Abstract
This experiment was designed with the objective of developing a simple, practical, and high repeatability technique for the simultaneous evaluation of the integrity of the plasmatic and acrosomal membranes, as well as funcional mitochondria of domestic fowl spermatozoa using an association of fluorescent probes. Four ejaculates (motility > 80% and abnormal morphology < 10%) from each of six Ross male broiler breeder (n=24) were diluted in TALP sperm medium (25x10(6) spermatozoa/mL) and split into two aliquots, and one of these aliquots was flash frozen in liquid nitrogen and thawed to damage all cellular membranes. Three treatments were prepared from these aliquots, with the following ratios of Fresh semen:Flash frozen semen: 100:0 (T100), 50:50 (T50), and 0:100 (T0). A 150-µL aliquot of diluted semen was placed in a microcentrifuge tube with the addition of 2-µL PI, 2-µL MITO, and 50-µL FITC-PSA, and incubated at 38.5º C/8 min in the dark. An 8-µL sample was placed on a slide, coverslipped, and examined by epifluorescence microscopy. Each sample had 200 cells counted and classified based on the fluorescence emitted by each probe. By regression analysis, plasma membrane integrity, as detected by PI, was determined as: v=4.17+0.82X (R²=0.95). Acrosome integrity, as detected by FITC-PSA, generated the equation: v=4.19+0.84X (R²=0.96). Functional mitochondria was estimated by the equation v=3.20+0.83X (R²=0.96). This is an efficient technique to simultaneously evaluate plasmatic, acrosomal, and mitochondrial membranes in fowl sperm. It is suggested that its application in flow cytometry systems allows this methodology to be applied in large scale.
Fluorescent probes; membranes; rooster; sperm
Utilization of fluorescent probe association for simultaneous assessment of plasmatic, acrosomal, and mitochondrial membranes of rooster spermatozoa
Celeghini ECCI; Arruda RPI; Albuquerque RII; Silva FHAIII; Faria DEIII; Andrade AFCI; Nascimento JI; Raphael CFI
ILaboratório de Biotecnologia do Sêmen e Andrologia, Departamento de Reprodução Animal, Faculdade de Medicina Veterinária e Zootecnia (FMVZ), Universidade de São Paulo (USP)
IIDepartamento de Nutrição e Produção Animal, FMVZ, USP
IIIDepartamento de Zootecnia, Faculdade de Zootecnia e Engenharia de Alimentos (FZEA), USP
Mail Address Mail Address RP Arruda Av. Duque de Caxias Norte, 225 Post-box 23 13.635-900. Pirassununga, SP, Brazil. Telephone: +55 019 3565-4221 Fax: +55 019 3565-4060 E-mail: arrudarp@usp.br
ABSTRACT
This experiment was designed with the objective of developing a simple, practical, and high repeatability technique for the simultaneous evaluation of the integrity of the plasmatic and acrosomal membranes, as well as funcional mitochondria of domestic fowl spermatozoa using an association of fluorescent probes. Four ejaculates (motility > 80% and abnormal morphology < 10%) from each of six Ross male broiler breeder (n=24) were diluted in TALP sperm medium (25x106 spermatozoa/mL) and split into two aliquots, and one of these aliquots was flash frozen in liquid nitrogen and thawed to damage all cellular membranes. Three treatments were prepared from these aliquots, with the following ratios of Fresh semen:Flash frozen semen: 100:0 (T100), 50:50 (T50), and 0:100 (T0). A 150-µL aliquot of diluted semen was placed in a microcentrifuge tube with the addition of 2-µL PI, 2-µL MITO, and 50-µL FITC-PSA, and incubated at 38.5º C/8 min in the dark. An 8-µL sample was placed on a slide, coverslipped, and examined by epifluorescence microscopy. Each sample had 200 cells counted and classified based on the fluorescence emitted by each probe. By regression analysis, plasma membrane integrity, as detected by PI, was determined as: v=4.17+0.82X (R2=0.95). Acrosome integrity, as detected by FITC-PSA, generated the equation: v=4.19+0.84X (R2=0.96). Functional mitochondria was estimated by the equation v=3.20+0.83X (R2=0.96). This is an efficient technique to simultaneously evaluate plasmatic, acrosomal, and mitochondrial membranes in fowl sperm. It is suggested that its application in flow cytometry systems allows this methodology to be applied in large scale.
