Abstract
Trachea, lung, and conjunctive samples from 51 commercial layer farms from Bastos region, São Paulo, Brazil, were submitted to nested-PCR and virus isolation in SPF chicken embryos for ILT diagnosis. This region experienced an outbreak characterized by respiratory signs, decrease in egg production and increased mortality. Out of the 51 tested field samples, 23 were positive for ILT by nested-PCR, 22 were positive after the virus isolation, and 24 were positive when both techniques were used. Newcastle disease virus, Avian pneumovirus, or Mycoplasma gallisepticum were not detected. Infectious bronchitis virus was detected in one farm and Mycoplasma synoviae was detected in eight farms. The high incidence of infectious laryngotracheitis virus (ILTV) detection, the high correlation between the observed clinical signs and the ILTV detection, and the results of differential diagnosis demonstrated that ILTV was the causative agent of the outbreak of respiratory disease observed in Bastos region, São Paulo, Brazil.
Infectious laryngotracheitis; nested-PCR; Virus isolation
Survey of infectious laryngotracheitis outbreak in layer hens and differential diagnosis with other respiratory pathogens
Chacón JLVI; Brandão PEBII; Villarreal LYBI; Gama NMIII; Ferreira AJPI, *
ILaboratório de Ornitopatologia - Departamento de Patologia, FMVZ/USP
IIDepartamento de Medicina Veterinária Preventiva e Saúde Animal, FMVZ/USP
IIIInstituto Biológico, Bastos, SP
Mail Address Mail Address Antonio J. Piantino Ferreira Departamento de Patologia Faculdade de Medicina Veterinária e Zootecnia Universidade de São Paulo Av. Prof. Dr Orlando Marques de Paiva, 87 Cidade Universitária, Butantã 5.508-900. São Paulo, SP, Brasil E-mail: ajpferr@usp.br
ABSTRACT
Trachea, lung, and conjunctive samples from 51 commercial layer farms from Bastos region, São Paulo, Brazil, were submitted to nested-PCR and virus isolation in SPF chicken embryos for ILT diagnosis. This region experienced an outbreak characterized by respiratory signs, decrease in egg production and increased mortality. Out of the 51 tested field samples, 23 were positive for ILT by nested-PCR, 22 were positive after the virus isolation, and 24 were positive when both techniques were used. Newcastle disease virus, Avian pneumovirus, or Mycoplasma gallisepticum were not detected. Infectious bronchitis virus was detected in one farm and Mycoplasma synoviae was detected in eight farms. The high incidence of infectious laryngotracheitis virus (ILTV) detection, the high correlation between the observed clinical signs and the ILTV detection, and the results of differential diagnosis demonstrated that ILTV was the causative agent of the outbreak of respiratory disease observed in Bastos region, São Paulo, Brazil.
Keywords: Infectious laryngotracheitis, nested-PCR, Virus isolation.
INTRODUCTION
Infectious laryngotracheitis (ILT) is a highly contagious acute respiratory disease of chickens caused by a herpesvirus of the family Herpesviridae. This disease is common in areas of intensive poultry production, and its outbreaks result in high economics losses due to increased mortality, decreased growth rates, and lower egg production (Guy & Bagust, 2003; Humberd et al., 2002).
Although Infectious laryngotracheitis virus (ILTV) strains are antigenically homogenous, ILTV strains naturally vary in virulence, from highly virulent strains, causing high morbidity and mortality, to strains with low virulence, which that produce mild-to-unapparent infection (Bauer et al., 1999; Guy & Bagust, 2003). Clinical signs associated with the severe form of the disease include gasping, depression, nasal discharge, conjunctivitis, and expectoration of bloody mucus. Upon gross examination of the trachea, characteristic severe hemorrhages and mucus plugs are observed (Cover, 1996; Sellers et al., 2004). The clinical signs associated with less severe forms of the disease include conjunctivitis, swelling of the infraorbital sinuses, closed eyes, persistent nasal discharge and mild tracheitis (Timurkaan et al., 2003). Although some clinical signs of the disease are characteristic, in less severe episodes many symptoms are common to other respiratory diseases of poultry.
