the initial results.7171. Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168-79.
An 8-year follow-up of MTA children, however, did not find differences between the four treatment groups, and children with ADHD combined subtype showed poorer functioning than non-ADHD children, despite the improved outcomes compared with baseline.7272. Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484-500. These results suggest that an initial period of randomly assigned treatment does not change the disorder trajectory. Conclusions from these results are widely discussed. It is possible that: a) treatment was not effective in the long-term; b) medication and behavioral interventions are equally effective; or c) community care is effective in the long-term, and intensive medication management or intensive behavioral therapy improve its effectiveness, but the benefit weakens once the controlled treatment stops.7373. Banaschewski T, Buitelaar J, Coghill DR, Sergeant JA, Sonuga-Barke E, Zuddas A, et al. The MTA at 8. J Am Acad Child Adolesc Psychiatry. 2009;48:1120-1; author reply 3-4. It is also argued that the naturalistic design of the follow-up after 1 year (children returned to community care) may have influenced the results.7474. Pliszka SR. The MTA at 8. J Am Acad Child Adolesc Psychiatry. 2009;48:1122; author reply 3-4. In conclusion, long-term controlled studies are needed in order to better understand the continuing effects of different modalities of treatment on symptoms and functioning of individuals with ADHD, and also to explore potential moderators of long-term outcomes.
A recent systematic review summarized findings from studies assessing long-term outcomes of treatment vs. non-treatment of ADHD.7575. Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99. It was found that ADHD individuals left untreated had poorer long-term outcomes compared to treated individuals in nine major categories, but treatment did not result in normalization. Benefits were more prominent in driving, obesity, self-esteem, social function, academic, and drug use/addictive behavior outcomes. Although the superiority of specific treatment modalities still needs to be further studied, it appears that in general ADHD treatment improves long-term outcomes. However, the field still demands studies to clarify the association between shot-term and long-term effects.
Functional outcomes
Another challenge for ADHD treatment is determining functional outcomes of treatment (e.g., academic and occupational outcomes). Although few studies have investigated this topic, there is evidence of positive impact on specific outcomes. In regard to academic outcomes, Hechtman et al.7676. Hechtman L, Abikoff H, Klein RG, Weiss G, Respitz C, Kouri J, et al. Academic achievement and emotional status of children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43:812-9. found improvement of school performance and homework behaviors in children with ADHD treated with medication associated or not with other treatment modalities (psychosocial treatment or academic intervention). A recent review by Prasad et al.7777. Prasad V, Brogan E, Mulvaney C, Grainge M, Stanton W, Sayal K. How effective are drug treatments for children with ADHD at improving on-task behaviour and academic achievement in the school classroom? A systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2013;22:203-16. examined children's on-task behavior and academic performance and found that drug treatment improved children's time spent on task and amount of schoolwork they completed. Scheffler et al.7878. Scheffler RM, Brown TT, Fulton BD, Hinshaw SP, Levine P, Stone S. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123:1273-9. also found that medicated children with ADHD had higher mathematics and reading scores. In regard to criminality, during periods under treatment with medication, individuals with ADHD presented lower criminality rates (measured as any conviction for a crime) compared to non-medicated periods.7979. Lichtenstein P, Halldner L, Zetterqvist J, Sjolander A, Serlachius E, Fazel S, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367:2006-14. Brook et al.8080. Brook JS, Brook DW, Zhang C, Seltzer N, Finch SJ. Adolescent ADHD and adult physical and mental health, work performance, and financial stress. Pediatrics. 2013;131:5-13. recently studied the relationship between ADHD in adolescence and several outcomes in adulthood, and found that adolescents with ADHD are more likely to have impaired physical and mental health, antisocial personality disorder, impaired work performance, and high financial stress in adulthood. Raman et al.8181. Raman SR, Marshall SW, Haynes K, Gaynes BN, Naftel AJ, Sturmer T. Stimulant treatment and injury among children with attention deficit hyperactivity disorder: an application of the self-controlled case series study design. Inj Prev. 2013;19:164-70. documented that the treatment with stimulants decrease the risk for injuries in children (see slide attached).
