Abstract
The aim of this study was to determine the effects of incubation temperature (34.5; 35.5; 36.5; 37.5 and 38.5ºC), on incubation period, embryonic mortality, hatching rate, water loss and chick weight at hatch, using daily incubation of partridge (Rhynchotus rufescens) eggs. The highest hatching percentage was obtained between 35.5 and 36.5ºC. Incubation length and temperature were inversely proportional. Water loss was lower in eggs incubated at low temperatures as compared to high temperatures. There was no difference among incubation temperatures in absolute and relative hatchling weights. Early embryonic mortality increased at low temperatures (<35.5ºC), whereas intermediate and late embryonic mortality increased at high temperatures (>36.5ºC). Our results show that, under conditions of daily incubation of eggs in the same incubator, higher hatching rate can be obtained using temperatures between 35.5ºC and 36.5ºC; incubation temperature is inversely proportional to incubation length, and absolute and relative weights of partridge chicks are not affected by incubation temperature.
Incubation; hatchability; mortality; temperature; water loss
Effect of temperature on incubation period, embryonic mortality, hatch rate, egg water loss and partridge chick weight (Rhynchotus rufescens)
Nakage ES; Cardozo JP; Pereira GT; Queiroz SA; Boleli IC
Faculdade de Ciências Agrárias e Veterinárias UNESP- Jaboticabal
Correspondence Correspondence to Isabel Cristina Boleli Departamento de Morfologia e Fisiologia Animal Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias Via de Acesso Prof. Paulo Donato Castellani, Km 5 Jaboticabal , SP Brasil 14874-900 E-mail: icboleli@fcav.unesp.br
ABSTRACT
The aim of this study was to determine the effects of incubation temperature (34.5; 35.5; 36.5; 37.5 and 38.5ºC), on incubation period, embryonic mortality, hatching rate, water loss and chick weight at hatch, using daily incubation of partridge (Rhynchotus rufescens) eggs. The highest hatching percentage was obtained between 35.5 and 36.5ºC. Incubation length and temperature were inversely proportional. Water loss was lower in eggs incubated at low temperatures as compared to high temperatures. There was no difference among incubation temperatures in absolute and relative hatchling weights. Early embryonic mortality increased at low temperatures (<35.5ºC), whereas intermediate and late embryonic mortality increased at high temperatures (>36.5ºC). Our results show that, under conditions of daily incubation of eggs in the same incubator, higher hatching rate can be obtained using temperatures between 35.5ºC and 36.5ºC; incubation temperature is inversely proportional to incubation length, and absolute and relative weights of partridge chicks are not affected by incubation temperature.
Keywords: Incubation, hatchability, mortality, temperature, water loss.
INTRODUCTION
Temperature is one of the physical factors that determine the success of incubation. Therefore, it is essential to determine and use a temperature that promotes the highest hatchability (Swann & Brake, 1990b; French, 1997) and the best hatchling quality (Wilson, 1991; Decuypere & Mitchels, 1992), known as optimum incubation temperature.
The optimum incubation temperature of wild fowl eggs is within a wide range of values, varying from 33ºC to 39ºC, whereas a narrower range (37ºC to 38ºC) is considered as optimum for domestic poultry (Visschedijk, 1991).
The effect of incubation temperature on egg hatchability and hatchling quality may be related to its influence on incubation length and water loss during incubation. However, such effects depend on how long and how intense is the shift from optimum temperature. According to Givisiez et al. (2000) an increase of 1ºC (38.8ºC) above the optimum incubation temperature (37.8ºC) starting at day 13 of incubation causes a significant reduction of the hatching rate of broiler eggs, whereas such effect is not observed when the temperature is reduced in 1ºC (36.8ºC).
