Abstract
The aim of this study was to follow-up the physiological variations in the development of the bone tissue, associating them with the egg production curve. This study was carried out in the facilities of the Faculdade de Medicina Veterinária e Zootecnia of the UNESP, Botucatu, Brazil. Twenty-three families of Ross broiler breeders were used, each family consisting of 13 females and 1 male, distributed in 23 pens of 5.0m² each. The management was that recommended by the genetic company manual (Agroceres Ross, 2003), with daily feeding until 6th week of age; and birds were fed according to a 5:2 schedule (5 days fed, 2 days of fasting) between 7 and 17 weeks of age, returning to daily feeding starting at 18 weeks of age. Birds did not receive afternoon calcium supplementation. On the fourth week of rearing, 84 females were removed for bone analyses of the right tibia and femur, using optical densitometry in radiographic images technique. These analyses were sequentially carried out in 4, 8, 12, 15, 20, 24, 30, 35, 42, 47, and 52 week-old birds. The egg production curve of the birds was followed-up and associated to bone mineral density results. For bone mineral density evaluation (BMD) birds were divided by weight categories as light, intermediate, or heavy within each data age. BMD values of the tibias were not influenced by weight range, but by the age at collection. On the other hand, interactions were found among femur BMD values and weight and age categories. There was no correlation between eggshell quality and femur BMD. A negative correlation (-0.15) was observed between tibia BMD and eggshell percentage. It was possible to conclude that the egg production has little influence on bone mineral density of the birds probably because there was no need of bone mineral mobilization during the production period, since the observed egg production was below that observed under commercial conditions.
Bone; bone density; broiler breeders; egg production; shell quality
Bone mineral density of tibae and femura of broiler breeders: growth, development and production
Almeida Paz ICLI; Mendes AAII; Quinterio RRI; Vulcano LCIII; Takahashi SEI; Garcia RGI; Komiyama CMI; Balog AIV; Pelícia KI; Wescheler FSII; Scudeller PSOV; Piccinin AVI
IStudent of the Post-Graduation Program in Animal Science of the School of Veterinary Medicine and Animal Science, UNESP/Botucatu
IIProfessor of the Dept. of Animal Production of the School of Veterinary Medicine and Animal Science, UNESP/Botucatu
IIIProfessor of the Dept. of Veterinary Reproduction and Radiology of the School of Veterinary Medicine and Animal Science, UNESP/Botucatu
IVUndergraduate student of the Animal Science Course of the School of Veterinary Medicine and Animal Science, UNESP/Botucatu
VPh. D. student of the Post-Graduation Program in Animal Science of the School of Veterinary Medicine and Animal Science, UNESP/Botucatu
VIStudent of the Post-Graduation Program in Genetics of the Biosciences Institute of UNESP/Botucatu
Mail Address Mail Address Ariel Mendes Faculdade de Medicina Veterinária e Zootecnia UNESP Fazenda Experimental Lageado, s/nº Departamento de Produção e Exploração Animal 18.618-000. Botucatu, SP, Brazil E-mail: arielmendes@fca.unesp.br
ABSTRACT
The aim of this study was to follow-up the physiological variations in the development of the bone tissue, associating them with the egg production curve. This study was carried out in the facilities of the Faculdade de Medicina Veterinária e Zootecnia of the UNESP, Botucatu, Brazil. Twenty-three families of Ross broiler breeders were used, each family consisting of 13 females and 1 male, distributed in 23 pens of 5.0m² each. The management was that recommended by the genetic company manual (Agroceres Ross, 2003), with daily feeding until 6th week of age; and birds were fed according to a 5:2 schedule (5 days fed, 2 days of fasting) between 7 and 17 weeks of age, returning to daily feeding starting at 18 weeks of age. Birds did not receive afternoon calcium supplementation. On the fourth week of rearing, 84 females were removed for bone analyses of the right tibia and femur, using optical densitometry in radiographic images technique. These analyses were sequentially carried out in 4, 8, 12, 15, 20, 24, 30, 35, 42, 47, and 52 week-old birds. The egg production curve of the birds was followed-up and associated to bone mineral density results. For bone mineral density evaluation (BMD) birds were divided by weight categories as light, intermediate, or heavy within each data age. BMD values of the tibias were not influenced by weight range, but by the age at collection. On the other hand, interactions were found among femur BMD values and weight and age categories. There was no correlation between eggshell quality and femur BMD. A negative correlation (-0.15) was observed between tibia BMD and eggshell percentage. It was possible to conclude that the egg production has little influence on bone mineral density of the birds probably because there was no need of bone mineral mobilization during the production period, since the observed egg production was below that observed under commercial conditions.
