Abstract
Objective:
Schizophrenia is one of the most severe psychiatric disorders, and its current treatment relies on antipsychotic medications with only partial effectiveness. Clozapine is an atypical antipsychotic with a specific profile of action indicated for treatment-resistant schizophrenia. Neuroimaging studies assessing the effects of clozapine could help shed light on the neural underpinnings of the effects of this drug in the brain. The objective of this study was to review the available literature on the structural and functional neuroimaging findings associated with use of clozapine.
Method:
We conducted a systematic review of the indexed literature using the PubMed, BIREME, and ISI Web of Knowledge search engines and the following keywords: clozapine, neuroimaging, computed tomography, MRI, functional magnetic resonance, PET, SPECT, and DTI.
Results:
A total of 23 articles were included in the review. In structural studies, the use of clozapine was associated with volume reductions in the basal ganglia, especially the caudate nucleus, where functional neuroimaging studies also found decreased perfusion. In the frontal lobe, clozapine treatment was associated with increased gray matter volume and reduced perfusion.
Conclusion:
The results of the studies reviewed suggest that the use of clozapine is associated with distinctive structural and functional neuroimaging findings that are not shared with other antipsychotics.
Clozapine; neuroimaging; schizophrenia; magnetic resonance; computed tomography
Introduction
Schizophrenia is a chronic and often disabling disorder that affects about 1% of the world population, with no significant differences between countries, cultures, or gender, and poses an important challenge to clinical psychiatry.11. Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063-72.,22. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187-93. In general, onset occurs in late adolescence and early adulthood, causing severe impairment to the personal, social, and occupational functioning of patients, affecting their productivity, and generating high costs to health systems. Schizophrenia has a heterogeneous presentation and different psychopathological dimensions, classified as positive, negative, disorganized, affective, and cognitive domains, the expression of which varies across individuals and over the course of the disease.33. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1-23.
Despite numerous studies aimed at understanding the disorder, its etiology and pathophysiology are still unknown, and available treatments are only partially effective.44. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100:4-19. Evidence suggests that schizophrenia is the result of the interaction of genetic, environmental, and social aspects, and most researchers view it as a neurodevelopmental disorder.55. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409-32.
6. Pilowsky LS, Kerwin RW, Murray RM. Schizophrenia: a neurodevelopmental perspective. Neuropsychopharmacology. 1993;9:83-91.-77. Waddington JL. Schizophrenia: developmental neuroscience and pathobiology. Lancet. 1993;341:531-6.
Antipsychotic drugs are the main treatment for schizophrenia, and are classified as typical or atypical according to their profile of action and extrapyramidal side effects. These medications are of the utmost importance in reducing psychotic symptoms and preventing relapse.88. Kane JM, Marder SR. Psychopharmacologic treatment of schizophrenia. Schizophr Bull. 1993;19:287-302. However, in contrast with their clear effects on psychotic symptoms, antipsychotics have modest effects on negative symptoms and cognitive impairment.22. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187-93.
Clozapine, the first atypical antipsychotic, represents the main advance in the pharmacological treatment of schizophrenia since the introduction of antipsychotics in clinical practice in the 1950s. It has unique potential for treatment of refractory positive symptoms, negative symptoms, suicidal risk, and cognitive functioning without triggering extrapyramidal symptoms,99. Buchanan RW. Clozapine: efficacy and safety. Schizophr Bull. 1995;21:579-91.
10. Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60:82-91.-1111. Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156:990-9. with the important drawback of its potential to cause agranulocytosis. Due to its superior efficacy profile, clozapine has been investigated for a better understanding of its effects on the central nervous system in the search for novel, more efficient antipsychotic drugs.1212. Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115-24.
Major technical advances in imaging techniques achieved in recent decades have enabled the in vivo study of patients with schizophrenia, and become an important tool to investigate its pathophysiological mechanisms. Recent developments in this area have also vastly expanded our knowledge of the effects of pharmacological compounds in the brain and their action on the central nervous system without the use of invasive methods.1313. Leslie RA, James MF. Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci. 2000;21(8):314-8.
The objective of this study was to review available data on the structural and functional correlates of the use of clozapine in schizophrenia, assessed through neuroimaging techniques, namely computed tomography (CT), structural (MRI) and functional (fMRI) magnetic resonance imaging, magnetic resonance spectroscopy (MRS), single-photon emission computed tomography (SPECT), positron emission tomography (PET), and diffusion tensor imaging (DTI).