Incubation temperature correlates directly to the duration of in ovo development both in turkeys (French, 1994) and broilers (Byerly, 1938; Decuypere et al., 1979). The development is delayed in temperatures below optimum and accelerated in temperatures above optimum (Romanoff, 1960; Wilson, 1991). Such difference in embryo development rate as a result from changing the incubation temperature seems to explain body weight differences reported by some authors (Givisiez et al., 2000; Decuypere et al., 1979; Swan & Brake, 1990a).
Water loss is a normal process during incubation, usually 12 to 14% of water is lost in broilers and turkeys eggs (Rahn et al., 1981). However, too low or too high water loss influences embryo development (Rahn & Ar, 1974), and consequently, egg hatchability (Meir et al., 1984). Incubation temperatures above the optimum cause excessive egg water loss (higher than 14%), leading to embryo mortality by dehydration. On the other hand, temperatures below the optimum decrease hatchability due to reduced water loss (< 12%), which causes an over-hydration of the embryo and an impairment of gas exchange (Romanoff, 1930).
The partridge (Rhynchotus rufescens) is a wild bird that has significantly reduced natural populations nowadays due to predatory hunting and destruction of natural habitats. Some poultry producers have shown increasing interest in raising partridges commercially as an alternative source of exotic meat. However, there is little information in literature regarding egg incubation parameters for this specie.
One of the major problems in partridge production is the low egg production, which is due to the short laying period (6 months; spring and summer). In addition, this species is undergoing the first stages of taming process, and therefore, it is still not well adapted to captivity conditions. Thus, with the objective of determining adequate handling of fertile eggs and also to increase partridge chick production, the present study analyzed the effects of incubation temperature on incubation length, water loss, embryonic mortality, hatching rate, as well as on the weight of the chick partridge by using daily egg incubation in a same incubator.
MATERIAL AND METHODS
Eggs and incubation
In the present study, 182 fertile eggs (56 ± 3g) were collected (3 times/day) during the intermediate laying period (November-December/2001) from partridges (Rhynchotus rufescens) given pelleted diet (15% crude protein, 2,800 kcal ME/kg) and water ad libitum. All eggs were manually sprayed using a disinfecting solution of 1% formaldehyde + 0.5% quaternary ammonium (Branco, 1990; Morita, 1990). After drying, 2 to 4 eggs were transferred to incubators (Premium Ecológica, Model IP120) three times per day until a maximum of 65 eggs per incubator.
On day 16 of incubation, eggs were weighed and transferred to hatchers (Premium Ecológica, Model NP120). Temperature and relative humidity in the incubators and hatchers were monitored and recorded 3 times/day by direct observation of dry and wet bulb thermometers, respectively, throughout the incubation period.
Eggs were divided into five experimental groups with different incubation temperatures: group 1 (34.5ºC, N: number of fertile eggs=26), group 2 (35.5ºC, N=40), group 3 (36.5ºC, N=62), group 4 (37.5ºC, N=31), and group 5 (38.5ºC, N=23). All groups were submitted to the same relative humidity (60%) until hatching.
Parameters
The following parameters were analyzed for each experimental group: hatching percentage (number of hatched eggs/total number of incubated fertile eggs x 100); percentage of water loss up to the transference to the hatcher [(egg weight before incubation egg weight at transference)/egg weight before incubation x 100]; total incubation period (days); absolute and relative hatchling weight (absolute chick weight/egg weight before incubation x 100); total mortality rate [(number of incubated fertile eggs number of hatched eggs)/number of incubated fertile eggs x 100]. Non-hatched eggs were opened and embryo stage was determined according to Hamburger & Hamilton (1951). Then, it was determined early mortality rate (number of embryos dead between 1 and 7 days/number of incubated fertile non-hatched eggs x 100); intermediate mortality rate (number of embryos dead between 8 and 15 days/number of incubated fertile non-hatched eggs x 100) and late mortality rate (number of embryos dead between 16 days and hatching/number of incubated fertile non-hatched eggs x 100).
