Abstract
The objective of this experiment was to evaluate the effect of a probiotic (Bacillus subtilis, strain DSM 17299) in broiler diets on feed intake, weight gain, and feed conversion ratio. The experiment included 1,200 male Ross broilers from 1 to 42 days of age. Birds were randomly allocated to 4 treatments, with 10 replicates of 30 birds. The following treatments were applied: T1 - Negative Control (basal diet, with no added growth promoter; T2 - Negative Control + Bacillus subtilis (8 x 10(5) CFUs/g feed); T3 - Negative Control + Bacillus subtilis (3 x 10(5) CFUs/ g de feed) and T4 - Positive Control (avilamycin + anticoccidial from 1 to 35 days of age). At 21, 35, and 42 days of age, there was an increase of antibiotic-free diet intake as compared to the diets with growth promoters (p<0.05), but there was no difference, however, as compared to the diets with probiotic as a growth promoter (p>0.05). The use of growth promoter did not improve weight gain at the studied ages. There was a marked improvement in the feed conversion ratio of broilers fed the diet with antibiotics and of broilers fed the diet with added B. subtilis. It is concluded that the Bacillus subtilis probiotic can be used as a growth promoter in broiler diets.
Broilers; performance; probiotic
On the use of a probiotic (Bacillus subtilis - strain DSM 17299) as growth promoter in broiler diets
Opalinski M; Maiorka A* * CNPq Researcher ; Dahlke F; Cunha F; Vargas FSC; Cardozo E
Universidade Federal do Paraná Setor de Ciências Agrárias
Mail Address Mail Address A Maiorka Rua dos Funcionários 1540 Bairro Cabral 80.035-050. Curitiba, PR, Brazil Phone: (55) 41 33505732 E-mail: amaiorka@ufpr.br
ABSTRACT
The objective of this experiment was to evaluate the effect of a probiotic (Bacillus subtilis, strain DSM 17299) in broiler diets on feed intake, weight gain, and feed conversion ratio. The experiment included 1,200 male Ross broilers from 1 to 42 days of age. Birds were randomly allocated to 4 treatments, with 10 replicates of 30 birds. The following treatments were applied: T1 Negative Control (basal diet, with no added growth promoter; T2 Negative Control + Bacillus subtilis (8 x 105 CFUs/g feed); T3 Negative Control + Bacillus subtilis (3 x 105 CFUs/ g de feed) and T4 Positive Control (avilamycin + anticoccidial from 1 to 35 days of age). At 21, 35, and 42 days of age, there was an increase of antibiotic-free diet intake as compared to the diets with growth promoters (p<0.05), but there was no difference, however, as compared to the diets with probiotic as a growth promoter (p>0.05). The use of growth promoter did not improve weight gain at the studied ages. There was a marked improvement in the feed conversion ratio of broilers fed the diet with antibiotics and of broilers fed the diet with added B. subtilis. It is concluded that the Bacillus subtilis probiotic can be used as a growth promoter in broiler diets.
Keywords: Broilers, performance, probiotic.
INTRODUCTION
The use of antibiotics as growth promoters has been a common practice in poultry production since the 1950s (Dibner & Richards, 2005). The first indications of their positive effects in broilers were described by Moore et al. (1946), and growth promoters have been constantly used since then due to significant enhancements in weight gain, feed conversionratio, and livability. The indiscriminate use of antibiotics, however, led to international consumer market pressure, which limited their traditional use, mainly due to possible resistance of pathogenic bacteria strains or opportunistic flora (Fuller, 1989), as well as to changes in the existing symbiosis between animals and the desirable flora (Mulder, 1991). In addition, there are evidences of the presence of antibiotic residues in animal tissues that will be consumed by humans, which may cause resistance of human flora to these groups of antibiotics. Moreover, cross-resistance to antibiotics used in the therapy of humans and other animals could also result (Van den Bogaard et al., 1997; Van den Bogaard, 1998; Van den Bogaard & Stobberingh, 2000; Caprioli et al., 2000; Pelicano et al., 2004, Bywater, 2005). Starr & Reynolds (1951) published one of the first reports on antimicrobial resistance caused by the presence of antibiotics in animal products by feeding turkeys with streptomycin. Resistance to tetracycline was also shown after its use as growth promoter (Barnes, 1958; Elliott & Barnes, 1959). Kolár et al. (2002) described an E. coli strain resistant to 21 of the 23 antibiotics used by the poultry industry.