In regard to substance use, there is no evidence that medication treatment increases the chance of developing dependence.7575. Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99. Results are conflicting with respect to the protective potential of treatment. Biederman et al.8282. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. found a reduced risk for substance use disorders in medicated individuals with ADHD after 4 years. This was consistent with an earlier meta-analysis.8383. Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179-85. However, a 36-month follow-up of the MTA study8484. Molina BS, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007;46:1028-40. and a 10-year follow-up study of a clinical sample8585. Biederman J, Monuteaux MC, Spencer T, Wilens TE, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165:597-603. found no association between ADHD treatment and rates of substance use. Additional studies assessing large samples and following up participants into adolescence and/or adulthood are necessary to determine whether treatment has a protective effect on substance use rates and development of dependence.
Treatment of preschool children
Treatment plan for preschoolers must take into account the intensity of symptoms, pervasiveness, but also the velocity and intensity of changes that occur in the brain during this phase. There is scarce data about efficacy and safety of stimulants for this age group, and most of the studies are restricted to methylphenidate.8686. Ghuman JK, Arnold LE, Anthony BJ. Psychopharmacological and other treatments in preschool children with attention-deficit/hyperactivity disorder: current evidence and practice. J Child Adolesc Psychopharmacol. 2008;18:413-47. The largest randomized controlled trial conducted for this age period is the Preschool ADHD Treatment Study (PATS),8787. Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284-93. which included 165 children aged 3 to 5.5 years in the medication phase. The inclusion criteria for this study were very rigorous: only children with moderate to severe ADHD-related impairment for at least 9 months were included; it was also required a level below 55 on the Children's Global Assessment of Functioning Scale; and a cross-site panel of clinicians had to agree about the inclusion and exclusion criteria for the enrollment of each participant. Furthermore, all participants underwent a Parent Training phase and only participants with continued impairment were allowed to begin medication.8888. Kollins S, Greenhill L, Swanson J, Wigal S, Abikoff H, McCracken J, et al. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS). J Am Acad Child Adolesc Psychiatry. 2006;45:1275-83. Therefore, children who participated in the study, especially in the medication phase, exhibited severe ADHD, which may explain the high rates of comorbidities they presented.8787. Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284-93. The mean optimal daily dose of methylphenidate (0.7±0.4 mg/kg/day) was lower than the dose recommended for school age children.8787. Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284-93. There were significant reductions in ADHD symptoms, but the effect sizes (0.4-0.8) were smaller than the ones found for school age children. Despite the relative good tolerability of methylphenidate, adverse effects were more frequent.8686. Ghuman JK, Arnold LE, Anthony BJ. Psychopharmacological and other treatments in preschool children with attention-deficit/hyperactivity disorder: current evidence and practice. J Child Adolesc Psychopharmacol. 2008;18:413-47. The most common described adverse effects are decreased appetite, stomachache, sleep difficulties, social withdrawal, lethargy, dysphoria, crying, whining, and irritability.
In respect to non-stimulant treatment, there is a randomized controlled trial of atomoxetine in 5- and 6-year old children with ADHD, demonstrating significant improvement in symptom scores compared to placebo.8989. Kratochvil CJ, Vaughan BS, Stoner JA, Daughton JM, Lubberstedt BD, Murray DW, et al. A double-blind, placebo-controlled study of atomoxetine in young children with ADHD. Pediatrics. 2011;127:e862-8. One-third to one-fourth of subjects reported adverse events, which included weight loss, decreased appetite, sedation, and gastrointestinal discomfort, but they were not related to treatment discontinuation.