Method
The article search and selection process was based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. We searched for articles indexed in the PubMed, BIREME, and ISI Web of Knowledge electronic databases using the following search terms and their equivalents in Portuguese: clozapine AND (neuroimaging OR computed tomography OR MRI OR functional magnetic resonance OR PET OR SPECT OR DTI), published until December 2012. References of selected articles were also hand-searched for possible additional citations.
To be included in the review, studies had to meet the following criteria: 1) involve only human samples; 2) be an original article published in English, Portuguese, or Spanish; and 3) describe brain structural and/or functional features associated with the use of clozapine in schizophrenia. Exclusion criteria were: 1) case reports, literature reviews, or letters to the editor; 2) studies that did not use clozapine; 3) reports on the side effects of clozapine or about its effects on organs other than the brain; 4) studies that did not use neuroimaging techniques; 5) investigations on the use of clozapine in other psychiatric and/or neurological disorders; 6) analyses of previous structural and functional changes predicting response to clozapine; and 7) studies assessing only receptor occupancy.
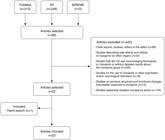
The article search and selection process and reasons for the exclusion of references are shown in Figure 1.
Results
Of the 23 articles examined in this review, 15 included healthy control groups for comparison. Regarding experimental samples, eight studies included only patients treated with clozapine and 15 involved patients on other antipsychotics in addition to participants on clozapine.
For heuristic purposes, the included studies were divided into “structural” and “functional” according to the neuroimaging technique used. Seven studies used structural MRI and the remaining 16 used functional neuroimaging techniques (two used fMRI, three used MRS, five used SPECT, and seven used PET). One of the functional studies used two techniques: spectroscopy and SPECT.
Structural studies
Studies evaluating the effects of clozapine by means of MRI are described in Table 1. The interval between MRI scans before and after clozapine initiation ranged from 24 weeks to 5 years. Of the seven MRI studies included, six described the use of typical antipsychotics prior to clozapine. Clozapine was the only medication used by patients during image acquisition in three studies, while the remaining four also included patients on other antipsychotics. Four studies in this group assessed basal ganglia volume, mainly focusing on the caudate nucleus, and the three remaining studies assessed white and gray matter volumes, white and gray matter density, and gray matter cortical thickness respectively.
In comparison with typical antipsychotic therapy, clozapine treatment was associated with volume reductions in the caudate nucleus in all studies assessing this region. Regarding white and gray matter volume, Molina et al.1818. Molina V, Reig S, Sanz J, Palomo T, Benito C, Sanchez J, et al. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res. 2005;80:61-71. found increased gray matter volume and reduced white matter volume after comparing a group on clozapine treatment and a healthy control group, with changes observed in total, frontal, parietal, and occipital volumes in comparison with controls.1818. Molina V, Reig S, Sanz J, Palomo T, Benito C, Sanchez J, et al. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res. 2005;80:61-71. Van Haren et al.1919. van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057-66. investigated white and gray matter density in a longitudinal study and reported that the dose of clozapine accumulated per year during the 5-year follow-up study was related to a smaller reduction in density in the region of the left superior frontal gyrus compared with typical antipsychotic therapy. In a study comparing patients on clozapine and olanzapine, another atypical antipsychotic, Mattai et al.2020. Mattai A, Chavez A, Greenstein D, Clasen L, Bakalar J, Stidd R, et al. Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr Res. 2010;116:44-8. assessed gray matter cortical thickness and found the use of clozapine to be associated with thickness reduction in an area of the right prefrontal cortex.
Functional studies
The included functional studies were divided in accordance with the neuroimaging technique used.