Many techniques have been described for the detection of ILTV. The detection of antibodies by serum neutralization or enzyme-linked immunosorbent assay (ELISA) can be used, but serological tests do not provide a timely diagnosis (Bauer et al., 1999; Sander & Thayer, 1997). Histopathology is used for the detection of syncytial cells and intranuclear inclusion bodies, but, as necrosis and sloughing destroy the infected epithelium, ILTV inclusion bodies can only be detected in tracheal sections from days 1 to 5 post-infection (Abbas et al., 1996; Humberd et al., 2002; Tinurkaan et al., 2003). The virus can be isolated from field material in specific-pathogen-free (SPF) chickens embryos inoculated via the chorioallantoic membrane (CAM), or by isolation in primary chicken embryo kidney (CEK) cells, chicken embryo liver (CELi) cells, or chicken kidney cells (Hugest et al., 1991; Schnitzlein et al., 1994). Virus isolation is widely used, and provides adequate; however, it is time-consuming (Hugest et al., 1988).
Polymerase chain reaction (PCR) has been used successfully to detect ILTV DNA from the trachea and extratracheal sites, such as the conjunctiva and the trigeminal ganglia (Abbas et al., 1996; Alexander et al., 1998; Alexander & Nagy, 1997; Williams et al., 1992).
The first report on ILTV in Brazil was published in 1974, and since then, some cases based on virological and serological tests were reported (Hipólito et al., 1974; Vargas, 1995). By the end of 2002, there was an outbreak of respiratory disease characterized by respiratory signs, decreased egg production, and increased mortality in commercial layer farms in the region of Bastos, São Paulo, Brazil. ILT was clinically diagnosed, based on the observed clinical signs and lesions.
This article describes the first outbreak of severe ILT in commercial layer hens in the region of Bastos, and establishes its differential diagnosis with other respiratory diseases.
MATERIALS AND METHODS
Samples
Trachea, lung, and conjunctive samples were collected from 11- to 104-week-old commercial laying hens housed in 51 farms in the region of Bastos, São Paulo, during an outbreak of respiratory disease for diagnostic and epidemiological studies. Samples from randomly selected 14 and 37 farms with and without clinical signs, respectively, were collected during 2003. The clinical signs observed were depression, decreased egg production, nasal discharge, moist rales, coughing, gasping, dyspnea, expectoration of blood-stained mucus, and increased mortality. A pool of the three tissues mentioned above from eight birds in each farm were homogenized in phosphate-buffered saline solution at 0.1 M, pH 7.4 (PBS), suspended in a 10% concentration (w/v), and centrifuged at 3000 x g for 20 min.
A field isolate of ILTV was obtained from the Agriculture Department Laboratory of the state of São Paulo. La Sota vaccine strain of the Newcastle disease virus (NDV), Holland H120 vaccine strain of the infectious bronchitis virus (IBV) , RTV vaccine strain 8544 of the Avian Pneumovirus (APV), 6/85 vaccine strain of Mycoplasma gallisepticum, and WVU-1853-ATCC vaccine strain of Mycoplasma synoviae were obtained from Intervet Laboratories (Brazil), and used as positive controls. Ultra pure water was used as negative control.
DNA and RNA extraction
Total RNA was extracted with TRIzol reagent (InvitrogenTM), according to the manufacturer's instructions, and DNA was extracted as described by Chomksinsky (1993).
ILTV detection by Nested-PCR
DNA was directly extracted from clinical samples of 51 farms, and submitted to Nested-PCR for ILTV DNA detection, aiming at amplifying a 219-bp fragment of the ILTV glycoprotein E gene. Two sets of specific primers were designed from one published sequence in GenBank (accession number: NC006623) with FAST PCR 3.3.64 (© 1999-2004 PCR Team, Institute of Biotechnology, University of Helsinki, Filand): GE1S (5'CGTATACCATCCTACAGACGGCA 3'), GE2AS (5'CGTACAATGGTTCGGTCTTGGA3'), for PCR; and GE3S (5'AGTCCTCTTATAGCCATCCCCA3'), and GE4AS (5' CACCCCCGCGACGACGAAGT 3') for nested-PCR.