Group-based parent training is a psychosocial intervention tested for preschool ADHD. Sonuga-Barke et al.9090. Sonuga-Barke EJ, Daley D, Thompson M, Laver-Bradbury C, Weeks A. Parent-based therapies for preschool attention-deficit/hyperactivity disorder: a randomized, controlled trial with a community sample. J Am Acad Child Adolesc Psychiatry. 2001;40:402-8. performed a controlled trial with 3-year-old children with ADHD, who were randomized to parent training, parent counseling and support, or a waiting list group. They found that parent training significantly improved ADHD symptoms (in clinical and direct observation measures) and mother's sense of well-being, and 53% of children in the parent training group met criteria for recovery by the end of the trial. The beneficial effects of parent training were still present 15 weeks after treatment. Another study by Jones et al.9191. Jones K, Daley D, Hutchings J, Bywater T, Eames C. Efficacy of the Incredible Years Programme as an early intervention for children with conduct problems and ADHD: long-term follow-up. Child Care Health Dev. 2008;34:380-90. evaluated the effectiveness of parent training for reducing ADHD symptoms in preschool children with ADHD symptoms (not a formal ADHD diagnosis) and conduct problems, and found similar results. Parent training was associated with significant greater reduction in ADHD symptoms, and 52% of children in the parent training group presented symptom scores below clinical threshold by the end of the intervention (against 21% for the waiting list control group). However, Barkley et al.9292. Barkley RA, Shelton TL, Crosswait C, Moorehouse M, Fletcher K, Barrett S, et al. Multi-method psycho-educational intervention for preschool children with disruptive behavior: preliminary results at post-treatment. J Child Psychol Psychiatry. 2000;41:319-32. found no effect of parent training on ADHD symptoms in kindergarteners, which may be explained by the low adherence to treatment, inclusion of children with symptom scores above a cutoff in a dimensional rating scale but without a formal diagnosis of ADHD, lack of impaired functioning indicated by parents and teachers, and the training format (delivered in a didactic format). Evidence for the effectiveness of parent treatment for improving ADHD symptoms in preschoolers is still limited and additional controlled trials with children formally diagnosed with ADHD are needed.8686. Ghuman JK, Arnold LE, Anthony BJ. Psychopharmacological and other treatments in preschool children with attention-deficit/hyperactivity disorder: current evidence and practice. J Child Adolesc Psychopharmacol. 2008;18:413-47.
Clinical guidelines indicate psychosocial intervention as first-line treatment for preschool children with ADHD. The guidelines by the American Academy of Pediatrics,1717. Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-22. the Preschool Psychopharmacology Working Group,9393. Gleason MM, Egger HL, Emslie GJ, Greenhill LL, Kowatch RA, Lieberman AF, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46:1532-72. and the NICE Guideline9494. Atkinson M, Hollis C. NICE guideline: attention deficit hyperactivity disorder. Arch Dis Child Educ Pract Ed. 2010;95:24-7. recommend specifically group-based parent training, reserving methylphenidate for the more impaired cases or for those children who do not benefit significantly from behavior treatment.1717. Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-22.,8686. Ghuman JK, Arnold LE, Anthony BJ. Psychopharmacological and other treatments in preschool children with attention-deficit/hyperactivity disorder: current evidence and practice. J Child Adolesc Psychopharmacol. 2008;18:413-47.,9393. Gleason MM, Egger HL, Emslie GJ, Greenhill LL, Kowatch RA, Lieberman AF, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46:1532-72.,9494. Atkinson M, Hollis C. NICE guideline: attention deficit hyperactivity disorder. Arch Dis Child Educ Pract Ed. 2010;95:24-7. Future studies should assess the efficacy and safety of other stimulants, of stimulants compared to parent training, and their short- and long-term effects in this age range, as well as long-term effects of psychosocial interventions.
Treatment of adults
Literature about the treatment of adults with ADHD is not as extensive as for school-age children, but a considerable amount of data is already available. Pooled estimation from meta-analysis shows that stimulants decrease adults' ADHD symptoms in short-term bases with a medium to large effect size. When stimulants as a group are compared to placebo, the effect size found is 0.67.9595. Meszaros A, Czobor P, Balint S, Komlosi S, Simon V, Bitter I. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-47. Other meta-analyses found effect sizes of 0.9 for methylphenidate,9696. Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2004;24:24-9. 0.73 for mixed amphetamine salts,9797. Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;CD007813. 0.8 for lisdexamfetamine (extracted from a single study),9797. Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;CD007813. and 0.6 for dextroamphetamine.9797. Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;CD007813. Non-stimulants, when considered as a group, seem to be inferior to stimulants, with pooled effect size of 0.395353. Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19:353-64. and 0.59.9595. Meszaros A, Czobor P, Balint S, Komlosi S, Simon V, Bitter I. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-47. Meta-analytic data also showed bupropion to be superior to placebo but less effective than stimulants.5858. Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl). 2008;197:1-11.,9898. Maneeton N, Maneeton B, Srisurapanont M, Martin SD. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65:611-7.,9999. Verbeeck W, Tuinier S, Bekkering GE. Antidepressants in the treatment of adult attention-deficit hyperactivity disorder: a systematic review. Adv Ther. 2009;26:170-84.