Keywords: Bone; bone density; broiler breeders; egg production; shell quality.
INTRODUCTION
Brazilian poultry industry has grown faster than other sector of the economy, presenting significant advances both in genetics and nutrition with consequent better production (Nicolau, 1996). In this context, broiler breeder production is paramount, as its objective is to produce high quality hatchable eggs at increasingly lower costs. Therefore, adequate nutrition is essential to allow birds to express their full genetic potential. Nutritional requirements of most nutrients are based on production responses, but, in some cases, health is the main criterion. A vitamin- and mineral-deficient feed may cause characteristic lesions, although the first symptoms of deficiency of these nutrients are also accompanied by a decrease in production parameters (Calderón, 1994; Hudson et al., 2000).
Breeder broiler calcium requirement is very high, particularly during the period of active eggshell formation. Calcium used for eggshell formation derives directly from the duodenum and jejunum, and indirectly from medullar bone through a bone resorption process (Kienholz et al., 1961; Landauer, 1967; Wilson et al., 1980; Wilson, 1983; Luquetti et al., 2002; Julian, 2005). The proportion of calcium derived from these two sources varies according to the period of the day. During the night, when dietary calcium sources are not available, birds mobilize calcium from the bones, whereas during the day, most calcium comes from the diet. It is known that the higher the contribution of bone calcium for eggshell formation, the worst is eggshell quality.
Medullar bone undergoes through periods of bone deposition and bone resorption. Bone resorption can be minimized when larger particles are fed, such as oyster meal, because these are digested more slowly during the night, extending calcium supply directly from the gastrointestinal tract. According to Julian (2005), the use of calcium in large particles (larger than 0.75mm) is good to maintain eggshell quality, as these particles are stored in the gizzards, ensuring the presence of calcium in the digestive tract during eggshell formation.
However, the supplementation of calcium during the afternoon is controversial. Some authors carried out studies using different particle sizes, forms, and times of calcium particle availability. In some cases, no improvement in eggshell specific weight and resistance, or in hatchability and chick viability were observed (Reis et al., 1995; Novo et al., 1997; Zhang & Coon, 1997; Maggioni, 1998).
Phosphorus also has an important role in the normal bone system development of broiler breeders and of embryos, as well as in egg hatchability (Harms et al., 1964; Julian, 2005). Singsen et al. (1962) suggested that 0.4% available phosphorus is required for maximum hatchability of birds housed in cages. Later, Wilson et al. (1980) determined that broiler breeders required 0.31% phosphorus for normal hatchability. Current recommendations are of 0.35 - 0.45% available phosphorus, depending on the rearing stage of broiler breeders (Agroceres Ross, 2003). Excessive phosphorus consumption, resulting from a combination of dietary phosphorus and other sources, may cause poor eggshell quality, and indirectly worse hatchability (Wilson et al., 1980).
Eggshell quality can be estimated by specific gravity and its relationship with shell porosity, as there is a positive correlation between eggshell quality and eggshell thickness, and a negative correlation with the concentration of pores (Peebles & Brake, 1987). Therefore, specific weight is an easy method to assess eggshell thickness, and it is widely used to determine its relation with hatchability in broiler breeders.
As the laying period progresses, egg weight increases, eggshell becomes thinner, and internal quality worsens. There is also a high incidence of eggs with no shell. At a determined moment, the hen stops laying eggs. Eggshell quality decreases as egg production is reduced. These changes are associated with an increase in egg size and with a lower intestinal absorption of minerals by the birds (Leeson & Summers, 2000; Luquetti et al., 2002). Therefore, the bone system is used to supply the mineral needs of the body, ensuring the balance between calcium and phosphorus absorption and requirements.