Amplification and nested-PCR Five µL of the extracted DNA were added to the PCR mix [1x PCR BufferTM (InvitrogenTM), 0.2 mM of each dNTP, 0.5 pmol/µL of each primer (GES1 and GEAS2), 1.5 mM MgCl2, 25.25 µL ultra-pure water and 1.25U Taq DNA polymerase to a 50µL final reaction], and submitted to 94ºC/5', 40 cycles of 94ºC/1', 58ºC/2' and 72ºC/2', followed by 72ºC/10' for final extension. The second round (nested) amplification was carried out with 5µL of the first PCR product added to the PCR mix, similarly as to first amplification, but employing GES3 and GEAS4 primers, using the same cycles.
Each step (DNA extraction, PCR and nested-PCR, and finally electrophoresis) was carried out in different rooms. Then, 10 µL of the nested product were analyzed in 1.5% agarose gel electrophoresis stained with 0.5 µg/ml ethidium bromide. The samples that produced the expected 219 bp nested-PCR product were considered as positive.
Virus isolation
A pool of trachea, lung, and conjunctive from hens in each farm was used for virus isolation in embryonated chicken eggs. A suspension of each sample was filtered through 0.45 mm and 0.22 mm membranes, and 30 µm gentamycin (1mg/mL) were added before the suspension was inoculated in the chorioallantoic membrane (CAM) of five 9-day-old specific-pathogen-free (SPF) embryonated chicken eggs (Biovet Laboratory). The inoculated eggs were incubated at 37ºC, and daily candled to check for embryo viability. CAM was harvested 6 days postinoculation. Field samples were submitted to at least 5 serial passages.
Differential diagnosis
Primers and reaction conditions described by Pang et al. (2002), Cavanagh et al. (2002), Cavanagh et al. (1999), Nascimento et al. (1991), and Lauerman (1998) were used for the detection of Newcastle disease virus, infections bronchitis virus, avian pneumovirus, Mycoplasma gallisepticum, and Mycoplasma synoviae, respectively.
RESULTS
ILTV detection by Nested-PCR
The nested-PCR technique amplified the expected 219 bp-long fragment length from isolated and clinical sample strains (Figure 1). The standardized reaction did not amplify DNA of any of the other avian pathogens included in this study. This reaction presented 95.45% sensitivity, and 93.1% specificity as compared to virus isolation technique.
Standardized PCR directly amplified ILTV DNA from clinical samples of 23 of the 51 farms (Table 1). In 11 of these 23 positive cases, respiratory signs, reduced egg production, and increased mortality were observed. Twenty-one positive cases were from hens in lay. As to analyzed tissues, ILTV DNA was detected by PCR in trachea, lung, and conjunctive in 18, 5 and 12 cases respectively.
Virus isolation
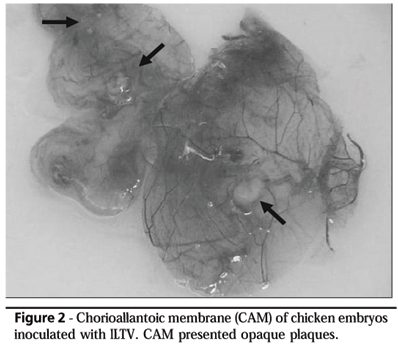
ILTV was isolated from 22 farms when inoculated via chorioallantoic membrane (CAM). ILTV typical lesions, such as opaque plaques on the CAMs, were observed five days post-inoculation (Figure 2). Field samples were isolated after first (6 cases), second (3 cases), third (3 cases), fourth (3 cases), and fifth (7 cases) passages (Table 1).