Other non-stimulants were assessed by clinical trials with no meta-analysis yet available. The superiority of atomoxetine to placebo has been demonstrated in a pooled post-hoc estimation combining data from six double-blind trials100100. Adler LA, Wilens T, Zhang S, Dittmann RW, D'Souza DN, Schuh L, et al. Atomoxetine treatment outcomes in adolescents and young adults with attention-deficit/hyperactivity disorder: results from a post hoc, pooled analysis. Clin Ther. 2012;34:363-73. and also by additional more recent clinical trials.101. Adler LA, Liebowitz M, Kronenberger W, Qiao M, Rubin R, Hollandbeck M, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26:212-21. 102. Adler LA, Spencer T, Brown TE, Holdnack J, Saylor K, Schuh K, et al. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29:44-50. 101-103103. Durell TM, Adler LA, Williams DW, Deldar A, McGough JJ, Glaser PE, et al. Atomoxetine treatment of attention-deficit/hyperactivity disorder in young adults with assessment of functional outcomes: a randomized, double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol. 2013;33:45-54. Modafinil was not superior to placebo in a well conducted clinical trial that included more than 300 patients.104104. Arnold VK, Feifel D, Earl CQ, Yang R, Adler LA. A 9-week, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study to evaluate the efficacy and safety of modafinil as treatment for adults with ADHD. J Atten Disord. 2012 May 22. [Epub ahead of print] Limited evidence is available for alpha-2-agonists for adults.105105. Strange BC. Once-daily treatment of ADHD with guanfacine: patient implications. Neuropsychiatr Dis Treat. 2008;4:499-506. A randomized, double blind, placebo-controlled trial compared the efficacy of guanfacine with dextroamphetamine for the treatment of ADHD in adults.106106. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21:223-8. The study found that guanfacine was similar to dextroamphetamine in reducing ADHD symptoms, compared to placebo, but only guanfacine improved performance on the Color-Word subtest of Stroop, indicating an enhancement of focused attention by guanfacine. A nicotinic agonist has also been studied for treating adult ADHD.107107. Bain EE, Robieson W, Pritchett Y, Garimella T, Abi-Saab W, Apostol G, et al. A randomized, double-blind, placebo-controlled phase 2 study of alpha4beta2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology. 2013;38:405-13. A randomized, double-blinded, placebo-controlled trial, with a crossover design, selected adults with ADHD to receive placebo and ABT-894, α4β2 (a subtype of neuronal nicotinic receptors) agonist, or atomoxetine.107107. Bain EE, Robieson W, Pritchett Y, Garimella T, Abi-Saab W, Apostol G, et al. A randomized, double-blind, placebo-controlled phase 2 study of alpha4beta2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology. 2013;38:405-13. ABT-894 4 mg twice-daily was comparable to atomoxetine and superior to placebo in reducing ADHD symptoms, but additional studies, with larger sample sizes, greater power, and a parallel design (to avoid results susceptibility to carryover effects) are need to better evaluate the safety and efficacy of this drug.
Psychosocial interventions for adults do not appear to be effective as sole treatment, but evidence shows that they may be effective as adjunctive treatment to psychopharmacological therapy for individuals with residual symptoms.108108. Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugue M, Carpentier PJ, et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67. The best evidence comes from a trial conducted by Safren et al.109109. Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-80. The study enrolled 86 adults with ADHD who were already being prescribed medication and randomized them to cognitive behavioral therapy or relaxation with attentional support. Cognitive behavioral therapy was associated with greater improvement in ADHD symptoms - with improved scores in the ADHD-rating scale, Clinical Global Impression severity, and self-report ADHD scale -, and the benefits were maintained after 12 months of follow-up.
As children and adolescents mature, further studies should be conducted to assess the continuous efficacy of treatment in adulthood, as well as for newly diagnosed individuals. Additionally, new compounds with already available preliminary data should be further tested.
