Abstract
Rotaviruses have been identified as one of the main etiological agents of diarrhea and enteritis in mammals, including humans, and in avian species. Few studies have been published about enteric viruses in Brazilian poultry, including those related to rotavirus infection. Such studies demonstrate significant occurrence and the importance of enteric viruses in poultry presenting intestinal problems. Enteric viruses are the primary cause of injuries to the gut, allowing other agents, especially bacteria, to attach, to penetrate, and to replicate in the enteric tissue, leading to further damage. The aim of the present study was to detect rotavirus in the intestinal contents of layers and broilers by polyacrylamide gel electrophoresis (PAGE) and virus isolation in MA-104 cell culture. A total of 45.3% of all samples were positive to rotavirus; rotavirus frequencies were 48.7% in samples from flocks with diarrhea, 46.4% in flocks with delayed growth, and 30% in asymptomatic flocks. It was possible to isolate rotavirus in MA-104 cells from the nine rotavirus-positive randomly chosen samples. These results indicate that rotavirus may have an important role in pathogenesis of enteric disease.
Enteric viruses; rotavirus; isolation; MA104 cells; chicken
Rotavirus detection and isolation from chickens with or without symptoms
Villarreal LYBI; Uliana GI; Valenzuela CI; Chacón JLVI; Saidenberg ABSI; Sanches AAII; Brandão PEII; Jerez JAII; Ferreira AJPI
IPathology Department, FMVZ USP
IIPreventive Veterinary Medicine and Animal health Department, FMVZ USP
Mail Address Mail Address: Antônio J. Piantino Ferreira. Departamento de Patologia - Faculdade de Medicina Veterinária e Zootecnia - Universidade de São Paulo Avenida Prof. Dr. Orlando M. de Paiva, 87 - Cidade Universitária, Butantã 05.508-900. São Paulo, SP, Brazil E-mail: af.piantino@fmvz.usp.br
ABSTRACT
Rotaviruses have been identified as one of the main etiological agents of diarrhea and enteritis in mammals, including humans, and in avian species. Few studies have been published about enteric viruses in Brazilian poultry, including those related to rotavirus infection. Such studies demonstrate significant occurrence and the importance of enteric viruses in poultry presenting intestinal problems. Enteric viruses are the primary cause of injuries to the gut, allowing other agents, especially bacteria, to attach, to penetrate, and to replicate in the enteric tissue, leading to further damage. The aim of the present study was to detect rotavirus in the intestinal contents of layers and broilers by polyacrylamide gel electrophoresis (PAGE) and virus isolation in MA-104 cell culture. A total of 45.3% of all samples were positive to rotavirus; rotavirus frequencies were 48.7% in samples from flocks with diarrhea, 46.4% in flocks with delayed growth, and 30% in asymptomatic flocks. It was possible to isolate rotavirus in MA-104 cells from the nine rotavirus-positive randomly chosen samples. These results indicate that rotavirus may have an important role in pathogenesis of enteric disease.
Keywords: Enteric viruses, rotavirus, isolation, MA104 cells, chicken.
INTRODUCTION
Rotaviruses have been identified as one of the main etiological agents of diarrhea and enteritis in mammals, including humans (Tzipori, 1985), and in avian species (Bergeland et al., 1977; Jones et al., 1979). Rotaviruses are classified as a genus of the Reoviridae family, having a different morphology that easily allows distinguishing them from other enteric viruses and a characteristic 11-segmented RNA. This virus has been isolated from a wide variety of avian species, including turkeys, chickens, and pheasants (Gough et al., 1985; Gough et al., 1986; McNulty, 2003; Reynolds et al., 1987a; Reynolds et al., 1987b; Theil et al., 1986). Rotaviruses that possess an antigenic A group are considered typical of mammals, while typical avian rotaviruses are those designated as groups D, F, and G (McNulty, 2003, McNulty et al., 1981). However, avian rotaviruses from group A have already been isolated from the intestinal contents of chickens, turkeys, and other avian species (Sugiyama et al., 2004; Brüssow et al., 1992).