Differential diagnosis
Twenty-four farms were positive when PCR and virus isolation were used in a parallel diagnosis. Infectious bronchitis virus (IBV) and Mycoplasma synoviae (MS) were detected by PCR in one and eight farms, respectively. One case of IBV-MS concurrent infection and six cases of ILTV-MS co-infections were detected. Newcastle disease virus, avian pneumovirus, and Mycoplasma gallisepticum were not detected in any of the samples. All positive controls resulted in the expected fragments, while no amplifications were found in the reactions corresponding to negative controls.
DISCUSSION
Diagnosis is the first step to establish control procedures and to prevent virus dissemination during an ILT outbreak. Therefore, laboratorial tests offering fast and highly specific results are necessary.
The standardized nested-PCR performed in this study yielded high analytical sensitivity and specificity. DNA detection limit was between 3 and 6 ng/µl, which is higher than the limit reported by Abbas et al. (1996) and Humberd et al. (2002), and lower than the results obtained by Alexander & Nagy (1997) (62.5 ng/µl). This technique did not amplify DNA or cDNA of the other avian pathogens included in this study. In addition, when two PCR products were submitted to DNA sequencing, the identity of amplified fragment was confirmed (data not shown).
As Standardized nested-PCR was used due to its high efficiency to detect ILTV. Samples were collected from commercial laying hens from Bastos region, São Paulo State. Poultry from this region had been suffering respiratory disease, with high mortality and lower egg production due to lesions in respiratory tract. ILT was suggested based on clinical signs and lesions observed in the field, and by the fact that no ILT vaccine had been used before, as this disease had never been reported in this area.
Out of the 51 tested samples, 23 field samples were positive for ILT in nested-PCR, 22 samples were positive in virus isolation, and 24 were positive when both techniques were performed.
Most research studied focusing on laboratorial diagnosis and standardization of new techniques used almost exclusively trachea samples. In this study, trachea, lung, and conjunctive tissues were included, and separately tested by nested-PCR because these are the target organs involved in replication and infection cycles.
In this work, out of 23 ILT positive farms diagnosed by nested-PCR, ILTV was detected in trachea in 78% of the positive cases, which explains the choice of this organ for ILT diagnosis. In addition, 22% of ILT-positive cases were detected in lungs, and 52% were detected in conjunctive samples, suggesting the importance of including conjunctive samples for ILT diagnoss. When using nested-PCR, samples consisting only of trachea were not efficient to detect all positive cases. Nevertheless, when trachea and conjunctive sample results were pooled, it was possible to detect 100% of the positive cases. This shows the importance of conjunctive samples for the accuracy of ILT diagnosis.
Nested-PCR results were compared to isolation in embryonated chicken egg CAM results for ILT diagnosis at the farm level. Nested-PCR, as standardized in the present study, presented high relative sensitivity (95.45%) and specificity (93.1%) as compared to virus isolation. Therefore, the nested-PCR described here may be useful to confirm ILT in suspect cases. In addition, results can be obtained in less than 24 hours, which is essential for decision-making during outbreaks.
The correlation between results of virus isolation and nested-PCR was very significant, that is,in all samples from which ILTV was isolated, viral DNA was detected by nested-PCR, with one exception (in this case, ILTV was isolated in the fifth passage, but the farm was negative in nested-PCR when directly tested in clinical samples). This finding may be explained by the low concentrations of viral particles in the original sample. This is common in early and late infection stages. It was reported that the highest virus titer is recovered from experimentally infected birds between days 2 and 4 post-infection (PI), which then decreases until day 6 PI, after which no virus is recovered (Bagust 1986, Bagust, et al. (1986). Inflammation-associated factors and Taq polimerase inhibitors present in tissues can also produce false-negative results in PCR reactions.
In two farms positive for ILT in nested-PCR, it not only possible to isolate ILTV until the fifth passage. These samples might have had inactivated viral particles, and consequently replication in embryonated eggs did not occur. Other possibilities are low virus concentration in original sample and the need of additional passages.