Statistical analysis
Data were submitted to one-way analysis of variance (temperature) and subsequently expressed by polynomial functions to justify the differences between the treatment means. Mortality data were evaluated by Fisher's Test (5%). All statistical analysis were performed using GLM procedure of SAS (Statistical Analysis System, 1998).
RESULTS AND DISCUSSION
Figure 1 shows a significant (p<0.05) quadratic effect for hatchability as a function of incubation temperature (y= -7970 + 450.5x 6.312x2; R2= 0.998), thus the highest hatching rate was observed within the temperature range of 35.5ºC and 36.5ºC.
The optimum incubation temperature depends on the avian species. The best hatchability is obtained at 37.8ºC for broilers (Barott, 1937), 37.2ºC 37.5ºC for quails (Albino & Neme, 1998),39.0ºC 39.5ºC for waterfowl and 38.3ºC 38.6ºC for turkey (Reis, 1942a,b),39.0ºC for ducks and 39.5ºC for geese (Cullington, 1975). Moreng & Avens (1990) reported that the best hatchability for European partridges was found at an incubation temperature of 37.4ºC. Thus, our findings also suggest that the optimum incubation temperature is lower for wild birds when compared to domestic birds.
The mortality of partridge eggs (Figure 1) was also described by a quadratic effect as a function of incubation temperature (y= 8070 450.5x + 6.312x2; R2= 0.998). According to this equation, opposite to what happens to hatching rate, temperatures below 34.5ºC and above 36.5ºC increase mortality. However, incubation at a temperature lower than 35.5ºC increased late mortality, whereas incubation at a temperature above 36.5ºC showed predominantly early and intermediate mortality (Figure 2). These data differ from that reported for broilers, since broiler embryos are more sensitive to temperatures below optimum (37.8ºC) in the beginning of incubation (French, 1997), and to temperatures above optimum during late incubation period (Ono et al., 1994). Primmett et al. (1988) also reported that embryo survival was lower at early stages of development when incubation temperature is set to at extremes of the temperature range. Thus, our findings revealed that the partridge embryo is also susceptible to incubation temperature, but mortality at early stages is higher at low temperatures, differing from broiler embryos.
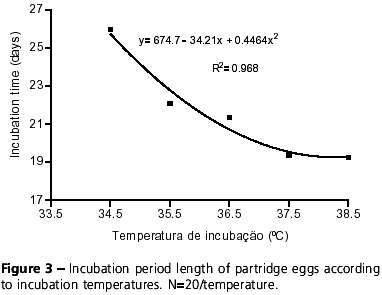
In the present study, incubation of partridge eggs lasted 26 and 19 days at 34.5ºC and 38.5ºC, respectively. Within this range of temperature, the incubation period of partridge eggs changes as a function of incubation temperatures (y= 674.7 34.21x + 0.4464x2; R2= 0.968) (Figure 3). French (1994) also observed in turkeys that incubation time is reduced as incubation temperature increases, showing that this is a common feature in different bird specie.
The plasticity of broiler embryos to respond to shifts in incubation temperature, which might delay or accelerate their development, may be used in hatcheries as a means to manipulate hatching rate. Nevertheless, such technique must be carefully considered for partridges, since data presented here evidenced that incubation temperatures below 34.5ºC and above 36.5ºC result in a significant decrease in hatching rate, opposite to the main objective of the incubation process, which is to maximize hatching. The risk of using such procedure to manipulate hatching day in broilers was already mentioned by Wilson (1990). Therefore, incubation length or hatching day must be analyzed simultaneously with the hatchability of fertile eggs, because optimum incubation temperature is determined by their synchronism (Visschedijk, 1991).