Keywords: Fluorescent probes, membranes, rooster, sperm.
INTRODUCTION
Fertility is one of the most important economic traits in poultry production, together with egg hatchability. Male fertility potential may be defined as the capability to produce and to ejaculate spermatozoa that are able to fertilize eggs, which includes accomplishment of all steps of the fertilization process: sperm moving across the female reproductive tract and reaching the sperm storage tubule, binding and penetration into the perivitelline layer, and fertilization (Celeghini et al., 2001).
Sire selection in most commercial poultry breeds is performed on a subjective basis, determined by secondary sexualtraits, such as the comb development (Celeghini et al., 2001). Given the importance of fertility rate in a breeder flock, studies have investigated more precise evaluations of male fertility capacity, since one male is responsible for fertilizing several females. Thus, the analysis of fowl semen characteristics can be used as a tool for the selection of sires kept either under natural mating, or as semen donors in artificial insemination systems (Wilson et al., 1979). Some researchers found significant correlations between semen characteristics and egg fertility (Harris Jr. et al., 1984), sustaining that male fertilizing potential is dependent on semen quality. It is known that, in order to ensure high egg fertility rate, the semen needs to present some characteristics, which evaluation is based on physical and morphological examination. More objective techniques to evaluate semen-fertilizing potential have been proposed, such as tests of sperm penetration in the blastodisc region (Bramwell et al., 1995; Barbarato et al., 1998), and sperm membrane evaluations (Bilgili & Renden, 1984; Chalah & Brillard, 1998).
Despite being highly correlated to fertility, the sperm penetration test is labor-intensive, and difficult to apply in large breeding stocks (Bramwell et al., 1995; Barbarato et al., 1998). However, as membranes play an essential role in maintaining sperm ability to fertilize, they have been evaluated by more objective techniques, such as the use of fluorescent probes (Bilgili & Renden, 1984; Graham et al., 1990; Chalah & Brillard, 1998; Celeghini et al., 2005).
Sperm plasma membrane is responsible for establishing a barrier between intracellular and extracellular environments, which is important to maintain osmotic equilibrium and cellular homeostasis. Damages in this structure lead to cellular instability caused by homeostasis loss, resulting in cellular death. Therefore, plasma membrane integrity exerts a crucial role on sperm survival in the female reproductive tract and its fertilizing ability (Parks & Graham, 1992).
Earlier studies evaluating the integrity of the sperm plasma membrane in fowl, utilizing fluorescent probes, mention the use of ethidium bromide (Bilgili & Renden, 1984). Nevertheless, due to its high toxicity, ethidium bromide application is limited. Propidium iodide (PI), a fluorescent dye with properties similar to ethidium bromide, but less toxic, has DNA affinity, and stains damaged plasma membrane cell nucleus in red (Bayyari et al., 1990; Graham et al., 1990; Chalah & Brillard, 1998). Because it is a stable fluorescent stain, PI has been the most frequently utilized probe in research, with successful results in several species, both by fluorescent microscopy (Garner et al., 1997; Sukardi et al., 1997; Thomas et al., 1997; Chalah & Brilllard, 1998) and by flow cytometry system (Bayyari et al., 1990; Graham et al., 1990; Pintado et al., 2000; Gillan et al., 2005).