Bone tissue resistance results from calcium and phosphorus deposition as hydroxyapatite during the process of bone mineralization (Field, 1999; Bruno, 2002).
In the present study, it was possible to better understand broiler breeder development during growth, development, and production stages with the aid of bone densitometry and other analysis. This study aimed at establishing a relation between bone density, egg production, and eggshell quality, taking into account broiler breeders development stages.
MATERIAL AND METHODS
This study used 280 Ross 308 females and 40 Ross 308 males. Birds were housed in the experimental facilities of Faculdade de Medicina Veterinária e Zootecnia of UNESP-Botucatu, from August 2003 until August 2004, in a masonry house divided in 24 pens of 5m² each, with an average density of 2.6 birds/m² in a at an average density of 2.6 birds/m². The house was darkened with a cover with a light retention capacity of 80%, and special dark curtains (black-out curtains). Starting on the 18th week of rearing, birds were submitted to natural light, curtains remained opened during the day, and the cover was removed.
During grower and developer periods, males were kept in pens separate from females. On week 18, birds were mated, and divided in 24 pens, with a density of 2.8 birds/m2, with a total number of 14 birds per pen. Twenty-two pens were allocated to the experiment, whereas the two remaining pens housed replacement males.
Feed nutritional levels and rearing stages were determined according to the genetic company manual (Agroceres Ross, 2003) recommendations. Rearing was divided in 6 stages: grower (0 to 5 weeks), developer I (6 to 13 weeks), developer II (14 to 19 weeks), pre-lay (20 to 24 weeks), lay starter (25 to 45 weeks), lay finisher (46 to 52 weeks), whereas cockerels were offered a specially formulated feed.
Feeds were manufactured at the feed mill of FMVZ-UNESP/Botucatu, in an automatic mixer with 500-kg capacity. Feed preservative BHT was added at 100 g BHT/1000 kg feed.
The genetic line manual recommendations were adopted for feeding and weight control. Feed was offered ad libitum on the first week, and controlled thereafter. Birds were fed daily from 1 to 6 weeks, and, from week 7 to week 17, a 5:2 feeding regime was adopted 5 days feeding, and 2 days fasting) at the beginning of he 7th week. Feed was again daily offered daily starting on week 18. No afternoon supplementation of calcium was offered, and calcium particle size was the same as that offered to broilers.
Data were collected 11 times for evaluation of optical density in radiographic images of broiler breeder tibiae and femura in order to obtain bone mineral density values. Data were collected on weeks 4, 8, 12, 15, 20, 24, 30, 35, 42, 47, and 52, considering critical rearing ages of physiological development and of egg production.
On the forth rearing week, 84 females were randomly designated to analysis groups. The same individual birds were evaluated during the entire experiment, and 5 birds were sacrificed per radiographic collection. These birds were transported to the Veterinary Hospital of FMVZ, where they were submitted to x-ray examination using a portable commercial xray apparatus, which was calibrated and applied a focus-film distance of 63 cm. The 47kVp X 2mAs radiographic technique was applied to the samples collected on weeks 4, 8, and 12 weeks, and this technique was later changed to 47kVp X 4mAs for samples collected on weeks 15, 20, 24, 30, 35, 42, 47, and 52.
All radiographic films were of the same brand, with green background and 18x24 cm frames, equipped with rare earth metal frames. A phantom aluminum scale was placed in the central area of the frame, in parallel and 3.0 cm distant from the studied region. This scale was used as densitometric reference. The phantom consists of 20 degrees, with the first degree being 0.5 mm thick, followed by 0.5 mm variations up to the 20th degree. Each degree presented an area of 15 x 5 mm. Radiological procedures were commonly used in clinical routines, and development and fix were performed in a standard automatic processor.