In field conditions, rotavirus infections in poultry may induce subclinical manifestations, or they may be associated with enteritis, dehydration, anorexia, low weight gain, and increased mortality (McNulty, 2003; Tamehiro et al., 2003).
Symptoms of rotavirus infection may vary from a mild disease in young chickens to a more severe manifestation in 12 to 21-day-old chickens, characterized by unrest, litter ingestion, watery feces, wet litter, and severe diarrhea (Barnes, 1997).
Few studies have been published related to enteric viruses in Brazilian poultry, including those related to rotavirus infection. Such studies demonstrate significant occurrence and the importance of enteric viruses in poultry presenting enteric problems (Alfieri et al., 1988; Alfieri et al., 1989a; Alfieri et al., 1989b).
This study aimed at isolating avian rotavirus field strains in MA-104 cells in order to determine its electrophoretic profile, and to discuss the importance of rotavirus in chickens with delayed growth and diarrhea.
MATERIAL AND METHODS
Sample collection
Between April 2004 and July 2005, 128 samples of intestinal contents were collected during necropsy of poultry (layers and broilers). Twenty eight of these samples were from chickens with delayed growth, 80 from birds presenting diarrhea and high feed conversion ratio, and 20 samples from asymptomatic flocks (without diarrhea and delayed growth). These samples came from different Brazilian states (CE, MG, PA, PR, RS, RJ, SP, and SC) with bird ages varying from 36 to 43 days. Each sample consisted of a pool of the intestinal contents of five birds, randomly chosen in each flock.
The samples were submitted to the Avian Pathology Laboratory (LABOR) FMVZ-USP under refrigeration. The enteric contents were collected, processed as 20% suspensions in PBS 0.01 M pH 7.2, and clarified at 12,000 x g/ 30 minutes at 4ºC in order to obtain the supernatant, which was kept frozen at -80ºC until the remaining processing was performed.
Polyacrylamide gel electrophoresis (PAGE)
RNA extraction from fecal suspensions was performed with phenol-chloroform, followed by discontinuous polyacrylamide gel electrophoresis at 3.5%/7.5% dyed with silver nitrate (Herring et al., 1982). Samples classified as positive were those presenting RNA band migration patterns similar to those observed with the NCDV rotavirus strain (White et al., 1970), included as a positive control.
Isolation in cell culture
Nine samples diagnosed as positive to rotavirus by PAGE were selected for cultivation in 48-hour-old confluent monolayer of MA-104 cell (Rhesus monkey kidney) in 25 cm2 plastic flasks.
The supernatants of fecal suspensions were filtered with 0.22 µM Millex-pore (Millipore) filters and added up with virus activation solution (VAS), consisting on 5 mg/mL crystalline trypsine (Sigma) in MEM EAGLE medium (Cultilab) in a 4:1 proportion in order to cleave VP4 to VP5 and VP8 (Arias et al., 1996) and incubated at 37ºC for 30 minutes.
Cell culture growth medium was discarded and monolayers were rinsed with sterile PBS 0.01 M pH 7.4. Next, 1 mL of the inoculum (sample treated with VAS) was incubatedat 37ºC for 60 minutes, and then the maintenance medium (MEM-EAGLE Cultilab) with crystalline trypsine (Sigma) at 5 µg/ml was added without discarding the inoculum, as described by Rodriguez et al. (2004).
The flasks were then incubated at 37ºC, and the monolayers were observed until cytopathic effect appeared. Those monolayers presenting cytopathic effect after incubation for up to 96 hours were frozen at -80ºC, and submitted to at least five serial passages.
All passages presenting cytopathic effect resembling rotavirus infection (cell rounding and cell elongation with gradual cell and monolayers destruction similar to a "string of pearls") were monitored by PAGE in order to determine if the observed effect was actually caused by rotavirus and not by the trypsine.
Statistical analysis
PAGE results were categorized according to the disease status of the flocks, i.e., flocks only with diarrhea, flocks only with delayed growth, flocks with diarrhea and delayed growth (diseased flocks), and asymptomatic flocks. All categories were compared with the Chi-square test using the Minitab® Release 14.1 software (© 1972 - 2003 Minitab Inc.), with one degree of freedom and a-level of 0.05.