Adverse events
Pharmacological treatment of ADHD may be associated with a range of adverse effects. Adverse effects are mostly mild and/or transitory, and although serious adverse events are rare, they are a subject of concern and debates.110110. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22:213-37. The European ADHD Guidelines Group (EAGG) recently reviewed the most common adverse effects and proposed recommendations for clinical management.111111. Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227-46. The most common adverse effects associated with stimulants are reduction of appetite, sleep disturbance, tics, seizures, and psychotic symptoms. The impact of stimulants on growth and its cardiovascular risks prompted significant concerns among clinicians and authorities and debate in the media.
Growth delay is likely caused by inappropriate nutrition due to loss of appetite.112112. Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994-1009. A review found deficit in height associated with stimulant use, but also found that the deficit tends to attenuate over time.112112. Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994-1009. Some studies also found that growth delay is dose-dependent and can be compensated within 2 years after discontinuing treatment.112112. Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994-1009. Recommendations from the EAGG to manage growth deficit involve: managing loss of appetite (high-caloric snacks, medication after meals, late evening meals); monitoring appetite, weight, height and body mass index regularly; alternative options (i.e., medication holidays, pausing medication on weekends); and referring to a growth specialist if necessary.111111. Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227-46. Table 2 displays clinical recommendations for managing appetite loss and growth delay in children treated with ADHD medications.
Management of loss of appetite and growth delay during treatment with ADHD medications111111. Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227-46.
Because stimulants can potentially increase blood pressure and heart rate, a lot has been speculated about the risk of serious cardiac events of stimulant users. Nevertheless, there is evidence that stimulants are not associated with changes in electrocardiographic parameters or with serious cardiovascular events (meaning sudden cardiac death, acute myocardial infarction and stroke).113. Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50:978-90. 114. Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896-904. 113-115115. Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306:2673-83. Habel et al.115115. Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306:2673-83. conducted a retrospective cohort study, evaluating electronic health records of 443,198 adults, of whom 150,359 were users of ADHD medications (methylphenidate, amphetamine, or atomoxetine) at baseline. The study investigated cardiovascular events such as myocardial infarction, sudden cardiac death, and stroke. No evidence was found for the association of current use with increased risk of cardiovascular events, compared with nonuse or remote use (risk ratio of any cardiovascular event for current use = 1.03; 95% confidence interval [95%CI] 0.86-1.24). Similar results were found in another retrospective cohort study that analyzed automated data from health plans from more than 1,200,000 children and young adults.114114. Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896-904. The results showed that current use of ADHD drugs was not associated with increased risk for cardiovascular events (hazard ratio of cardiovascular events for current use = 0.75, 95%CI 0.32-1.85), but the upper limit of 1.85 for the wide confidence interval found requires attention. It is recommended to investigate history of heart disease and family history of sudden death under the age of 40, and to refer the patient to a complete cardiologic evaluation before commencing treatment if either of those is present.111111. Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227-46. Heart rate and blood pressure should be assessed at baseline and monitored regularly during the course of treatment, but there is no evidence to support routine electrocardiogram before prescribing ADHD medication.111111. Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227-46. Table 3 shows recommendations regarding monitoring and managing cardiovascular risk in children and adolescents treated with ADHD medications.
Management of cardiovascular risk during treatment of children and adolescents with ADHD medications111111. Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227-46.,113113. Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50:978-90.
The presence of adverse events should be evaluated and managed carefully when prescribing ADHD medications but, although all effort should be made to avoid exposing patients to unnecessary harm, it is important to adequately treat individuals who need treatment. It is also fundamental to perform a detailed assessment of pretreatment conditions and family history that increases risk for severe adverse events, avoiding negative events that can be anticipated.
Final considerations
There has been a growing investment in research on ADHD over the years. As a result, accumulating evidence supports the validity of the diagnosis. Current data indicate that ADHD is the outcome of deviant developmental processes and complex interactions between genetic variants and environmental exposures. There is no identifiable risk factor at the present moment that is either sufficient or necessary for the occurrence of the disorder. Therefore, it is hypothesized that different combinations of risk factors can lead to ADHD. The underlying etiological heterogeneity is likely to result in heterogeneity in the clinical presentation; countless different combinations of cognitive impairments and behavioral manifestations can be identified in individuals classified under the diagnosis of ADHD.