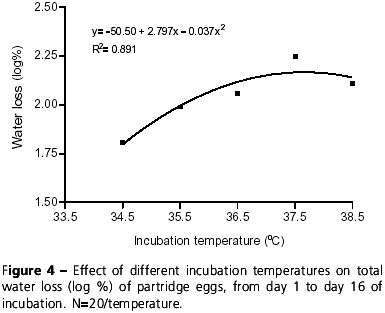
With respect to water loss percentage in partridge eggs, there was a quadratic effect as a function of incubation temperatures from day 1 to day 16 of incubation (y= -50.5 + 2.797x 0.037x2; R2= 0.891), that is, the higher the temperature, the higher the percentage of egg water loss (Figure 4). Similar effects of incubation temperature on water loss were reported in broiler eggs by Swann & Brake (1990b).
Results showed that water loss during the first 16 days of incubation was approximately 8% of the initial weight of partridge eggs incubated at 35.5ºC and 36.5ºC, and 9.94% when incubated at 37.5ºC. These values are similar to water loss for broilers eggs (10.8% at 37.8ºC) (Deeming, 1993). Water loss of partridge eggs incubated at 34.5ºC was lower when compared to eggs incubated at higher temperatures (Figure 4), which suggests that chicks hatched at 34.5ºC would be heavier than those hatched at higher temperatures, considering that water percentage in the albumen determines chick weight at hatching (Simkiss, 1980). However, as shown in Figure 5, there was no significant difference between the incubation temperatures for chick weight, showing that incubation temperature and, consequently, water loss in the first 16 days of incubation did not influence absolute and relative chick weights in partridge. Data shown in Figures 4 and 5 suggest that possibly all partridge eggs may lose the same percentage of water (p>0.05) until the end of incubation. Thus, in such case, incubation temperature may influence the velocity of water loss, but not the total percentage of water loss. Swann & Brake (1990a) also did not report effects of incubation temperature on the weight of broiler hatchlings.
The relative weight of partridge chick was about 72.3 to 73.14% of the weight of fresh eggs (Figure 5). A broader variation (62 to 71%) was found in broiler chick (Merritt & Gowe, 1965). The smaller variation in the relative weight of partridge chicks compared to broiler chicks may be related to a higher uniformity of egg weight in the present study.
In summary, the present study shows that, when partridge eggs (Rhynchotus rufescens) are incubated daily in the same incubator, the highest hatching rate is obtained at 35.5ºC and 36.5ºC. Incubation temperature is inversely proportional to incubation length, the higher incubation temperature, the higher the rate of egg water loss in the first 16 days of incubation. Also, it was shown that absolute and relative weights of partridge chicks are not affected by incubation temperature, and that temperature below 35.5ºC result in late mortality, whereas temperatures above 36.5ºC causes an increase in early and intermediate mortality.
Acknowledgment
The authors thank the Wild Animal Sector of FCAV - UNESP/ Jaboticabal, for the partridge eggs, and CNPq for M. Sc. scholarship granted to E.S.N.
Arrived: july 2002
Approved: march 2003
- Albino LFT, Neme R. Codornas. Manual Prático de Criação. Viçosa (MG):Ed. Aprenda Fácil; 1998.
- Barott HG. Effect of temperature, humidity, and other factors on hatch of hens'eggs and on energy metabolism of chick embryos. In. USDA Technical Bulletin 1937. nş553.
- Branco JAD. Métodos de desinfecção de ovos incubáveis. In. Curso de Atualização em Incubação. FACTA; 1990. p.91-100.
- Byerly TC. Effect of different incubation temperatures on mortality of chick embryos. Poultry Science 1938; 17:200-205.
- Christensen VL, Donaldson WE, Nestor KE. Incubation temperature effects on metabolism and survival of turkey embryos. In. Proceedings of 9th European Poultry Conference; 1994; Glasgow, UK:Word's Poultry Science Association; 1994. Vol II. p. 399-402.
- Cullington JM. Patos y Gansos. Zaragoza (Espanha):Ed. Acribia; 1975.
- Decuypere E, Mitchels H. Incubation temperature as a management tool:a review. World's Poultry Science Journal 1992; 48:28-38.
- Decuypere E. Incubation temperature and postnatal development. In. Proceedings of 9th European Poultry Conference; 1994; Glasgow, UK:Word's Poultry Science Association; 1994. Vol II. p. 407-410.