Functional magnetic resonance (fMRI)
The two fMRI studies assessing the effects of clozapine in the central nervous system are described in Table 2. Both studies used single scans and tasks simultaneous to image acquisition. Wenz et al.2121. Wenz F, Schad LR, Knopp MV, Baudendistel KT, Flomer F, Schroder J, et al. Functional magnetic resonance imaging at 1.5 T: activation pattern in schizophrenic patients receiving neuroleptic medication. Magn Reson Imaging. 1994;12:975-82. compared patients taking clozapine with healthy controls during a motor task to assess the sensorimotor cortex. A substantial reduction in motor activation signal was found in patients taking clozapine in comparison with controls, especially in association with left hand movements.2121. Wenz F, Schad LR, Knopp MV, Baudendistel KT, Flomer F, Schroder J, et al. Functional magnetic resonance imaging at 1.5 T: activation pattern in schizophrenic patients receiving neuroleptic medication. Magn Reson Imaging. 1994;12:975-82.
Besides patients on clozapine and healthy controls, Schmitt et al.2222. Schmitt A, Otto S, Jatzko A, Ruf M, Demirakca T, Tost H, et al. Parieto-prefrontal dysfunction during visuo-auditory information processing in elderly, chronic schizophrenic patients and medication effects. Rev Psiquiatr Clin. 2009;36:89-96. also assessed patients using typical antipsychotics during visual and auditory stimulation tasks, with the objective of assessing prefrontal and parietal areas and the anterior cingulate gyrus. In this study, clozapine was associated with greater activation in these areas in comparison with atypical antipsychotics.
None of these studies provided specific information concerning the use of typical antipsychotics prior to clozapine.
Magnetic resonance spectroscopy (MRS)
Three studies assessed the effects of clozapine on the central nervous system through MR proton spectroscopy (Table 3). Two of these investigations included groups of patients on typical and other atypical antipsychotics and healthy control groups to be compared with patients on clozapine in a single scan. The remaining study was a longitudinal investigation of a group of patients without medication (baseline) and after 8 weeks of clozapine treatment. All patients taking clozapine in these studies had been previously treated with typical antipsychotics. The brain areas assessed using MRS were the left frontal lobe, left temporal lobe, caudate nucleus, left thalamus, and left dorsolateral prefrontal cortex (LDLPFC). Metabolites measured with MRS included N-acetylaspartate (NAA), choline, and creatine, but the use of clozapine was not associated with any significant changes in their levels.
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT)
The functional neuroimaging studies that used PET and SPECT (n=12) are listed respectively in Tables 4 and 5. Of all studies included in this group, nine were longitudinal studies. Six of the studies in this category used tasks during image acquisition protocols and six recorded activity at rest. The use of typical antipsychotics prior to clozapine was reported in eight articles. Four of the studies included only samples of patients on clozapine, whereas the remaining eight included groups using other typical and/or atypical antipsychotics.
Six articles described reduced frontal lobe perfusion with the use of clozapine, especially in prefrontal areas.2626. Rodriguez VM, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, et al. Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res. 1997;76:51-61.,2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54.,3131. Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry. 1997;54:481-6.,3232. Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40.,3535. Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26.,3636. Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157-62. Three of these were longitudinal studies in which the first scan was obtained while patients were on treatment with typical antipsychotics2626. Rodriguez VM, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, et al. Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res. 1997;76:51-61.,3535. Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26. or atypical antypsychotics2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54. and the second scan was performed after a period of treatment with clozapine. Cohen et al.3232. Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40. described reduced frontal lobe perfusion with the use of clozapine in comparison with controls, and Molina et al.3636. Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157-62. found similar results comparing a group on clozapine with neuroleptic-naive patients and controls.3232. Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40.,3636. Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157-62. Lastly, Cohen et al.3131. Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry. 1997;54:481-6. also found reduced frontal lobe perfusion in patients on clozapine compared with patients on fluphenazine and controls.3131. Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry. 1997;54:481-6.
Conversely, one study found significantly increased perfusion in regions of the frontal lobe comparing patients without medication and the same group of patients 8 weeks after the initiation of clozapine treatment.2525. Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res. 2009;174:121-9. Clozapine treatment was also associated with increased cerebral blood flow (CBF) in the dorsolateral prefrontal cortex and reduced CBF in the ventrolateral frontal cortex in comparison with haloperidol treatment and untreated controls.3333. Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601-8.
A pattern of decreased activation in the region of the basal ganglia in association with the use of clozapine was found in four studies.2626. Rodriguez VM, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, et al. Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res. 1997;76:51-61.,2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54.,3535. Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26.,3636. Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157-62. Two of these compared patients on clozapine and patients on typical antipsychotics,2626. Rodriguez VM, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, et al. Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res. 1997;76:51-61.,3535. Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26. one compared patients on clozapine and risperidone,2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54. and the last one compared a group of patients on clozapine with neuroleptic-naive patients and healthy controls.3636. Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157-62.