The region standardized for reading was the right tibia proximal epiphysis and the right femur distal epiphysis. Radiographic optical density readings (bone mineral density) were carried out using the software CROMOX® ATHENA 3.1, and all readings followed the same pattern. Radiographs were scanned, and the images were analyzed using a 10-mm high and a 35-55-mm wide (depending on bone size) opening reading window. Bone density readings of the tibiae used a 0° axis, whereas the reading axis of the femura followed the inclination of the bone diaphysis, which varies between 27° and 32°, based on a horizontal axis with 0° inclination. Birds were submitted to fasting until the end of each radiograph collections.
Individual weights of broiler breeders used for other analyses were also utilized to follow up their growth curve. Duly identified breeders were weighed in semi-analytical scales, with 2-g precision, in the experimental house. Birds were weighed on the same dates as those of bone mineral density evaluation.
In order to follow up the production curve and to associate it to bone development, five daily egg collections were carried out. Weekly averages were calculated, and egg production was expressed as percentage. Eggs were submitted to specific weight and eggshell percentage analysis using a method adapted from Castelló et al. (1989). Eggs for eggshell quality analyses were collected using nest traps, which allowed egg identification. Nest traps were placed two days before and two days after collection of eggs for radiographic image. All eggs laid by the breeders of the studied group were analyzed, and therefore the number of analyzed eggs per collection was different. Eggs, duly identified, were submitted to specific gravity and eggshell percentage analyses in order to establish the relation between these measures and bone development measures. The method suggested by Castelló et al. (1989) was used to mearure specific gravity and eggshell percentage.
Data were submitted to analysis of variance for repeated measures of the SAEG (1998) statistical package. Egg quality curves were fitted using regression equations. Correlations between these characteristics and bone mineral density were analyzed using the test of Pearson (Gomes, 1982) at 5% significance level.
RESULTS AND DISCUSSION
Body weight data shows that females were about 10% lighter as compared to the weight recommended by the genetic line manual (Agroceres Ross, 2003), and therefore, a growth curve was specially calculated for this flock, following the curve trend, and avoiding sudden weight increase, and production problems On the other hand, males were heavier than the manual recommendations, probably because birds were housed in small pens, which allowed dominance of the males that hence did not suffer the stress of hierarchy establishment. Table 1 shows the average body weight values of all birds included in the study, according to week of age. Table 2 presents weekly egg production values, expressed as percentage per weekly bird number, and total number during the weeks.
It is important to stress here that the egg production of the experimental birds (analyzed group) cannot be compared to that of a commercial flock, as these breeders were submitted to significant stress due to transportation of 12 km to the Veterinary Hospital for radiographic image collections. Another source of stress was the visits of undergraduate student classes of the School of Veterinary Medicine and Animal Science of FMVZ-UNESP, campus de Botucatu. Despite maintaining complete silence, they caused a drop in egg production. Therefore, egg production of these birds was much lower than the estimated egg production of the genetic line manual (Agroceres Ross, 2003), in addition to starting lay later, on week 27 instead of on week 25. Egg production peak was also lower and not persistent.
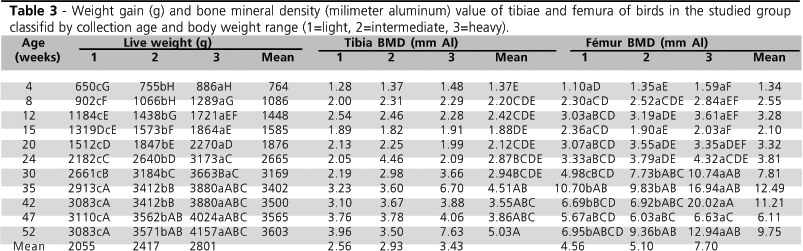
Only with the purpose of evaluating bone mineral density results using the technique of optical densitometry in radiographic images, birds were divided into three weight classes: light (1), intermediate (2), and heavy (3) within each collection age. The results are presented in Table 3. as expected, age influenced live weight. Weight increase as the bird aged, within each weight range (p<0.05).
Bone mineral density (BMD) of the tibiae was not affected by weight class, only by collection age. The lowest values were found at four weeks of age, but these were not statistically different from those found up to 30 weeks of age, when the breeders started to produce eggs (19.98%). Tibia BMD between 35 and 52 weeks of age were not significantly different, despite a trend for higher values for the ages of 35 and 52 weeks. At 35 weeks of age, birds were entering the period of peak of egg production (60.74%), which may indicate an increase in calcium deposition in the tibiae to supply the requirement for laying. On 52 weeks of age, production was 43.31%, possibly indicating that breeders were depositing calcium in the tibiae because production was declining.