In order to meet market and international health organization demands, the poultry industry is studying alternatives to antibiotics that could be both economically feasible, and maintain performance levels. Probiotics can be listed among these products. Probiotics are live organisms that favorably affect the animal body when constantly provided in the diet, and act by balancing the intestinal microbiota (Fuller, 1989). As a consequence, the intestinal environment can be improved for the processes involving nutrients digestion and absorption.
Several studies found in literature report the inclusion of probiotics in broiler diets (Jin et al., 1998; Loddi et al., 2000; Maiorka et al., 2001; Edens, 2003; Pelicano et al., 2004; Pelícia et al., 2004; Dibner & Richard, 2005; Huff et al., 2005; Ricke et al., 2005). The results, however, are not unanimous, and the efficacy of these products has not been fully proved yet.
Thus, the objective of the present study was to evaluate the effect of adding a probiotic, Bacillus subtilis (strain DSM 17299), as a growth promoter on broiler performance.
MATERIAL AND METHODS
The experiment included a total number of 1,200 male broiler chicks, hybrid Ross commercial line, reared from 1 to 42 days of age, distributed in pens with a wood-shavings litter (second flock litter) in a brick poultry house, according to Brazilian traditional broiler management principles.
Experimental diets
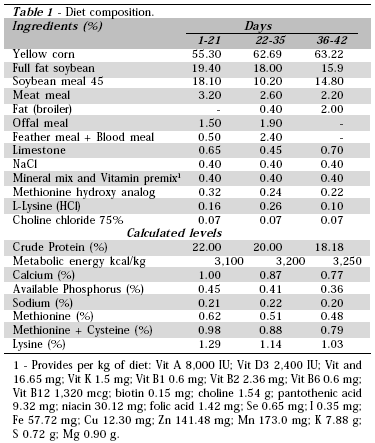
All feeds had the same nutritional value, and were formulated with ingredients commonly used by the Brazilian poultry industry (Table 1), and provided ad libitum. Antibiotic growth promoters were not added to the diets, except in the positive control treatment (T4), which used Avilamycin (10 ppm) from 1 to 35 days + Monensin (110 ppm from 1 to 21 days), and Salinomycin (66 ppm from 22 to 35 days).
Treatments and experimental design
The experiment had 4 treatments with 10 replicates of 30 birds each. The following treatments were applied: T1 Negative Control (basal diet, with no added growth promoter; T2 Negative Control + Bacillus subtilis (8 x 105 CFUs/g feed); T3 Negative Control + Bacillus subtilis (3 x 105 CFUs/g feed) and T4 Positive Control (avilamycin + anticoccidial from 1 to 35 days of age). Birds were randomly allocated to the treatment groups.
Determination of microbial concentration
A quantitative method was used to determine the microbial concentration of Bacillus subtilis, and the results were expressed as CFU/g. One or more samples were homogenized with sterile dilutant, and this new dilution was prepared for each sample and submitted to heat treatment of 80ºC / 10 minutes. Decimal dilutions (dilutant: casein peptone, NaCl, and antifoaming agent) are prepared from the heat-treated samples, transferred to TBA agar plates (trypticase soy agar containing blood), and incubated.
Evaluated parameters
At 21, 35, and 42 days of age, the birds and the remaining feed were weighed to obtain data for the performance evaluation. Feed Intake (FI), weight gain (WG), and feed conversion ratio (FCR) were analyzed to determine the performance.
Statistical analysis
The obtained results were submitted to analysis of variance (SAS, 1998), and the test of Tukey (5% significance level) was used to analyze differences among means.
RESULTS AND DISCUSSION
Performance results from 1 to 21 days of age are presented in Table 2. Feed intake increased in the treatment group with no growth promoter as compared to the group fed diets containing antibiotic as growth promoter (p<0.05). There were no feed intake differences among the other treatment groups (p>0.05), nor in weight gain of broilers fed the different diets. As a result, the best feed conversion ratios were found for the groups in which the broilers were fed the diet containing antibiotic and that containing Bacillus subtilis (8 x 105 CFUs/g feed) as growth promoter.
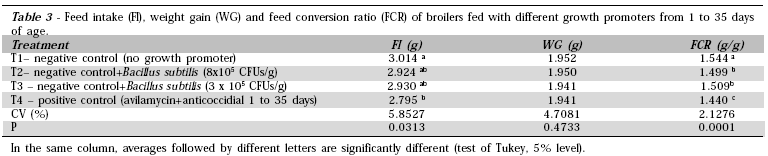
From 1 to 35 days of age, feed intake was higher in broilers fed the basal diet as compared with those fed the diet with antibiotics (Table 3). Also in this period, treatments did not affect weight gain, and broilers that consumed the feed containing antibiotic (Treatment 4) presented better feed conversion ratios followed by the broilers that consumed those containing Bacillus subtilis as compared to those fed the negative-control diet.