Increased perfusion in regions of the temporal, occipital, and parietal cortices was reported in five studies.2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54.,3131. Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry. 1997;54:481-6.
32. Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40.-3333. Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601-8.,3535. Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26. Two of the studies found increased perfusion in the occipital cortex of patients on clozapine compared with haloperidol treatment.3333. Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601-8.,3535. Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26.
Molina et al.2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54. reported increased posterior temporal and occipital cortex perfusion in patients treated with clozapine compared with controls, in addition to increased perfusion in the medial occipital cortex in clozapine patients compared with risperidone patients.2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54. Finally, Cohen et al.3232. Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40. described increased perfusion in the occipital and parietal cortices when comparing patients treated with clozapine and healthy controls.3232. Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40.
Discussion
We reviewed empirical articles dealing with the structural and functional correlates of the use of clozapine in schizophrenia assessed through neuroimaging techniques. Taken together, the results of these studies suggest that clozapine treatment may be associated with changes in the structure and activity of specific brain areas, mainly the basal ganglia and frontal cortex.
The area of the basal ganglia, especially the caudate nucleus, was shown to develop volume reductions associated with the use of clozapine in structural studies, in addition to decreased perfusion in functional studies. This may reflect the weak antagonism of clozapine on D2 receptors, which are abundant in this region, and its significant action on D3 and D4 receptors. This profile of action may cause a down-regulation of D2 receptors and consequent volumetric and metabolic reductions in a given brain region.3737. Lee H, Tarazi FI, Chakos M, Wu H, Redmond M, Alvir JM, et al. Effects of chronic treatment with typical and atypical antipsychotic drugs on the rat striatum. Life Sci. 1999;64:1595-602. The previous use and discontinuation of typical antipsychotics could be implicated in such findings, since their strong antagonist action on D2 receptors could stimulate upregulation of these receptors, as well as cause volume and perfusion increases.
Accordingly, the discontinuation of these medications could lead to reductions in volume and activity that could be mixed with the effects of clozapine introduction.3838. Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry. 1996;153:41-9.
Regarding gray matter, the results available suggest that clozapine may have a neuroprotective effect, as suggested by descriptions of increased gray matter volume and decreased loss of density in the left frontal gyrus in the articles reviewed. Previous studies using olanzapine, which is an atypical antipsychotic similar to clozapine, showed an increase in glial cell division and cortical hypertrophy in rats.3939. Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29:1230-8. Such effects of clozapine may also explain its action on gray matter.
Another possibility is that the use of typical antipsychotics such as haloperidol has been associated with progressive loss of gray matter.4040. Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361-70. Therefore, the previous use of typical antipsychotics and their subsequent discontinuation could be involved in the increase of volume and density of gray matter.
The frontal lobe, and mainly its prefrontal portion, has been assessed in many functional studies included in this review because of its likely involvement in the etiology and pathophysiology of schizophrenia. Half of the studies that used SPECT and PET described reduced activity in frontal regions. Clozapine increases extracellular dopamine in frontal areas, and this increase stimulates the release of GABA, an inhibitor that reduces perfusion in the region.4141. Yamamoto BK, Pehek EA, Meltzer HY. Brain region effects of clozapine on amino acid and monoamine transmission. J Clin Psychiatry. 1994;55:8-14.,4242. Grobin AC, Deutch AY. Dopaminergic regulation of extracellular gamma-aminobutyric acid levels in the prefrontal cortex of the rat. J Pharmacol Exp Ther. 1998;285:350-7. The fact that clozapine blocks D1 receptors more efficiently than D2 receptors may also be involved in the metabolic patterns observed.4343. Meltzer HY, Bastani B, Ramirez L, Matsubara S. Clozapine: new research on efficacy and mechanism of action. Eur Arch Psychiatry Neurol Sci. 1989;238:332-9. D1 receptors are densely distributed in the prefrontal cortex, and clozapine-induced D1 blockade could contribute to lower prefrontal activity. Clozapine has also been found to reduce prefrontal hyperfunction in patients with schizophrenia during task performance.2828. Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54.