BMD values found for broiler breeders are higher as compared to broiler values. Tibia BMD values found by Almeida Paz et al. (2004) for broilers were between 1.46 and 1.77 mm Al. When studying tibia BMD of 53-day-old broilers, Louzada (1997) found values between 1.77 and 1.96 mm Al. These values are consistent to those found in 4 to 15-week-old female breeders. However, in an experiment carried out by Oliveira et al. (2005), bone density values found for 42-day-old broilers tibiae were similar to those found in the present study, ranging from 2.47 and 3.50 mm Al.
Weight class influenced only femur bone mineral density (BMD), with an interaction (p<0.05) between collection age, live weight, and BMD. Weight increased as bird aged, but live weight did no influence BMD until 24 weeks of age (pre-laying period). After 30 weeks of age, body weight affected BMD, with higher BMD values observed with heavier weights. There was an exception at 47 weeks of age, when weight class had no effect on BMD values at this age, there was a sudden increase in egg production (from 42.55% in the previous week to 51.94% during this week), when a decrease in BMD level was also observed.
As previously mentioned, female weight in the present study was lower than the average weight recommended by the genetic company. Taking into consideration that only the collection at 30 weeks of age represents the laying period with a 19.98% production rate, and that the light-weight breeders were not producing eggs, it is possible that the increase in femur BMD values at this age and weight class were due to a higher calcium mobilization for eggshell formation (Wilson et al., 1980; Wilson, 1991). This may be an indication of a possible reduction in BMD values on week 47, when egg production increased 9.34% in a single week.
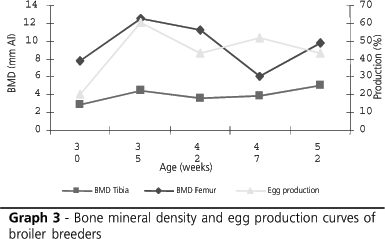
Graphs 1 and 2 show the behavior of tibia and femur BMD values according to collection ages. These value followed a linear trend, both for tibae and femura. It is possible to observe that tibia BMD values changed less as compared to femur DMO. This may indicate that femura deposit more minerals, and make them more easily available if needed. According to Julian (2005), the femur of layers is the main bone responsible for calcium supply for eggshell formation when dietary calcium is not available. The author observed that the femura of calcium-deficient layers are fragile, porous, and have thin walls.
Table 4 shows results of eggshell quality traits during the production period, egg weight increased as bird aged, but these differences were marked only between the first two weeks and the other weeks. Eggshell percentage had a similar behavior as that observed for egg weight, with a gradual increase in these values as a function of collection age. Similar results were found by Luquetti et al. (2002), when studying the effect of breeder age on eggshell quality. In the study of Pedroso et al. (2005), an increase in egg weight and in eggshell percentage as the breeder aged was also found; however, eggshell percentage values were much lower than those found in the present study, ranging from 5.88 to 6.30%. In an experiment on the influence of breeder age on egg quality, Ferreira et al. (2005) also found increase in egg weight, but lower eggshell percentage as breeder aged. These authors found eggshell percentages ranging between 8.85 and 9.22%, which were similar to those found in the present study. Brito et al. (2004) also observed a 9.40-9.48% range in eggshell percentage in an experiment testing different feeds for commercial layers.
Specific gravity presented the highest values in the first and in the last collection (30 and 52 weeks), whereas weeks 35 and 42 presented the lowest values, and collection on week 47 was not different from the other weeks. Similar results were found by Gomes et al. (2005) in a study of the effect of broiler breeder genetic line and age on eggshell percentage. In that trial, eggshell percentage with specific gravity higher than 1080 decreased as age increased; however, the studied ages were 30, 45, and 60 weeks.