The comparison of PCR to virus isolation a widespread method for diagnosing ILT indicates that, very early in infection, PCR is not as efficient as virus isolation in identifying infected birds. Later, PCR yields higher numbers of positive samples as compared to virus isolation (Alexander & Nagy, 1997). In some cases, the failure to isolate ILTV may be due to low virus concentrations present in carrier birds (Abbas & Andreasen, 1996; Williams et al., 1994). Latent ILTV was detected in trigeminal ganglia of birds that recovered from the disease 61 days after infection by PCR, but it was not possible to isolate the virus (Williams et al., 1992).
A high frequency of ILTV infection was detected in this study: 47% (24/51). The positive results in the nested-PCR are consistent with the characteristic laryngotracheitis symptoms, such as tracheitis, watery eyes, and dyspnea, observed in the surveyed farms. In 13 of the 24 positive farms, no clinical signs were observed during sample collection. Clinical signs usually appear between 6 and 12 days PI, but the PCR technique can detect DNA virus already on the third day PI (Alexander & Nagy 1997). Consequently, it is possible that samples were collected in the early stages of infection, before to the appearance of clinical signs. Eleven of the 14 farms with respiratory disease (79%) were ILT-positive. This high correlation between possible ILT clinical signs and ILTV detection suggests that this pathogen caused the clinical manifestations observed.
ILT was more frequently diagnosed in farms with hens in lay (87.5% of cases), while only three of the 12 farms with younger birds included in the study were ILT-positive. When considering farms with respiratory disease, 93% of cases included adult birds, whereas only one from three ILT-positive farms with younger birds showed clinical signs during sample collection. These results are consistent with those obtained by other authors. Cover (1996) observed that all birds are susceptible to infection, but clinical disease is more frequent in adult or in lay birds.
The signs and lesions observed in field, and the high frequency of ILTV, detected by nested-PCR and confirmed by virus isolation, suggest that ILTV was probably responsible for the outbreak. However, as the observed signs could have been caused by other pathogens, differential diagnosis with other respiratory agents was performed. Infectious bronchitis virus was detected in one farm, which presented respiratory disease and was ILT-negative. Mycoplasma synoviae (MS) was detected in eight farms with clinical disease, but in six cases, ILTV was also detected. However, considering the high correlation between clinical disease and ILTV detection in the present study, MS probably acted as secondary agent. Avian influenza was ruled out by laboratory tests performed by the Official Services of São Paulo State (Data not shown). Avibacterium paragallinarum, Pasteurella multocida, and Ornitobacterium rhinotracheale were not included in the differential diagnosis of this study because the disease caused by these agents does have not epidemic characteristics, as those observed during the outbreak in the region of Bastos.
The results obtained herein demonstrate high ILTV incidence , high correlation between clinical findings and detection of this agent, using virus isolation and nested-PCR. It was shown that this testis a fast, sensitive, and highly specific tool for ILT diagnosis.
Therefore, based on these results, as well as on the clinical history and on differential diagnosis results, we conclude that ILTV was responsible for the outbreak of respiratory disease that began by the end of 2002 in commercial layers in the region of Bastos (Sao Paulo State), and that the use of the PCR described the present study in both trachea and conjunctive samples may allow, in addition to diagnosis during outbreaks, epidemiological surveillance,and possibly molecular epidemiology by DNA sequencing, for instance.
Acknowledgements
The authors thank CDA SAA, SP, for the ILTV field sample, and CNPq for the post-graduation student fellowship.
Arrived: December / 2005
Approved: January / 2007
This study is part of the M. Sc. dissertation presented by Jorge Luis Chacón Villanueva in the Experimental and Comparative Pathology Post-Graduation Program, Department of Pathology, Faculty of Veterinary Medicine University of São Paulo, Brazil.
- Abbas F, Andreasen JR, Jackwood MW. Development of a Polymerase chain reaction and a nonradioactive DNA probe for Infectious laryngotracheitis virus. Avian Diseases 1996; 40(1): 56-62.
- Abbas F, Andreasen JR. Comparison of diagnostic test for Infectious laryngotracheitis. Avian Diseases 1996; 40(2):290-295.
- Alexander HS, Key DW, Nagy E. Analysis of Infectious laryngotracheitis virus isolates from Ontario and New Brunswick by the Polimerase chain reaction. Canadian Journal Veterinary Research 1998; 62(1):68-71.