The increase in cortical activity in the parietal and temporal cortices and, mainly, in the occipital cortex observed with the use of clozapine may be a result of the tendency of atypical antipsychotics to increase cortical perfusion.4444. Davis CE, Jeste DV, Eyler LT. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophr Res. 2005;78:45-60. However, it may also be a consequence of the discontinuation of previous therapy with typical antipsychotics, the use of which has been associated with decreased cortical activity. In general, however, patients taking clozapine showed greater activation in the cortical regions assessed when compared with patients using typical antipsychotics. This can probably be explained by an improvement in glutamatergic transmission caused by clozapine, which is supported by findings from animal studies that show an increase in the activity of NMDA glutamate receptors with clozapine treatment that did not occur with the administration of haloperidol.4545. Arvanov VL, Liang X, Schwartz J, Grossman S, Wang RY. Clozapine and haloperidol modulate N-methyl-D-aspartate-and non-N-methyl-D-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J Pharmacol Exp Ther. 1997;283:226-34.,4646. Melone M, Bragina L, Conti F. Clozapine-induced reduction of glutamate transport in the frontal cortex is not mediated by GLAST and EAAC1. Mol Psychiatry. 2003;8:12-3. Therefore, typical antipsychotics seem unable to counteract glutamatergic hypofunction in schizophrenia.
Levels of NAA, a marker of neuronal integrity assessed through spectroscopy, were found to be reduced in prefrontal and temporal regions in schizophrenia.2323. Bustillo JR, Lauriello J, Rowland LM, Jung RE, Petropoulos H, Hart BL, et al. Effects of chronic haloperidol and clozapine treatments on frontal and caudate neurochemistry in schizophrenia. Psychiatry Res. 2001;107:135-49.,4747. Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949-62. The absence of significant differences in NAA levels across the groups evaluated in the studies may suggest a normalization of this marker with the use of clozapine.2424. Szulc A, Galinska B, Tarasow E, Kubas B, Dzienis W, Konarzewska B, et al. N-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) study. Med Sci Monit. 2007;13:17-22. Previous use of typical antipsychotics should also be considered as a possible explanation, since this class of antipsychotics has been associated with lower NAA levels in the thalamus and frontal lobe of patients compared to controls.2323. Bustillo JR, Lauriello J, Rowland LM, Jung RE, Petropoulos H, Hart BL, et al. Effects of chronic haloperidol and clozapine treatments on frontal and caudate neurochemistry in schizophrenia. Psychiatry Res. 2001;107:135-49.,2424. Szulc A, Galinska B, Tarasow E, Kubas B, Dzienis W, Konarzewska B, et al. N-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) study. Med Sci Monit. 2007;13:17-22.
The structural and functional changes associated with clozapine treatment found in this review are different from those previously associated with typical antipsychotics and, albeit smaller, from those described in association with the use of other atypical antipsychotics. A consistent finding of MRI studies is that typical antipsychotics are associated with enlargement of the basal ganglia and reductions in cortical volume, mainly in the frontal and temporal lobes, while atypical antipsychotics seem to be mostly associated with enlargement of the thalamus.4848. Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763-77.
49. Dazzan P, Morgan KD, Orr K, Hutchinson G, Chitnis X, Suckling J, et al. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30:765-74.-5050. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156:1200-4. Spectroscopy studies have described reduced NAA levels in frontal areas in patients treated with typical antipsychotics compared to patients on atypical antipsychotics.5151. Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, et al. Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res. 2000;41:389-95.,5252. Heimberg C, Komoroski RA, Lawson WB, Cardwell D, Karson CN. Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect. Psychiatry Res. 1998;83:105-15. In addition, typical antipsychotics seem to reduce activity in the frontal cortex and increase activity in the basal ganglia, while atypical antipsychotics appear to be associated with decreased activity in frontal areas.4444. Davis CE, Jeste DV, Eyler LT. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophr Res. 2005;78:45-60.,5353. Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SC, Sharma T. Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci U S A. 1999;96:13432-7.,5454. Miller DD, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Comparison of the effects of risperidone and haloperidol on regional cerebral blood flow in schizophrenia. Biol Psychiatry. 2001;49:704-15.