In addition to plasmatic membrane integrity, it is important to consider acrosomal membrane integrity and the maintenance of its enzymes, as acrosomal reaction, characterized by the release of the acrosomal enzymes, is essential for the sperm to penetrate the blastodisc region, and to egg fertilization (Bakst & Howarth, 1977). Acrosome integrity can be checked by different fluorescent techniques. Among them, the use of marked lectins is emphasized (Graham et al., 1990), such as Pisum sativum agglutinin (PSA), Ricinus communis agglutinin (RCA), Arachis hypogea agglutinin (PNA) (Cross & Meizel, 1989), and Concanavalia ensiformis (ConA), which are fluorescein isothiocyanate-conjugates (FITC). PSA is an agglutinin from edible peas, and binds to the glycoconjugate of the acrosomal matrix (Cross & Meizel, 1989). It has affinity for terminal a-D-glucosyl and a-D-mannosyl residues of glycoproteins, and binds specifically to the sugar a-mannoside found in acrosomal contents (Sukardi et al., 1997). This agglutinin, when conjugated to FITC, marks the sperm acrosome in yellow-green, and identifies acrosome damage. It can be applied to spermatozoa of different species (Graham et al., 1990; Arruda et al., 2002).
On the other hand, mitochondria, arranged helicoidally in the spermatozoa midpiece, are responsible for producing the energy necessary for flagellar beat. It warrants sperm motility, essential for sperm to cross the female reproductive tract, to be stored in the sperm storage tubule, and to reach the fertilizing site. The energy produced by mitochondria is ATP, generated by internal membrane potential in the respiratory chain (Bereiter-Hahn, 1990). The ability to monitor changes in mitochondrial membrane potential in situ inside of cells can be crucial to interpret cellular physiology changes in various experimental situations. Mitochondrial function can be evaluated by different fluorescent probes, as rhodamine 123; 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolyl carbocyanine iodide (JC-1); 8-(4'-chloromethyl) phenyl-2,3,5,6,11,12,14,15 octahydro-1H,4H,10H,13H-diquinolizino-8H-xanthylium chloride (CMXRos or Mitotracker red); and MitoTracker green FM (MITO). MITO is non-fluorescent in water solutions, and becomes fluorescent once it accumulates in the lipid environment of mitochondria (Haugland, 2005). This probe has been utilized in human sperm to visualize mitochondrial sheath dysplasia (Rawe et al., 2001) and in bovine spermatozoa to verify cryopreservation effects (Garner et al., 1997). MITO stain preferentially accumulates in the mitochondria, independent of mitochondrial potential, and therefore can be used a tool for mitochondrial mass determination (Haugland, 2001). Contradictorily, in bovine cryopreserved sperm, a high correlation was observed between sperm stained by MITO and sperm motility (r = 0.96), as well as sperm viability, as detected by SYBR-14 (r = 0.97), indicating that this probe reflects the functional status of mitochondria (Garner et al., 1997).
Considering that, in order for to fertilize the oocyte, sperm need to have all its membranes intact, it is then vital that sperm evaluation be simultaneous, supplying information on the number of spermatozoa with intact plasmatic and acrosomal membranes, as well as preserved mitochondrial function. The association of fluorescent probes allows the simultaneous evaluation of more than one sperm cell compartment, as well as simultaneous evaluation of plasmatic and acrosomal membranes, using Hoechst 33258 and FITC-PSA association (Arruda et al., 2002), PI and LYSO-G or PI + SYTO-17 and FITC-PNA (Thomas et al., 1997), phycoerythrin (PE)-conjugated PNA associated to probes SYBR-14 and PI (NAGY et al., 2003), or PI and FITC-PSA association (Centola et al., 1990; Graham et al., 1990; Sukardi et al., 1997; Arruda et al., 2002).
Graham et al. (1990) demonstrated that at least three bovine sperm compartments can be simultaneously evaluated by addition of three probes. These authors utilized PI for plasmatic membrane integrity evaluation, PSA to determine acrosomal integrity, and R123 to verify mitochondrial function. For simultaneous evaluation, single flow cytometry apparatus was used, and the validation was performed separately for each probe.
The more sperm parameters are evaluated in a semen sample, the higher the value in the in vitro fertility prognostic. However, in commercial poultry production, a large number of samples must be processed and evaluated. Consequently, this technique may require to be faster and cheaper to be routinely applied.