Table 5 shows the shows the correlations between egg external quality traits and bone mineral density. Significant correlations were observed as to eggshell quality, with the highest value for the correlation between specific gravity and eggshell percentage. This was expected, because specific egg weight is influenced by eggshell weight (Castelló et al., 1989). Similar results were obtained by Peebles & Brake (1987) and North & Bell (1990), who mention the influence of eggshell percentage and eggshell weight on specific gravity.
According to Hudson et al. (2000), nutritional deficiencies may cause drop in egg production and reduced quality. This is corroborated by Calderón (1994), in a revision on this subject. Nevertheless, in the present study, no correlation (p>0.05) was found between eggshell quality traits and femur BMD, whereas there was a low-value negative correlation between tibia BMD and eggshell percentage. These findings support the notion that breeders did not use bone calcium and phosphorus for eggshell formation.
According to Maggioni (1998), the use of bone calcium for eggshell formation occurs when the available dietary calcium does not supply the requirements. This is corroborated by Julian (2005), who asserts that, when there is no available dietary calcium, the bird removes calcium from the medullar bones, which can cause bird's death. The feeds used in the present study followed the recommendations of the genetic line manual (Agroceres Ross, 2003), with no afternoon calcium supplementation. The results showed that birds do not need to remove bone calcium for eggshell formation, as tibia and femur bone mineral density presented low or no correlation with eggshell quality.
Graph 3 shows the egg production curve and the tibia and femur bone mineral density curves of the experimental birds. Tibia BMD curve follows the egg production curve, whereas the femur BMD curve decreases on week 47, when egg production increased.
CONCLUSION
It was possible to conclude that egg production had little influence on bone mineral density of broiler breeders, probably because there was no need to mobilize bone minerals during the production period as egg production was below to that usually found under commercial conditions.
Bone mineral density of broiler breeders showed a trend to increase as bird aged, until 30 weeks of age, when birds started to lay. On the 47th week of age, there was a 9.39% increase in egg production, with a significant decrease in femur bone mineral density, which may indicate that this bone is responsible for the supply of minerals for eggshell formation, if needed.
Arrived:
Approved:
- Agroceres Ross. Manual de manejo de matrizes. São Paulo: Agroceres Ross Melhoramento Genético de Aves ; 2003. 81p.
- Almeida Paz ICL, Mendes AA, Takita TS, Vulcano LC, Guerra PC, Wescheler FS, Garcia RG. Tibial dyschondroplasia and bone mineral density. Brazilian Journal of Poultry Science 2004; 6:207-12.
- Brito AB, Stringhini JH, Belém LM, Xavier SAG, Santos FCB, Barros FS. Avaliação nutricional do gérmen intergral de milho para poedeiras comerciais de 30 a 64 semanas. 2. Qualidade interna e de casca do ovo. In: Conferência Apinco de Ciência e Tecnologia Avícolas; 2004; Santos, SP. Brasil. p. 82.
- Bruno LDG. Desenvolvimento ósseo em frangos de corte: Influência da restrição alimentar e da temperatura ambiente [tese]. Jaboticabal (SP): UNESP; 2002.
- Calderon VC. Efectos nutricionales sobre la calidad de la cáscara. In: Conferência Apinco 1994 de Ciência e Tecnologia Avícolas; 1994; Santos, SP. Brasil. p. 35-66.
- Castelló JAC, Pontes MP, González FF. Producción de huevos. Barcelona: Real Escuela de Avicultura; 1999. 367p.
- Ferreira FC, Lara LJC, Baião NC, Chiarelli IM, Lana AMQ, Corrêa GSS. Influência da idade da matriz sobre a qualidade do ovo. In: Conferência Apinco de Ciência e Tecnologia Avícolas; 2005; Santos, SP. Brasil. p. 16.
- Field RA. Ash and calcium as measures of bone in meat and bone mistures. Meat Science 1999; 55:255-264.
- Gomes FP. Curso de estatística experimental. Piracicaba: Ed. Nobel; 1982. 430p.
- Gomes FS, Santos GCF, Silva PL. Efeito da linhagem e da idade de reprodutoras pesadas na qualidade dos ovos incubáveis. In: Conferência Apinco de Ciência e Tecnologia Avícolas; 2005; Santos, SP. Brasil. p. 20.