RESULTS
Fifty-eight samples out of the 128 tested by PAGE (45.3%) were positive for rotavirus. Rotavirus frequencies were 48.7% among samples from flocks with diarrhea, 46.4% among flocks with delayed growth, and 30% among asymptomatic flocks (Table 1).
Statistical analysis for rotavirus detection revealed no significant differences among samples from flocks with diarrhea and asymptomatic flocks (p=0.132), flocks with delayed growth and asymptomatic flocks (p=0.251), flocks with diarrhea and flocks with delayed growth (p=0.832), and diseased and asymtomatic flocks (p=0.134).
The eletropherotype obtained from the majority of the fecal samples did not show sufficient resolution to determine the rotavirus group.
It was possible to isolate rotavirus in MA-104 cells from the nine randomly chosen rotavirus-positive samples; these rotavirus strains were named AR-01 to AR-09.
In the first and second passages, all inoculated samples showed low intensity cytopathic effect, characteristic of rotavirus after 48 to 60 hours of inoculation, reaching the maximum effect 5 days post-inoculation.
By the fifth passage, an intense cytopathic effect was observed after 24 hours of inoculation, leading to the complete destruction of the monolayers two days after inoculation.
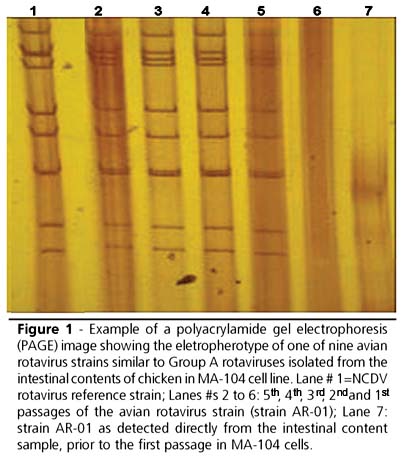
PAGE used for monitoring each passage was positive for all samples in all passages, presenting an electropherotype similar to group A rotaviruses, with the intensity of the bands increasing according to the successive passages. Figure 1 shows an example of PAGE results for strain AR-01 from the original intestinal content sample until the fifth passage in MA-104 cells.
DISCUSSION
Rotavirus with an electropherotype similar to that described for Group A rotaviruses was detected in layer hens and broilers with diarrhea and delayed growth, as well as in asymptomatic chickens, using polyacrylamide gel electrophoresis and cell culture isolation.
In poultry, both in layer hens and broilers, rotavirus has already been established as the etiological agent of enteritis, originated from viral replication in intestinal epithelium, resulting in diarrhea and nutrient malabsorption (Snodgrass et al., 1986), which causes an increase in feed conversion ratio and large economic losses to poultry industry (Barnes, 1997).
The finding of rotavirus in healthy poultry can be explained most probably by the fact that these chickens were in the beginning of the infection period, already eliminating the virus in feces, but still in the incubation period of two to five days. The hypothesis of the incubation period as an explanation for the presence of rotavirus in healthy poultry is supported by the absence of a significant difference between asymptomatic flocks and flocks with diarrhea (p=0.132), delayed growth (p=0.251), and the category "diseased flocks" (p=0.134), which included birds both with diarrhea and delayed growth. Another possibility is that the chickens had already gone through the clinical period of the disease, had recovered, but were still eliminating the virus (McNulty, 2003).
It is still possible that virus-associated factors, such as virulence, as previously established in mammalian rotaviruses caused by genetic reassortment events (Estes, 1996), as well as host-associated factors, such as natural resistance to rotavirus due to different genetic poultry lines, may have accounted for the different patterns of infection observed herein.