This experiment was designed with the objective of developing a simple, practical, and highly repeatable technique for simultaneous integrity evaluation of plasmatic and acrosomal membranes, as well as of mitochondrial function in domestic fowl spermatozoa by the association of fluorescent probes.
MATERIALS AND METHODS
Semen Preparation
Four ejaculates from six Ross male broiler breeder (n=24) were utilized, all presenting motility > 80%,and abnormal morphology < 10%. Immediately upon collection, the semen was diluted in TALP sperm medium (Bavister et al., 1983) to a final concentration of 25x106 spermatozoa/mL. The sample of diluted semen was split into 2 aliquots, and 1 aliquot was flash frozen in liquid nitrogen and thawed in 3 continuous cycles in order to damage cellular membranes and to disturb mitochondrial function. Three treatments were prepared from these aliquots, with the following ratios of Fresh semen:Flash frozen semen: 100:0 (T100), 50:50 (T50) and 0:100 (T0).
Spermatozoa Stain
After the preparation, the three samples, T100, T50 and T0, were submitted to a stain technique adapted from Arruda & Celeghini (2003). A 150-µL aliquot of diluted semen was placed in a microcentrifuge tube, and 2-µL PI (3 mM, Sigma, 28,707-5, in DPBS), 2-µL MITO (500 mM, Molecular Probes, M-7514, in DMSO), and 50-µL FITC-PSA (100 µg/mL, Sigma, L-0770, in DPBS) were added. The sample was incubated for 8 minutes at 38.5º C, in the dark.
Fluorimetric Assessment
An 8-µL sample of stained spermatozoa suspension was placed on a slide, a coverslip added, and the examination was immediately performed under epifluorescence microscopy (Nikon, model Eclipse 80i) in a triple filter (D/F/R, C58420), with the set: UV-2E/C (340-380 nm excitation and 435-485 nm emission), B-2E/C (465-495 nm excitation and 515-555 nm emission), and G-2E/C (excitation 540-525 and emission 605-655), at 1.000 x magnification. Each sample had 200 cells counted and classified based on the fluorescence emitted by each probe.
Statistical Analysis
Data obtained from T0, T50, and T100 treatment groups were evaluated by analysis of variance (ANOVA) (SAS, 1998). Treatment means across all samples were compared by Fisher's LSD test. The data of plasmatic and acrosomal membranes integrity, and mitochondrial function (dependent variables) in the treatments T0, T50, and T100 (independent variables) were submitted to simple linear regression analysis by StatView software (SAS, 1998).
RESULTS
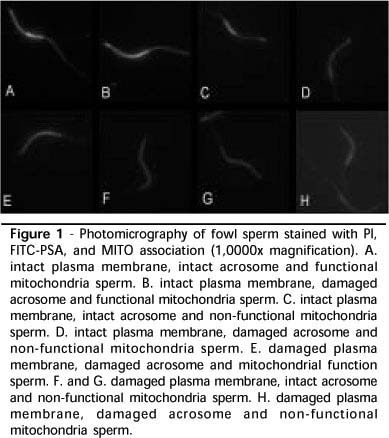
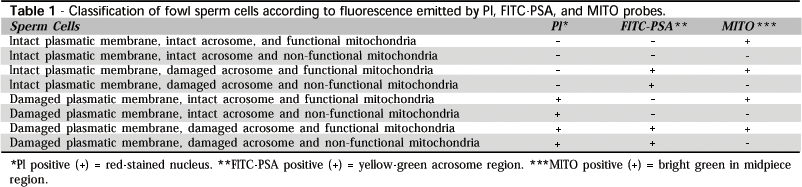
The association of PI, FITC-PSA and MITO probes resulted in eight cell categories, according to plasmatic and acrosomal integrity,and mitochondrial function, as shown in Table 1. The fluorescence standard presented by sperm stained by the fluorescent probes association is shown in Figure 1.
ANOVA and Fisher tests detected statistical differences (p<0.0001) between the T100, T50, and T0 treatment groups, as shown in Table 2, validating the submission of data to linear regression analysis.