ADHD is frequently associated with important negative outcomes during development and in adulthood. Therefore, early detection and intervention are fundamental for reducing the burden of the disorder for the individuals, families, and the society. The efficacy and safety of pharmacological agents has been extensively demonstrated for the short-term treatment of the disorder. Less data is available for the long-term treatment and for non-pharmacological interventions. Evidence for the treatment of preschoolers and adults with ADHD is also less abundant. In the near future, long-term follow-up studies, evaluation of less studied populations, and development and assessment of new interventions are expected to contribute to further advance the knowledge about ADHD.
-
Disclosure Taciana G. Costa Dias reports no conflicts of interest. Christian Kieling receives research grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo è Pesquisa do Estado do Rio Grande do Sul (FAPERGS). He has also received royalties from Artmed and Manole, and research support from Deva and Novartis. Ana Soledade Graeff-Martins has developed educational material for Janssen-Cilag. Tais Moryiama has received continuing medical education support from Astra Zeneca, Eli-Lilly, and Janssen-Cilag. Luis A. Rohde has worked on the speakers' bureau and/or acted as a consultant for Eli-Lilly, Janssen-Cilag, Novartis, and Shire in the last 3 years; received travel awards (air tickets and hotel costs) from Novartis and Janssen-Cilag in 2010 for taking part in two child psychiatric meetings; as chair of the ADHD and Juvenile Bipolar Disorder Outpatient Programs, he has received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Abbott, Eli-Lilly, Janssen-Cilag, Novartis, and Shire; he has also received authorship royalties from Oxford Press and ArtMed. Guilherme V. Polanczyk has served as a speaker and/or consultant to Eli-Lilly, Novartis, Janssen-Cilag, and Shire Pharmaceuticals; has developed educational material to Janssen-Cilag; receives authorship royalties from Manole Editors; and has received unrestricted research support from Novartis.
References
-
1Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942-8.
-
2Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619-23.
-
3Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159-65.
-
4Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJ, Jensen PS, Cantwell DP. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429-33.
-
5Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237-48.
-
6Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, et al. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69:1295-303.
-
7Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51:990-1002 e2.
-
8Merikangas KR, He JP, Burstein M, Swendsen J, Avenevoli S, Case B, et al. Service utilization for lifetime mental disorders in U.S. adolescents: results of the National Comorbidity Survey-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2011;50:32-45.
-
9Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013;54:3-16.
-
10Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215-20.
-
11Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010 27;6:155-79.
-
12Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038-55.
-
13Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275-89.
-
14Kieling C, Goncalves RR, Tannock R, Castellanos FX. Neurobiology of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2008;17:285-307, viii.
-
15Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649-54.
-
16Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72:191-7.
-
17Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-22.
-
18Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: continuity from ages 3 to 6. Am J Psychiatry. 2012;169:1157-64.
-
19Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816-8.
-
20Matte B, Rohde LA, Grevet EH. ADHD in adults: a concept in evolution. Atten Defic Hyperact Disord. 2012;4:53-62.
-
21Kieling C, Kieling RR, Rohde LA, Frick PJ, Moffitt T, Nigg JT, et al. The age at onset of attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:14-6.
-
22Polanczyk G, Caspi A, Houts R, Kollins SH, Rohde LA, Moffitt TE. Implications of extending the ADHD age-of-onset criterion to age 12: results from a prospectively studied birth cohort. J Am Acad Child Adolesc Psychiatry. 2010;49:210-16.
-
23Sayal K, Goodman R. Do parental reports of child hyperkinetic disorder symptoms at school predict teacher ratings? Eur Child Adolesc Psychiatry. 2009;18:336-44.
-
24Rohde LA. Is there a need to reformulate attention deficit hyperactivity disorder criteria in future nosologic classifications? Child Adolesc Psychiatr Clin N Am. 2008;17:405-20, x.
-
25American Psychiatric Association. Diagnostic and statistical manual of mental disorders - DSM-IV-TR¯. 4th ed. Arlington: American Psychiatric Publishing; 2000.
-
26Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490-9.
-
27Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336-46.
-
28Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183-213.
-
29Nikolas MA, Nigg JT. Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology. 2013;27:107-20.
-
30Toplak ME, Rucklidge JJ, Hetherington R, John SC, Tannock R. Time perception deficits in attention-deficit/ hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. J Child Psychol Psychiatry. 2003;44:888-903.