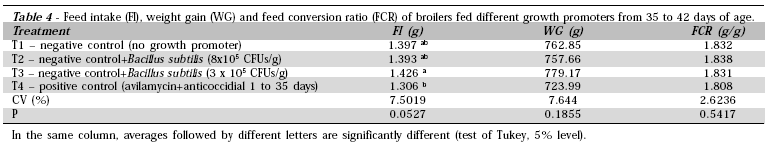
In the finisher phase (35 - 42 days), only birds fed the diets with antibiotic and with Bacillus subtilis (3 x 105 CFUs/g feed) presented different feed intake as compared to the other treatments (Table 4).
When the entire rearing period (Table 5) was evaluated, feed intake of broilers fed antibiotics was significantly lower than in the other treatments. It is also evidenced that the best feed conversion ratio in this period was presented by the broilers fed the diet with antibiotics, followed those receiving the diets with added B. subtilis.
The addition of antibiotic growth promoters to diets may influence broiler weight gain (Jones & Ricke, 2005). However, the main objective of using these compounds in broiler diets is to improve their feed conversion ratio (Dibner & Richards, 2005), as was observed in this study.
The mechanism that explains the action of antibiotics is focused on gastrointestinal tract, as most of these products are not absorbed, and are not efficient as growth promoters in germ-free animals (Coates et al., 1955; Coates et al., 1963). Therefore, it maybe speculated that there is a strong interaction between growth promoters and the intestinal microflora. This improvement in performance due to the action of antibiotics on the microflora can be interpreted in two ways: the first is related to the reduction in the utilization of nutrients by microorganisms, and the second is the decrease of microbial metabolites that interfere with host growth (Visek, 1978; Anderson et al., 1999). In addition, maintaining the integrity of the intestinal mucosa results in high energy requirements, and the decrease of pathogens and intestinal metabolites can also decrease intestinal cell turnover, resulting in more energy available for production. Finally, the reduction of opportunistic pathogens and subclinical infections can also be associated with the use of antibiotic growth promoters (Dibener & Richards, 2005).
At this time, however, the use of these products is being debated due to a possible relation with the resistance to antibiotics used in human antibiotic therapy (Maiorka et al., 2001). The improvement in performance (feed conversion ratio) of birds fed with diets containing the tested probiotic shows that the use of these products is a feasible alternative to antibiotics used as growth promoters. Similar results were also found by Maiorka et al. (2001), Pelicano et al. (2004), and Pelícia et al. (2004). Edens (2003) reported that the addition of a probiotic, with a predominance of Bacillus subtilis, did not affect weight gain of broilers at 42 days of age; however, it improved feed conversion ratio. Pedroso et al. (1999) found that the use of probiotics (Bacillus subtilis) in layers improve feed conversion ratio and egg shell quality. There was also a significant reduction in carcass contamination by enteric bacteria, potentially pathogenic for humans (Maruttta et al., 1996; Fritts et al., 2000), as they are present in smaller numbers in broilers feces.
The inclusion of desirable microorganisms (probiotics) in the diet allows the rapid development of beneficial bacteria in the digestive tract of the host, improving its performance (Edens, 2003). As a consequence, there is an improvement in the intestinal environment, increasing the efficiency of digestion and nutrient absorption processes (Pelicano et al., 2004), which may explain the improvement in feed conversion ratioobserved in the present study. The efficiency of probiotics, however, will depend on the quantitative and qualitative characteristics of microorganisms used in the production of growth promoters (Tournut, 1998), making it difficult to conduct comparative studies between different products.
CONCLUSIONS
The probiotic (Bacillus subtilis strain DSM 17299) improved feed conversion when added to broiler diets containing no antibiotic growth promoters. Despite being banned in some markets, antibiotic inclusion in broiler diet improves feed conversion ratio.
Arrived: June / 2006
Approved: July / 2007
- Anderson DB, McCracken VJ, Aminov RJ, Simpson JM, Mackie RJ, Vestegem MWA. Gaskins HR. Gut microbiology and growth-promoting antibiotics in swine. Pig News and Information 1999; 20:115N-122N.
- Barnes EM. The effect of antibiotics supplements on the faecal streptococci (Lancefiels group D) of poultry. British Veterinary Journal 1958; 114:333-344.