Regression analysis results for plasma membrane integrity, as detected by PI, are displayed in Figure 2. Acrosome integrity, as detected by FITC-PSA probe, generated the equation shown in Figure 3. Mitochondrial function estimated by MITO is displayed in Figure 4.
DISCUSSION
In this experiment, the association of fluorescent probes was tested and validated for the simultaneous evaluation of plasmatic (PI) and acrosomal (FITC-PSA) membranes integrity, as well as of mitochondrial function (MITO) in fowl sperm. These tests are important in order to obtain a highly repeatable technique that presents results reflecting the real status of each structure. It is still necessary to identify which probes associate better, as there may be different results with different associations due to changes in its characteristics and fluorescence standards.
The possibility of using the association of fluorescent probe for simultaneous assessment of plasmatic and acrosomal membranes, has already been well described in literature relative to mammalian sperm (Centola et al., 1990; Sukardi et al., 1997; Arruda et al., 2002; Nagy et al., 2003) and poultry sperm (Chalah & Brilllard, 1998). The PI and FITC-PSA probe association to assessplasmatic and acrosome membranes integrity, respectively, as utilized in this experiment, was reported in sheep (Sukardi et al., 1997), human (Centola et al., 1990), horse (Arruda et al., 2002), and cattle (Graham et al., 1990). PI was also associated with probes to evaluate mitochondrial function as R123, MITO and JC-1; these probes showed high positive correlation (r>0.96) with sperm motility (Garner et al., 1997). However, this experiment validated the association of PI and FITC-PSA with an additional probe for mitochondrial function evaluation in fowl sperm.
PI, FITC-PSA and MITO association resulted in very consistent marking of spermatozoa, making it easy to identify the evaluated structures. The association of PI and MITO probes was mentioned by Garner et al. (1997) in bovine spermatozoa assessment. T100, T50 and T0 treatment groups were prepared to validate the technique, according to the methodology described by Thomas et al. (1997). The results obtained were submitted to ANOVA, and means were compared using the Fisher test. The comparison of the results and the confirmation of differences among treatments were important to determine the efficacy of each treatment for technique validation before submitting them to linear regression analysis.
As confirmed by regression analysis results, this technique presented good results and high repeatability. Some MITO characteristics were observed: when it binds to mitochondria that have membrane potential, it emits bright green fluorescence. However, it is important to stress that MITO also binds to regions of the membrane of the head and tail of the sperm in a non-specific fashion, emitting less intense fluorescence, as observed by Garner et al. (1997). This unspecific binding of MITO is, in a way, beneficial to the evaluation, because it makes it possible to visualize the shape of cells with intact plasma membrane. However, it is important to differentiate midpiece stain nuances in functional and non-functional mitochondria. In an attempt to investigate more objectively these associations in bovine sperm, Celeghini et al. (2005) validated two fluorescent-probe techniques for simultaneous evaluation of plasmatic, acrosomal, and mitochondrial membranes, using PI, Hoechst 33342, FITC-PSA, and CMXRos or JC-1, and obtained very consistent results. Nevertheless, it is must be noted that the addition of one additional probe (H342) increases the technique cost.
In the regression analysis equation obtained for plasmatic membrane integrity (Figure 2), evaluated by the exclusion of PI from the nucleus, it is possible to verify that the value of point "a" (where the line crosses the Y axis, i.e., showing the value of Y when X = 0) is next to zero (a = 4.17), as would be expected from a sample that was submitted to flash freezing with the objective of damaging all membranes. The value of "b" (regression coefficient, i. e., how much X varies in relation to Y) is also near the expected (b = 0.82) for T100, representing a sample with at least 80% of motility. The regression coefficient of 95% confirms these observations. Similar results were found by Graham et al. (1990), who compared the efficiency of PI with the stain technique by eosin/nigrosin, and found a positive correlation (r = 0.78) between techniques, and confidence interval of 95%. Positive correlations between PI and eosin/nigrosin were also observed in dogs (r = 0.88) (Peña et al., 1998), boars (r = 0.71) and bulls (r = 0.83) (Pintado et al., 2000). Pintado et al. (2000) also observed high positive correlations between PI and H258 in swine (r = 0.96) and bovine (r = 0.94) sperm.