-
31Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Inhibitory deficits in attention-deficit/hyperactivity disorder are independent of basic processing efficiency and IQ. J Neural Transm. 2008;115:261-8.
-
32Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, Alderson RM. Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): a ubiquitous core symptom or manifestation of working memory deficits? J Abnorm Child Psychol. 2009;37:521-34.
-
33Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry 2006;67:21-6.
-
34Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57:1224-30.
-
35Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345-55.
-
36Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109:6769-74.
-
37Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785-806.
-
38Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231-8.
-
39Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29-36.
-
40Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 20105;68:1084-91.
-
41Mills KL, Bathula D, Dias TG, Iyer SP, Fenesy MC, Musser ED, et al. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front Psychiatry. 2012;3:2.
-
42Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:33-45.
-
43Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185-98.
-
44Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051-62.
-
45Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332-7.
-
46Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61-9.
-
47Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, et al. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7-14.
-
48Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, et al. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:884-97.
-
49Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51-90.
-
50Nigg J, Nikolas M, Burt SA. Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:863-73.
-
51Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33:159-80.
-
52Van der Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clin Psychol Rev. 2008;28:783-800.
-
53Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19:353-64.
-
54Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165:1475-88.
-
55Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4.
-
56Klassen A, Miller A, Raina P, Lee SK, Olsen L. Attention-deficit hyperactivity disorder in children and youth: a quantitative systematic review of the efficacy of different management strategies. Can J Psychiatry. 1999;44:1007-16.
-
57Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T. 2009;34:678-94.
-
58Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl). 2008;197:1-11.
-
59Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921.
-
60Cheng JY, Chen RY, Ko JS, Ng EM. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology (Berl). 2007;194:197-209.
-
61Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, Wyk GW. Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis. J Atten Disord. 2011;15:674-83.
-
62Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:1551-9.
-
63Kollins SH, Jain R, Brams M, Segal S, Findling RL, Wigal SB, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127:e1406-13.
-
64Jain R, Segal S, Kollins SH, Khayrallah M. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:171-179.
-
65Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-84.
-
66Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155-65.
-
67Spencer T, Biederman J, Wilens T, Harding M, O'Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry 1996;35:409-32.
-
68Lee PC, Niew WI, Yang HJ, Chen VC, Lin KC. A meta-analysis of behavioral parent training for children with attention deficit hyperactivity disorder. Res Dev Disabil. 2012;33:2040-49.
-
69Antshel KM, Barkley R. Psychosocial interventions in attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2008;17:421-37, x.
-
70A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073-86.
-
71Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168-79.
-
72Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484-500.
-
73Banaschewski T, Buitelaar J, Coghill DR, Sergeant JA, Sonuga-Barke E, Zuddas A, et al. The MTA at 8. J Am Acad Child Adolesc Psychiatry. 2009;48:1120-1; author reply 3-4.
-
74Pliszka SR. The MTA at 8. J Am Acad Child Adolesc Psychiatry. 2009;48:1122; author reply 3-4.
-
75Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99.
-
76Hechtman L, Abikoff H, Klein RG, Weiss G, Respitz C, Kouri J, et al. Academic achievement and emotional status of children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43:812-9.
-
77Prasad V, Brogan E, Mulvaney C, Grainge M, Stanton W, Sayal K. How effective are drug treatments for children with ADHD at improving on-task behaviour and academic achievement in the school classroom? A systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2013;22:203-16.
-
78Scheffler RM, Brown TT, Fulton BD, Hinshaw SP, Levine P, Stone S. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123:1273-9.
-
79Lichtenstein P, Halldner L, Zetterqvist J, Sjolander A, Serlachius E, Fazel S, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367:2006-14.
-
80Brook JS, Brook DW, Zhang C, Seltzer N, Finch SJ. Adolescent ADHD and adult physical and mental health, work performance, and financial stress. Pediatrics. 2013;131:5-13.
-
81Raman SR, Marshall SW, Haynes K, Gaynes BN, Naftel AJ, Sturmer T. Stimulant treatment and injury among children with attention deficit hyperactivity disorder: an application of the self-controlled case series study design. Inj Prev. 2013;19:164-70.
-
82Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20.
-
83Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179-85.
-
84Molina BS, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007;46:1028-40.