Some limitations of our review must be mentioned. First, the previous use of typical antipsychotics reported in most studies and the time of withdrawal varying widely from a few days to months could act as confounding factors in the interpretation of the results presented. These drugs can cause specific structural and functional changes of unknown intensity, direction, and course after the discontinuation of use. Further investigation is necessary to accurately determine whether the changes reported in the articles and summarized here are due to the introduction of clozapine or to the discontinuation of typical antipsychotics. Another limitation is that the dose and time of clozapine treatment in the different samples varied significantly, making comparisons more difficult. Another factor possibly affecting results is that most of the subjects enrolled in the studies were diagnosed with refractory schizophrenia, which, due to its severity and poor prognosis, can cause changes in the central nervous system. The heterogeneity in study designs and number of scans performed should also be considered as obstacles to global analyses and imaging comparisons. Finally, the lack of control groups of either healthy subjects or patients on antipsychotics other than clozapine is another factor that hinders the comparison of results.
An important point to be noted in our review was the inclusion of articles describing studies with the same sample or similar samples whose results were analyzed and presented separately. Molina et al.3535. Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26. and Molina et al.3636. Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157-62. worked with the same sample of treatment-resistant patients that were taking clozapine; however, the most recent publication included and compared a group of treatment-naive patients. Both studies found reduced frontal lobe perfusion with the use of clozapine. Cohen et al.3232. Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40. worked with the same sample of Cohen et al.3131. Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry. 1997;54:481-6. but included more subjects and found similar results. The two articles by Scheepers et al.1616. Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24:47-54.,1717. Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS. Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry. 2001;158:644-6. analyzed the same sample of patients, but with different durations of clozapine treatment. Both articles described volume reductions in the caudate nucleus. Finally, Lahti et al.3333. Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601-8. and Lahti et al.3434. Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Frey KN, Hardin M, et al. Clozapine but not haloperidol Re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171-8. also worked with the same sample, but investigated different brain areas in each article. If, on the one hand, studies with the same sample can be expected to reach the same conclusions, the inclusion of additional analyses and comparisons may lend strength to their findings.
Available evidence from neuroimaging investigations suggests that clozapine may have a specific profile of action on the central nervous system when compared with typical and other atypical antipsychotics, particularly in the prefrontal area of the frontal lobe and in the basal ganglia. Determination of the neural basis of the effects of clozapine in the brain may provide clues into the still-unknown etiology of schizophrenia and inform the development of novel, better medications to treat psychosis.
Acknowledgements
JEH and JAC are recipients of research fellowship awards from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). JPMS receives a postdoctoral grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
-
1Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063-72.
-
2Insel TR. Rethinking schizophrenia. Nature. 2010;468:187-93.
-
3Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1-23.
-
4Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100:4-19.
-
5Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409-32.
-
6Pilowsky LS, Kerwin RW, Murray RM. Schizophrenia: a neurodevelopmental perspective. Neuropsychopharmacology. 1993;9:83-91.
-
7Waddington JL. Schizophrenia: developmental neuroscience and pathobiology. Lancet. 1993;341:531-6.
-
8Kane JM, Marder SR. Psychopharmacologic treatment of schizophrenia. Schizophr Bull. 1993;19:287-302.
-
9Buchanan RW. Clozapine: efficacy and safety. Schizophr Bull. 1995;21:579-91.
-
10Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60:82-91.
-
11Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156:990-9.
-
12Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115-24.
-
13Leslie RA, James MF. Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci. 2000;21(8):314-8.
-
14Chakos M, Lieberman J, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456-7.
-
15Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, et al. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry. 1996;153:564-6.
-
16Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24:47-54.
-
17Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS. Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry. 2001;158:644-6.
-
18Molina V, Reig S, Sanz J, Palomo T, Benito C, Sanchez J, et al. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res. 2005;80:61-71.
-
19van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057-66.
-
20Mattai A, Chavez A, Greenstein D, Clasen L, Bakalar J, Stidd R, et al. Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr Res. 2010;116:44-8.
-
21Wenz F, Schad LR, Knopp MV, Baudendistel KT, Flomer F, Schroder J, et al. Functional magnetic resonance imaging at 1.5 T: activation pattern in schizophrenic patients receiving neuroleptic medication. Magn Reson Imaging. 1994;12:975-82.