Similar coefficients were found for the same probe association by regression equations that calculated for acrosome integrity (Figure 3), as verified by the FITC-PSA probe. The value of "a" (4.19) is close to the expected. The value of "b" (0.84) also reflects the desired value and the determination coefficient of 95% demonstrates the efficiency of the technique. The FITC-PSA efficiency was evaluated by Graham et al. (1990), comparing it to naphthol yellow/erythrosin b, finding a confidence interval of 95%.
The equation obtained for mitochondrial function, with MITO associated to PI and FITC-PSA, showed a similar characteristics to plasmatic membrane integrity and acrosomal integrity (a = 3.20, and b = 0.83). Nevertheless, Arruda & Celeghini (2003) obtained a regression equation, for the same association of probes in bovine sperm, (Y= 35.0 + 0.55X) different than expected, in spite of finding a high determination coefficient (R2=0.84). This same technique, with minor changes, was validated in equine sperm (Celeghini et al., 2004), using the same methodology, which yielded an equation (v = 9.57 + 0.78 X) with results similar to those obtained in the present experiment, with a determination coefficient of 93%. These differences could be explained by differences among species or by the greater ability of the technique to differentiate stain nuances of MITO when bound to mitochondrial membranes presenting potential or not.
This is an efficient and easy technique to simultaneously evaluate plasmatic, acrosomal, and mitochondrial membranes of fowl sperm. It is possible to suggest that the application of this methodology in large scale can be maximized by the use of flow cytometry systems, providing higher accuracy and swiftness, as it is to read approximately 10,000 cells in a few seconds.
Arrived: January / 2007
Approved: August / 2007
Financial support provided by FAPESP process n. 00/14653-6 and Predileto Pena Branca Alimentos S.A., Brazil, for birds and food.
- Arruda RP, Celeghini ECC. Validation of a technique to evaluation simultaneous of the plasmatic, acrosomal and mitochondrial membranes in bovine spermatozoa. Acta Scientiae Veterinariae 2003; 31:230-231.
- Arruda RP, Souza NL, Marques A, Celeghini ECC, Gobesso AAO, Meirelles FV, Binelli M, Blasques FJH. Evaluation of techiniques using CFDA/PI, H258/FITC-PSA and Trypan Blue/Giemsa for assessment of the viability and acrosomal Integrity of cryopreserved equine spermatozoa. Theriogenology 2002; 57(1):477.
- Bakst MR, Howarth B. Hydrolysis of hen's perivitelline layer by cock sperm. Biology of Reproduction 1977; 17:370-379.
- Barbarato GF, Cramer PG, Hammestedt RH. A pratical in vitro sperm-egg binding assay that detects subfertile males. Biology of Reproduction 1998; 58:686-699.
- Bavister BD, Leibfried ML, Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biology of Reproduction 1983; 28(1):235-47.
- Bayyari GR, Cook JR, Harris Jr GC, Macy LB, Slavick MF, Skeeles JK. Research note: The evaluation of chicken spermatozoa using fluorescent staining in a 96-well format. Poultry Science 1990; 69: 1602-1605.
- Bereiter-Hahn J. Behavior of mitochondria in the living cell. International Review of Cytology 1990; 122:1-63.
- Bilgili SF, Renden JA. Fluorimetric determination of avian sperm viability and concentration. Poultry Science 1984; 63(11):2275-77.
- Bramwell RK, Marks HL, Howarth B. Quantitative determination of spermatozoa penetration of the perivitelline layer of the hen's ovum as assessed on oviposited eggs. Poultry Science 1995; 74(11):1875-1883.
- Celeghini ECC, Albuquerque R, Arruda RP, Lima CG. Seminal characteristics evaluation of the male broiler breeder selected by comb development to reproduction. Brazilian Journal of Veterinary Research and Animal Science 2001; 38(4):177-183.