-
85Biederman J, Monuteaux MC, Spencer T, Wilens TE, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165:597-603.
-
86Ghuman JK, Arnold LE, Anthony BJ. Psychopharmacological and other treatments in preschool children with attention-deficit/hyperactivity disorder: current evidence and practice. J Child Adolesc Psychopharmacol. 2008;18:413-47.
-
87Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284-93.
-
88Kollins S, Greenhill L, Swanson J, Wigal S, Abikoff H, McCracken J, et al. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS). J Am Acad Child Adolesc Psychiatry. 2006;45:1275-83.
-
89Kratochvil CJ, Vaughan BS, Stoner JA, Daughton JM, Lubberstedt BD, Murray DW, et al. A double-blind, placebo-controlled study of atomoxetine in young children with ADHD. Pediatrics. 2011;127:e862-8.
-
90Sonuga-Barke EJ, Daley D, Thompson M, Laver-Bradbury C, Weeks A. Parent-based therapies for preschool attention-deficit/hyperactivity disorder: a randomized, controlled trial with a community sample. J Am Acad Child Adolesc Psychiatry. 2001;40:402-8.
-
91Jones K, Daley D, Hutchings J, Bywater T, Eames C. Efficacy of the Incredible Years Programme as an early intervention for children with conduct problems and ADHD: long-term follow-up. Child Care Health Dev. 2008;34:380-90.
-
92Barkley RA, Shelton TL, Crosswait C, Moorehouse M, Fletcher K, Barrett S, et al. Multi-method psycho-educational intervention for preschool children with disruptive behavior: preliminary results at post-treatment. J Child Psychol Psychiatry. 2000;41:319-32.
-
93Gleason MM, Egger HL, Emslie GJ, Greenhill LL, Kowatch RA, Lieberman AF, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46:1532-72.
-
94Atkinson M, Hollis C. NICE guideline: attention deficit hyperactivity disorder. Arch Dis Child Educ Pract Ed. 2010;95:24-7.
-
95Meszaros A, Czobor P, Balint S, Komlosi S, Simon V, Bitter I. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-47.
-
96Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2004;24:24-9.
-
97Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;CD007813.
-
98Maneeton N, Maneeton B, Srisurapanont M, Martin SD. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65:611-7.
-
99Verbeeck W, Tuinier S, Bekkering GE. Antidepressants in the treatment of adult attention-deficit hyperactivity disorder: a systematic review. Adv Ther. 2009;26:170-84.
-
100Adler LA, Wilens T, Zhang S, Dittmann RW, D'Souza DN, Schuh L, et al. Atomoxetine treatment outcomes in adolescents and young adults with attention-deficit/hyperactivity disorder: results from a post hoc, pooled analysis. Clin Ther. 2012;34:363-73.
-
101Adler LA, Liebowitz M, Kronenberger W, Qiao M, Rubin R, Hollandbeck M, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26:212-21.
-
102Adler LA, Spencer T, Brown TE, Holdnack J, Saylor K, Schuh K, et al. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29:44-50.
-
103Durell TM, Adler LA, Williams DW, Deldar A, McGough JJ, Glaser PE, et al. Atomoxetine treatment of attention-deficit/hyperactivity disorder in young adults with assessment of functional outcomes: a randomized, double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol. 2013;33:45-54.
-
104Arnold VK, Feifel D, Earl CQ, Yang R, Adler LA. A 9-week, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study to evaluate the efficacy and safety of modafinil as treatment for adults with ADHD. J Atten Disord. 2012 May 22. [Epub ahead of print]
-
105Strange BC. Once-daily treatment of ADHD with guanfacine: patient implications. Neuropsychiatr Dis Treat. 2008;4:499-506.
-
106Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21:223-8.
-
107Bain EE, Robieson W, Pritchett Y, Garimella T, Abi-Saab W, Apostol G, et al. A randomized, double-blind, placebo-controlled phase 2 study of alpha4beta2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology. 2013;38:405-13.
-
108Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugue M, Carpentier PJ, et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67.
-
109Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-80.
-
110Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22:213-37.
-
111Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227-46.
-
112Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994-1009.
-
113Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50:978-90.
-
114Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896-904.
-
115Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306:2673-83.
Publication Dates
-
Publication in this collection
2013