In addition, predisposing factors, such as diseases attributed to other pathogens like coronaviruses, reoviruses, enteroviruses, and adenoviruses (Dea & Tijssen, 1988; Hayhow & Saif, 1993); bacteria such as Escherichia coli and Salmonella spp (Porter, 1998); physiological stress; and toxic and environmental factors, such as temperature, ventilation, and husbandry, may directly interfere in the resolution of the disease after rotavirus infection. However, the importance of these asymptomatic chickens is that they are constantly shedding the virus, without being clinically detected, and disseminate the virus to susceptible chickens.
Polyacrylamide gel electrophoresis to demonstrate the viral RNA segment is a sensitive, fast, and low-cost technique, which can be easily implemented in low-outfitted laboratories and, as shown in the present study, may be used as a screening procedure for rotavirus infection in poultry.
However, it is not always possible to obtain the visualization of the 11-viral segments for a definitive diagnosis. In the present study, the association of isolation in cell culture and PAGE technique was successful to enhance the visualization of rotavirus electropherotypes.
In the nine samples examined, from which rotavirus was isolated in cell culture, it was possible to improve the resolution of the electropherotype by increasing 11-RNA bands intensity as compared to those observed in the same fecal samples before the isolation.
Although cell culture isolation did not increase the number of positive samples in the present study, it can be used in association with PAGE in order to improve the resolution of this method in cases of poorly-defined electropherotypes.
While rotavirus isolation frequently requires from four to six passages before a positive sample can be detected (Kang et al., 1986), in the cases presented here it was shown that two passages were sufficient to detect the virus. Moreover, it was already shown that MA-104 cell line is efficient for isolating avian rotavirus in field samples (Yason & Schat, 1986).
In this study a high frequency of rotavirus in Brazilian poultry was found using PAGE, indicating that this virus, although neglected as an important putative pathogen of poultry, may have a role in the pathogenesis of enteric disease of layers and broilers.
Further studies are needed in order to assess the real impact of rotavirus on Brazilian poultry, such as those focused on the genetic diversity of the detected strains and the effects after experimental inoculation of birds.
As a conclusion, it can be suggested that both diarrhea and the low performance presented by the birds studied here are due, at least in part, to the presence of rotavirus, which is largely disseminated in the Brazilian flocks, suggestion that rotavirus infection in broilers should be considered an important pathogen in a single manifestation or in association with another pathogen contributing to enteric problem onset.
Acknowledgments
The authors are grateful to FAPESP and PIBIC CNPq for the grants given to Villarreal LYB, Uliana G, Valenzuela C and Chacón JLV.
Arrived: January / 2006
Approved: September / 2006
- Alfieri AF, Alfieri AA, Resende JS, Resende M. A new bisegmented double stranded RNA virus in avian feces. Arquivos Brasileiros de Medicina Veterinária e Zootecnia 1988; 40:437-440.
- Alfieri AF, Alfieri AA, Resende JS, Resende M. Atypical rotavirus infections among broiler chickens in Brazil. Arquivos Brasileiros de Medicina Veterinária e Zootecnia 1989a; 41:81-82.
- Alfieri AF, Alfieri AA, Resende M, Resende JS. Detection and propagation of avian enteric reovirus in chicken. Arquivos Brasileiros de Medicina Veterinária e Zootecnia 1989b; 41:493-501.
- Arias CF, Romero P, Alvarez V, Lopéz S. Trypsin activation pathway of rotavirus infectivity. Journal of Virology 1996; 70:5832-5839.
- Barnes HJ. Viral enteric infections. In: SAIF, YF. Diseases of poultry. 10th ed. Ames: Iowa State University Press; 1997. p 685-686.
- Bergeland ME, McAdaragh JP, Stotz I. Rotaviral enteritis in turkey poults. In: Proceedings of the 26 th Western Poultry Diseases Conference; 1977; Davis, CA: University of California, 1977. p.129-120.
- Brüssow H, Nakagomi O, Gerna G, Eichhorn W. Isolation of an Avianlike group A rotavírus from a calf with diarrhea. Journal of Clinical Microbiology 1992; 30(1):67-73.
- Dea S, Tijssen P. Viral agents associated with outbreaks of diarrhea in turkey flocks in Quebec. Canadian Journal of Veterinary Research 1988; 52:53-57.