- Celeghini ECC, Arruda RP, Andrade AFC, Raphael CF, Nascimento J. Simultaneous evaluation of the plasmatic, acrosomal, and mitochondrial membranes in equine spermatozoa. Proceedings of the 15th Internacional Congress Of Animal Reproduction; 2004; p. 511. Porto Seguro, BA-Brazil.
- Celeghini ECC, Nascimento J, Andrade AFC, Raphael CF, Souza LWO, Arruda RP. Use of CMXRos and JC-1 on mitochondrial function evaluation, associated to fluorescent probes to plasmatic and acrosomal membranes evaluation in bovine spermatozoa. Acta Scientiae Veterinariae 2005; 33:321.
- Centola GM, Mattox JH, Burde S, Leary JF. Assessment of the viability and acrosome status of fresh and frozen-thawed human spermatozoa using single-wavelength fluorescence microscopy. Molecular of Reproduction and Development 1990; 27(2):130-135.
- Chalah T, Brillard JP. Comparison of assessment of fowl sperm viability by eosin-nigrosin and dual fluorescence (SYBR-14/PI). Theriogenologv 1998; 50:487-493.
- Cross NL, Meizel S. Methods for evaluating the acrosomal status of mammalian sperm. Biology of Reproduction 1989; 41:635-641.
- Garner DL, Thomas AC, Joerg HW, Dejarnette JM, Marshall CE. Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biology of Reproduction 1997; 57:1401-06.
- Gillan L, Evans G, Maxwell WMC. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology 2005; 63:445-457.
- Graham JK, Kunze E, Hammerstedt RH. Analysis of sperm cell viability, acrosomal integrity, and mitochondrial function using flow cytometry. Biology of Reproduction 1990; 43:55-64.
- Harris Junior GC, Benson JA, Sellers RS. The influence of daylength, body weight, and age on the reproductive ability of broiler breeder cockerels. Poultry Science 1984; 63(9):1705-1710.
- Haugland, RP. Introduction to fluorescence techniques. In: Spence, MTZ. The handbook: a guide to fluorescent probes and labeling technologies. 10th ed. Invitrogen Corp.; 2005. p.1-6.
- Nagy S, Jansen J, Topper EK, Gadella BM. A triple-stain flow cytometric method to assess plasma and acrosome membrane integrity of cryopreserved bovine sperm immediately after thawing in presence of egg-yolk particles. Biology of Reproduction 2003; 68:1828-1835.
- Parks JE, Graham JK. Effect of cryopreservation procedures on sperm membranes. Theriogenology 1992; 38:209-222.
- Pintado B, De La Fuente J, Roldan ERS. Permeability of boar and bull spermatozoa to the nucleic acid stains propidium iodide or Hoechst 33258, or the eosin: accuracy in the assessment of cell viability. Journal of Reproduction and Fertility 2000; 118:145-152.
- Rawe VY, Galaverna GD, Acosta AA, Olmedo SB, Chemes HE. Incidence of tail structure distortions associated with dysplasia of the fibrous sheath in human spermatozoa. Human Reproduction 2001; 16(5):879-886.
- SAS Institute. User's guide: statistic. version 6.11. 2nd ed. Cary; 1998.
- Sukardi S, Curry MR, Watson PF. Simultaneous detection of the acrosomal status and viability of incubated ram spermatozoa using fluorescent markers. Animal Reproduction Science 1997; 46:89-96.
- Thomas CA, Garner DL, Dejarnette JM, Marshall CE. Fluorometric assessments of acrosomal integrity and viability in Cryopreserved bovine spermatozoa. Biology of Reproduction 1997; 56:991-998.
- Wilson HR, Piesco NP, Miller ER, Nesbeth WG. Prediction of the fertility potential of broiler breeder males. World's Poultry Science Journal 1979; 35(2):95-118.
Mail Address
Publication Dates
-
Publication in this collection
08 Jan 2008 -
Date of issue
Sept 2007
History
-
Accepted
Aug 2007 -
Received
Jan 2007