- Coates ME, Davies MK, Kon SK. The effect of antibiotics on the intestine of the chick. British Journal Nutrition 1955; 9:110-119.
- Coates ME, Fuller R, Harrison GF, Lev M, Suffolk SF. Comparison of the growth of chick in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplementations of penicillin. British Journal Nutrition 1963; 17:141-151.
- Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: History and mode action. Poultry Science 2005; 84:634-643.
- Edens FW. An alternative for antibiotic use in poultry: Probiotics. Brazilian Journal of Poultry Science 2003; 5(2):75-97.
- Elliott SD, Barnes EM. Changes in serological type and antibiotic resistance on Lancefield group D streptococci in chickens receiving dietary chlortetracycline. Journal Genetics Microbiology 1959; 20:246-433.
- Fritts CA, Kersey JH, Moti MA, Kroger EC, Yan F, Si J, Jiang Q, Campos MM, Waldroup AL, Waldroup PW. Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens. Journal of Applied Poultry Research 2000; 9(2):149-155.
- Fuller R. Probiotics in man and animals. Journal of Applied Bacteriology 1989; 66:365-378.
- Huff WE, Huff GR, RATH NC, Balog JM, Donoghue AM. Alternatives to Antibiotics: Utilization of bacteriophage to treat colibacilosis and Prevent foodborne pathogens. Poultry Science 2005; 84:655-659.
- Jim LZ, Ho YM, Abdullah N, Jalaludin S. Growth perfermance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus Cultures. Poultry Science 1998; 77:1259-1265.
- Jones FT, Ricke SC. Antibiotics in anial feeds: are there viable alternatives? Poultry Science 2005; 84:663.
- Loddi MM, Gonzales E, Takita TS, Mendes AA, Roça RO. Uso de probiótico e Antibiótico sobre o desempenho, o rendimento e a qualidade de carcaça de frangos de corte. Revista Brasileira de Zootecnia 2000; 29:1124-1131.
- Maiorka A, Santin E, Sugeta S, Almeida JG, Macari M. Utilização de prebióticos, probióticos ou simbióticos em dietas para frangos. Revista Brasileira de Ciência Avícola 2001; 3:75-82.
- Maruta K, Miyazaki H, Masuda S, Takahashi M, Marubashi T, Tadano Y, Takahashi H. Exclusion of intestinal pathogens by continuous feeding with Bacillus subtilis C-3102 and its influence on the intestinal microflora in broilers. Animal Science and Technology 1996; 67(1-2):273-280.
- Moore PR, Evenson A, Luckey TD, McCoy E, Elvehjem, Hart EB. Use of sulphasuccidine, streptothricon and streptomycin in nutrition studies with the chick. Journal of Biological Chemistry 1946; 165:437-441.
- Mulder RWAW. Probiotics as a tool against Salmonella contamination. Misset World Poultry Science 1991; 7:36-37.
- Pedroso AA, Moraes VMB, Ariki J. Effects of protein and probiótico (Bacillus subitilis) levels in pullet and laying hen diets. Brazilian Poultry Science 1999; 1(1):49-54.
- Pelicano ERL, Souza PA, Souza HBA, Leonel FR, Zeola NMBL, Boiago MM. Productive traits of broiler chickens fed diets containing different growth promoters. Brazilian Journal of Poultry Science 2004; 6:177-182.
- Pelícia K, Mendes AA, Saldanha ESPB, Pizzolante CC, Takahashi SE, Moreira J, Garcia RG, Quinteiro RR, Paz ICLA, Komiyama CM. Use of prebiotics and probiotics of bacterial and yeast origin for free-range broiler chickens. British Journal of Poultry Science 2004; 6:163-169.
- Ricke SC, Kundinger MM, Miller DR, Keeton JT. Alternatives to antibiotics: chemical and physical antimicrobial interventions and foodborne pathogen response. Poultry Science 2005; 84:667-675.
- Starr MP, Reynolds DM. Streptomycin resistence of coliform bacteria from turkeys fed streptomycin. In: Proceedings of 51st General Meeting Society of American Bacteriology; 1951; Chicago, IL. p.15-34.
- Tournut JR. Probiotics. In: 35Ş Reunião Anual da Sociedade Brasileira de Zootecnia; 1998; Botucatu, São Paulo, Brasil. p.179-199.
- Visek WJ. The mode of growth promotion by antibiotics. Journal of Animal Science 1978; 46:1447-1469.
Publication Dates
-
Publication in this collection
09 Oct 2007 -
Date of issue
June 2007
History
-
Accepted
July 2007 -
Received
June 2006