- Estes MK. Rotaviruses and their replication, In: Fields BN, Knipe DM, Howley PM editor. Fields virology. 3rd ed. Philadelphia: Lippincott-Raven Publishers, Philadelphia; 1996.
- Gough RE, Wood GW, Collins MS, Spackman D, Kemp J, Gibson LAC. Rotavirus infection in pheasant poults. Veterinary Record 1985; 116:295.
- Gough RE, Wood GW, Spackman D. Studies with an atypical avian rotavirus from pheasants. Veterinary Record 1986; 118:611-612.
- Hayhow CS, Saif YM. Experimental infection of specific-pathogen-free turkey poults with single and combined enterovirus and group A rotavirus. Avian Diseases 1993; 37:546-557.
- Herring AJ, Inglis NF, Ojeh CK, Snodgrass DR, Menzies JD. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. Journal of Clinical Microbiology 1982; 16:473-7.
- Kang SY, Nagaraja KV, Newman JA. Primary isolation and identification of avian rotaviruses from turkeys exhibiting signs of clinical enteritis in a continuous MA-104 cell line. Avian Diseases 1986; 30:494-499.
- Jones RC, Hughes CS, Hentry RR. Rotavirus infection in commercial laying hens. Veterinary Record 1979; 104:22.
- McNulty MS, Allan GM, Todd D, McFerran JB, McCracken RM. Isolation from chickens of a rotavirus lacking the rotavirus group antigen. Journal of General Virology 1981; 55:405-413.
- McNulty MS. Rotavirus infections. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE. Diseases of poultry. 11th ed. AMES, IA: Iowa State University Press; 2003. p.308-317.
- Porter RE. Bacterial enteritis of poultry. Poultry Science 1998; 77 (8):1159-1165.
- Reynolds DL, Saif YM, Theil KW. A survey of enteric viruses of turkey poults. Avian Diseases 1987a; 31:89-98.
- Reynolds DL, Theil KW, Saif YM. Demonstration of rotavirus and rotavirus-like virus in the intestinal contents of diarrheic pheasant chicks. Avian Diseases 1987b; 31:376-379.
- Rodriguez CAR, Brandão PE, Ferreira F, Buzinaro MG, Jerez, JA. Improved Animal Rotavirus Isolation in MA-104 cells using different trypsine concentrations. Arquivos do Instituto Biológico 2004; 71: 437-441.
- Snodgrass DR, Terzolo HR, Sherwood D, Campbell I, Menzies JD, Synge BA. Etiology of diarrhoea in young calves. Veterinary Record 1986; 119:31-34.
- Sugiyama M, Goto K, Uemukai H, Mori Y, Ito N, Minamoto N. Attachment and infection to MA104 cells of avian rotaviruses require the presence of sialic acid on the cell surface. The Journal of Veterinary Medical Science 2004; 66(4):461-463.
- Tamehiro CY, Alfieri AF, Médici KC, Alfieri AA. Segmented double-stranded genomic RNA viruses in fecal samples from broiler chicken. Brazilian Journal of Microbiology 2003; 34(4):344-348.
- Theil KW, Reynolds DL, Saif YM. Isolation and serial propagation of turkey rotaviruses in a fetal rhesus monkey kidney (MA104) cell line. Avian Diseases 1986; 30:93104.
- Tzipori S. The relative importance of enteric pathogens affecting neonates of domestic animals. Advances in Veterinary Science and Comparative Medicine 1985; 29:103-206.
- White RG, Mebus CA, Twiehaus MJ. Incidence of herds infected with a neonatal Calf Diarrhea Virus (NCDV). Veterinary Medicine- Small Animal Clinician 1970; 65:487-490.
- Yason CW, Schat KA. Experimental infection of specific-pathogen-free chickens with avian rotaviruses. Avian Diseases 1986; 30:551-556.
Mail Address:
Publication Dates
-
Publication in this collection
05 Mar 2007 -
Date of issue
Sept 2006
History
-
Accepted
Sept 2006 -
Received
Jan 2006