-
22Schmitt A, Otto S, Jatzko A, Ruf M, Demirakca T, Tost H, et al. Parieto-prefrontal dysfunction during visuo-auditory information processing in elderly, chronic schizophrenic patients and medication effects. Rev Psiquiatr Clin. 2009;36:89-96.
-
23Bustillo JR, Lauriello J, Rowland LM, Jung RE, Petropoulos H, Hart BL, et al. Effects of chronic haloperidol and clozapine treatments on frontal and caudate neurochemistry in schizophrenia. Psychiatry Res. 2001;107:135-49.
-
24Szulc A, Galinska B, Tarasow E, Kubas B, Dzienis W, Konarzewska B, et al. N-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) study. Med Sci Monit. 2007;13:17-22.
-
25Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res. 2009;174:121-9.
-
26Rodriguez VM, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, et al. Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res. 1997;76:51-61.
-
27Zhao J, He X, Liu Z, Yang D. The effects of clozapine on cognitive function and regional cerebral blood flow in the negative symptom profile schizophrenia. Int J Psychiatry Med. 2006;36:171-81.
-
28Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948-54.
-
29Ergun EL, Volkan-Salanci B, Ertugrul A, Demir B, Erbas B. Evaluation of SISCOM in routine regional cerebral blood flow alterations after clozapine, in schizophrenia. Hell J Nucl Med. 2010;13:35-9.
-
30Buchsbaum MS, Potkin SG, Marshall JF, Lottenberg S, Teng C, Heh CW, et al. Effects of clozapine and thiothixene on glucose metabolic rate in schizophrenia. Neuropsychopharmacology. 1992;6:155-63.
-
31Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry. 1997;54:481-6.
-
32Cohen RM, Nordahl TE, Semple WE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology. 1999;21:632-40.
-
33Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601-8.
-
34Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Frey KN, Hardin M, et al. Clozapine but not haloperidol Re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171-8.
-
35Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl). 2005;178:17-26.
-
36Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157-62.
-
37Lee H, Tarazi FI, Chakos M, Wu H, Redmond M, Alvir JM, et al. Effects of chronic treatment with typical and atypical antipsychotic drugs on the rat striatum. Life Sci. 1999;64:1595-602.
-
38Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry. 1996;153:41-9.
-
39Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29:1230-8.
-
40Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361-70.
-
41Yamamoto BK, Pehek EA, Meltzer HY. Brain region effects of clozapine on amino acid and monoamine transmission. J Clin Psychiatry. 1994;55:8-14.
-
42Grobin AC, Deutch AY. Dopaminergic regulation of extracellular gamma-aminobutyric acid levels in the prefrontal cortex of the rat. J Pharmacol Exp Ther. 1998;285:350-7.
-
43Meltzer HY, Bastani B, Ramirez L, Matsubara S. Clozapine: new research on efficacy and mechanism of action. Eur Arch Psychiatry Neurol Sci. 1989;238:332-9.
-
44Davis CE, Jeste DV, Eyler LT. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophr Res. 2005;78:45-60.
-
45Arvanov VL, Liang X, Schwartz J, Grossman S, Wang RY. Clozapine and haloperidol modulate N-methyl-D-aspartate-and non-N-methyl-D-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J Pharmacol Exp Ther. 1997;283:226-34.
-
46Melone M, Bragina L, Conti F. Clozapine-induced reduction of glutamate transport in the frontal cortex is not mediated by GLAST and EAAC1. Mol Psychiatry. 2003;8:12-3.
-
47Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949-62.
-
48Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763-77.
-
49Dazzan P, Morgan KD, Orr K, Hutchinson G, Chitnis X, Suckling J, et al. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30:765-74.
-
50Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156:1200-4.
-
51Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, et al. Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res. 2000;41:389-95.
-
52Heimberg C, Komoroski RA, Lawson WB, Cardwell D, Karson CN. Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect. Psychiatry Res. 1998;83:105-15.
-
53Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SC, Sharma T. Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci U S A. 1999;96:13432-7.
-
54Miller DD, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Comparison of the effects of risperidone and haloperidol on regional cerebral blood flow in schizophrenia. Biol Psychiatry. 2001;49:704-15.
Publication Dates
-
Publication in this collection
Jan-Mar 2015
History
-
Received
10 Feb 2014 -
Accepted
2 May 2014