- Decuypere E, Nouwen EJ, Kühn ER, Gess R, Michels H. Iodohormones in the serum of chick embryos and post-hatching chickens as influence by incubation temperature. Relationship with the hatching process and thermogenesis. Annales de Biologie Animale, Biochimie, Biophysique 1979; 19:1713-1723.
- Deeming C. Relative humidity affects water loss from eggs. Weight loss from bird eggs and relative humidity. Misset- World Poultry 1993; 9(5):33-37.
- French NA. Effects of temperature incubation on the gross pathology of turkey embryos. British Poultry Science 1994; 35:363-371.
- French NA. Modeling incubation temperature:the effects of incubator design, embryonic development, and egg size. Poultry Science 1997; 76:124-133.
- Givisiez PEN, Bruno LDG, Machado JRSA, Secato ER, Freitas D, Ribeiro LT, Macari M. Desempenho e resposta ao estresse calórico gradativo de frangos submetidos a estresse de calor e frio durante a incubação. Revista Brasileira de Ciência Avícola (Suplemento) 2000; 2:1.
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology 1951; 217:313-325.
- Meir M, Nir A, Ar A. Increasing hatchability of turkey eggs by matching incubator humidity to shell conductance of individual eggs. Poultry Science 1984; 63:1489-1496.
- Moreng RE, Avens JS. Ciência e Produção de aves. 1Ş. ed. São Paulo (São Paulo):Roca; 1990.
- Morita P. Gerenciamento do incubatório. In. Curso de Atualização em Incubação. FACTA; 1990. p.9-32.
- Ono H, Hou PCL, Tazawa H. Responses of developing chicken embryos to acute changes in ambient temperature:Noninvasive study of hart rate. Israel Journal of Zoology 1994, 40:467-480.
- Primmett, DRN, Stern, CD, Keynes, R.J. Heat shock causes repeated segmental anomalies in the chick embryo. Development 1988; 104:331.
- Rahn H, Ar A. The avian egg:Incubation time and water loss. Condor 1974; 76:147-152.
- Rahn H, Christensen VL, Edens FW. Changes in shell conductance, pores, and physical dimensions of egg and shell during the first breeding cycle of turkey hens. Poultry Science 1981; 60:2536-2541.
- Reis J. Marrecos e Patos. São Paulo (SP):Ed. Melhoramentos; 1942a.
- Reis J. Os perus. 2Ş. ed. São Paulo (SP):Ed. Melhoramentos; 1942b.
- Romanoff AL. Biochemistry and biophysics of the development hen's egg. Memoirs of Cornell University Agricultural Experimental Station 1930; 132:1-27.
- Romanoff AL. The avian embryo:structural and functional development. New York, Macmillan. 1960.
- SAS Institute, SAS (Statistical Analysis System). User Guide. SAS Institute Inc. Cary, NC. 1998.
- Simkiss K. Eggshell porosity and the water metabolism of the chick embryo. Journal of Zoology 1980; 192:1-8.
- Swann GS, Brake J. Effect of incubation dry-bulb and wet-bulb temperatures on time of hatch and chick weight at hatch. Poultry Science 1990a; 68:887-897.
- Swann GS, Brake J. Effect of dry-bulb temperature, relative humidity, and eggshell conductance during the first three days of incubation on egg weight loss and chick weight. Poultry Science 1990b; 69:535-544.
- Visschedijk AHJ. Physics and physiology of incubation. British Poultry Science 1991; 32:3-20.
- Wilson HR. Physiological requirements of the developing embryo:temperature and turning. In. Avian incubation, SG Tullett. Butterworth-Heinemann; 1991. p.145-156.
Correspondence to
Publication Dates
-
Publication in this collection
12 Nov 2003 -
Date of issue
May 2003
History
-
Accepted
Mar 2003 -
Received
July 2002