- Alexander HS, Nagy E. Polymerase chain reaction to detect Infectious laryngotracheitis virus in conjuntival swabs from experimentally infected chickens. Avian Diseases 1997; 41(3):646-653.
- Bagust TJ. Laryngotracheitis (Gallid-1) Herpesvirus Infection in the chicken. 4. Latency establishment by wild and vaccine strains of ILT virus. Avian Pathology 1986; 15(3):581-595.
- Bagust TJ, Calnek B, WFahey KJ. Gallid-1 Herpesvirus Infection in the chicken. 3. Reinvestigation of the pathogenesis of Infectious Laryngotracheitis in acute and early post-acute respiratory diseases. Avian Diseases 1986; 30(1):179-190.
- Bauer B, Lohr JE, Kaleta EF. Comparison of commercial ELISA test kits from Australia and the USA with the serum neutralization test in cell cultures for the detection of antibodies to the Infectious Laryngotracheitis Virus of chickens. Avian Pathology 1999; 28(1): 65-72.
- Cover MS. The early history of Infectious laryngotracheitis. Avian Diseases 1996; 40(3):494-500.
- Guy JS, Bagust TJ. Laryngotracheitis. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editor. Diseases of poultry. 11 ed. Ames (IA): Iowa State University Press; 2003. p. 121-134.
- Hipólito O, Soares LA, Pereira OAC, Pinto AA, Bottino JA. Isolamento e identificação do vírus da Laringotraqueite infecciosa das galinhas no Brasil. In: Congresso Brasileiro de Microbiologia; 1974; Rio de Janeiro, RJ. Brasil. p. 16.
- Hughes CS, Williams RA, Gaskell RM, Jordan FT, Brandbury JM, Bennett M, Jones RC. Latency and reactivation of infectious laryngotracheitis vaccine virus. Archives of Virology 1991; 121(2): 213-218.
- Hughes CS, Jones RC. Comparison of cultural methods for primary isolation of Infectious Laryngotracheitis Virus from field material. Avian Pathology 1988; 17(3):295-303.
- Humberd J, García M, Ribler SM, Resurrección RS, Brown TP. Detection of Infectious Laryngotracheitis Virus in formalin-fixed, paraffin-embedded tissues by Nested polymerase chain reaction. Avian Diseases 2002; 46(1):64-74.
- Sander JE, Thayer SG. Evalutation of ELISA titers to Infectious Laryngotracheitis. Avian Diseases 1997; 41(2):426-432.
- Schnitzlein WM, Radzevicius J, Tripathy DN. Propagation of Infectious Laryngotracheitis Virus in an Avian liver cell line. Avian Diseases 1994; 38(2):211-217.
- Sellers H, Garcia M, Glisson J, Brown T, Sander J, Guy J. Mild infectious Laryngotracheitis in broilers in Southeast. Avian Diseases 2004; 48(2):430-436.
- Timurkaan N, Yilmaz F, Bulut H, Ozer H, Bolat Y. Pathological and immunohistochemical findings in broilers inoculated with a low virulent strain of Infectious laryngotraqueitis virus. Journal Veterinary Science 2003; 4(2):175-180.
- Vargas RES. Laringotraqueíte infecciosa das aves: Estudo epidemiológico em plantéis avícolas no Estado do Rio Grande do Sul [dissertação]. Porto Alegre (RS): Universidade Federal Rio Grande do Sul; 1995.
- Williams RA, Bennet M, Bradbury JM, Gaskell RM, Jones RC, Jordan FTW. Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction. Journal General Virology 1992; 73(9):2415-2430.
- Williams RA, Savage CE, Jones RC. A comparison of direct electron microscopy, virus isolation and a DNA amplification method for the detection of Avian Infectious Laryngotracheitis Virus in field material. Avian Pathology 1994; 23(4):709-720.
Publication Dates
-
Publication in this collection
24 July 2007 -
Date of issue
Mar 2007
History
-
Accepted
Jan 2007 -
Received
Dec 2005