- Harms RH, Ammerman CB, Waldroup PW. The effect of supplement phosphorus in the breeder diet upon hatchability of eggs and bone composition of chicks. Poultry Science 1964; 43:209-212.
- Hudson BP, Lien RJ, Hess JB. Effects of early protein intake on development and subsequent egg production of broiler breeder hens. Journal Appleid. Poultry Reserch 2000; 9:324-333.
- Julian RJ. Patologias ósseas em aves. In: Conferência Apinco de Ciência e Tecnologia Avícolas; 2005; Santos, SP. Brasil. p. 107-122.
- Leeson S, Summers JD. Broiler breeder production. Guelph: University Boocks; 2000. 329p.
- Louzada MJQ. Densidade de peças ósseas de frangos. Estudo pela densitometria óptica radiográfica. Veterinária e Zootecnia 1997; 9: 95-109.
- Luquetti BC, Bruno LDG, Giacheto PF, Furlan RL, Gonzales E, Macari M. Influência da idade da matriz sobre características da casca e parâmetros sanguíneos e cardíacos de pintos neonatos. In: Conferência Apinco de Ciência e Tecnologia Avícolas; 2002; Campinas, SP. Brasil. p. 5.
- Maggioni R. Efeito do fracionamento de cálcio dietético sobre o desempenho produtivo e a qualidade da casca do ovo de poedeiras semi-pesadas durante o verão [dissertação]. Pelotas (RS): Universidade Federal de Pelotas; 1998.
- Nicolau JA. Custo de transação e cooperação vertical na indústria de frango. Caderno de Ciência & Tecnologia 1996; 13(1):57-65.
- North MO, Bell DB. Commercial chicken production manual. 4th ed. New York: Van Nostrand Reinhold; 1990.
- Novo RP, Gama LT, Soares MC. Effects of oviposition time, hen age, and extra dietary calcium on egg characteristics and hatchability. Journal Appleid Poultry Reserch 1997; 6:335-343.
- Oliveira MC, Rodrigues EA, Bruno LDG, Marques RH, Gravena RA, Moraes VWB. Características ósseas de frangos de corte alimentados com dietas contendo mananologossacarídeo e complexo enzimático. In: Conferência Apinco de Ciência e Tecnologia Avícolas; 2005; Santos, SP. Brasil. p. 41.
- Peebles ED, Brake J. Eggshell quality and hatchability in broiler breeder eggs. Poultry Science 1987; 66:594-604.
- Pedroso AA, Stringhini JH, Leandro NSM, Andrade MA, Lima FG, Barbosa CE. Eclodibilidade, mortalidade embrionária e tempo de eclosão de pintos de corte oriundos de ovos de matrizes jovens de diferentes pesos. In: Conferência Apinco 2005 de Ciência e Tecnologia Avícolas; 2005; Santos, SP. Brasil. p. 7.
- Reis LH, Feio P. Extra dietary calcium supplement and broiler breeders. Jounal Appleid of Poultry Reserch 1995; 4:276-282.
- SAEG (Sistema para análise estatística e genéticas). Manual de utilização do programa SAEG. Viçosa: UFV; 2003. 59p.
- Singsen EP, Spandorf AH, Matterson LD, Serafin JA, Tlustohowics JJ. Phosphorus in the nutrition of the adult hen. 1. Minimum phosphorus requirements. Poultry Science 1962; 41:1401-1414.
- Wilson HR, Miller ER, Harms RH, Damron BL. Hatchability of chicken eggs by dietary phosphorus and calcium. Poultry Science 1980; 59: 1284-1289.
- Wilson HR, Interrelationships of egg size, chik sizr, posthatching growth and hatchability. World's Poultry Science Journal 1991; 47: 5-20.
- Zhang B, Coon CN. The relationship of calcium intake, source, size, solubility in Vitro and in vivo, and gizzard limestone retention in laying hens. Poultry Science 1997; 76:1702-1706.
Mail Address
Publication Dates
-
Publication in this collection
09 Oct 2006 -
Date of issue
June 2006
History
-
Received
Aug 2005 -
Accepted
Apr 2006