Abstract
The present study aimed at evaluating the effect of the prebiotic Bio Mos™ (2 kg/ton up to 10 days; 1 kg/ton from 10 to 21 days; and 0.5 kg/ton from 21 days to slaughter), of the probiotic Lac XCL 5x™ (by spray-mixing), of a combination of the two supplements (prebiotic + probiotic), and of one growth promoter antimicrobial agent (Avilamycin at 15 ppm). Birds were orally challenged with inoculated Salmonella Enteritidis (SE) 10(6) CFU at 3 days of age. Four hundred and eighty male Ross chicks were used. The experiment lasted 28 days, and the analyses were conducted at 15 and 28 days of age. Cecum and liver bacterial colonization of production of anti-SE antibiodies, intestinal micrometry and bird performance were assessed. Neither the prebiotic, nor the probiotic influenced performance or production of anti-SE antibodies in SE-challenged birds. Intestinal micrometry and bird mortality were not influenced by prebiotic or probiotic supplementation, or by the antimicrobial agent. The use of an antimicrobial agent produced higher SE CFUs in cecum bacterial counts, while prebiotic and probiotic yielded lower counts. The combination prebiotic+probiotic did not produce significantly different results from the individual use of the additives.
Additives; birds; immunity; salmonellosis
Effects of prebiotics and probiotics on the colonization and immune response of broiler chickens challenged with Salmonella Enteritidis
Ribeiro AMLI; Vogt LKII; Canal CWIII; Cardoso MRIIV; Labres RVV; Streck AF; Bessa MCII
IDepartamento de Zootecnia, bolsista CNPq
IIGraduated student, PPG-Zootecnia, UFRGS
IIIDepartamento de Medicina Animal, Professor, UFRGS cláudio.canal@ufrgs.br
IVDepartamento de Medicina Veterinária Preventiva. mcardoso@ufrgs.br. Bolsistas CNpq
VUndergraduate student, Veterinary, UFRGS
Mail Address Mail Address Universidade Federal do Rio Grande do Sul Andréa Machado Leal Ribeiro UFRGS, Professor Av. Bento Gonçalves, 7712 91.540-000. Porto Alegre, RS, Brasil E-mail: aribeiro@ufrgs.br
ABSTRACT
The present study aimed at evaluating the effect of the prebiotic Bio Mos (2 kg/ton up to 10 days; 1 kg/ton from 10 to 21 days; and 0.5 kg/ton from 21 days to slaughter), of the probiotic Lac XCL 5x (by spray-mixing), of a combination of the two supplements (prebiotic + probiotic), and of one growth promoter antimicrobial agent (Avilamycin at 15 ppm). Birds were orally challenged with inoculated Salmonella Enteritidis (SE) 106 CFU at 3 days of age. Four hundred and eighty male Ross chicks were used. The experiment lasted 28 days, and the analyses were conducted at 15 and 28 days of age. Cecum and liver bacterial colonization of production of anti-SE antibiodies, intestinal micrometry and bird performance were assessed. Neither the prebiotic, nor the probiotic influenced performance or production of anti-SE antibodies in SE-challenged birds. Intestinal micrometry and bird mortality were not influenced by prebiotic or probiotic supplementation, or by the antimicrobial agent. The use of an antimicrobial agent produced higher SE CFUs in cecum bacterial counts, while prebiotic and probiotic yielded lower counts. The combination prebiotic+probiotic did not produce significantly different results from the individual use of the additives.
Keywords: Additives; birds; immunity; salmonellosis.
INTRODUCTION
Considering avian salmonellosis, also called paratyphic salmonellosis, among infections caused by any Salmonella classified under non-specific serovar group for one given host, Salmonella Enteritidis (SE) is the most important. SE incidence has recently increased in several countries, and today, SE is the most commonly isolated serovar in human food-borne infections (Saif et al., 2003). In this scenario, the concerns on the negative impact of this bacterium on broiler chicken production, formerly the main focus of researchers and breeders, has given way to the organism importance relative public health hazard.
Foreign markets have increased the demands on microbiological quality of Brazilian poultry products. Today, in NAFTA members (USA, Canada, and Mexico) as well as European Union countries, the norm is to reject food products containing Salmonella in 25-g food samples (Saif et al., 2003).
Prebiotics and probiotics are being tested under different experimental conditions to study the pathways used by these substances to assist in the prevention of carcass contamination and in the elimination of pathogens present in the birds organisms. Their chief aim is to enhance broiler chicken performance and, often, to improve immune response (Spring et al, 2000; Huang et al., 2004).
A prebiotic was defined by Gibson & Roberfroid (1995) as a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon. The authors also argue that a prebiotic (i) must not be hydrolyzed or absorbed in the small intestine, (ii) must be a selective substrate for a specific class of beneficial commensal bacteria, and (iii) must be capable to change the gut microbiota and to induce luminal or systemic effects that benefit the host.
Among the most commonly studied substances in the family of prebiotics are the oligosaccharides, which are non-digestible carbohydrates, particularly fructoligosaccharides (FOS), glucoligosaccharides (GOS), and mannanoligosaccharides (MOS). Prebiotics may change gut microbiota in two ways: (i) by supplying the nutrients for beneficial bacteria, or (ii) by leading pathogenic bacteria to acknowledge attachment sites in oligosaccharides as intestinal mucosa sites, thus reducing undesired intestinal colonization, which in turn decreases the incidence of infections and improves the integrity of the mucosal surface of the gut wall (Iji & Tivey, 1998). Mannanoligosaccharides are able to block pathogen adherence and to prevent colonization by allowing bacteria to attach to the compound molecules, using their fimbriae, and not to the intestinal mucosa. Therefore, these harmful bacteria travel along with the passage of the ingesta, without causing diseases to animals (Collet, 2000).
On the other hand, the mode of action of probiotics is related to the competition for attachment sites, or competitive exclusion. The bacteria present in the probiotic attach to the intestinal mucosa, thus forming a physical barrier that blocks the attachment of pathogenic bacteria (Furlan, 2005). Additional roles played by probiotics are the production of antibacterial compounds and enzymes, as well as the stimulation of the immune system by increasing phagocyte population and activity. In bird's body, Peyer's patches, cecal tonsils, and the bursa of Fabricius are sites of lymphoid tissue accumulation. These organs capture antigens that circulate in the digestive tract and that stimulate the production of the immune system B and T cells.
In this scenario, this study aimed at evaluating and at comparing the use of a growth promoter (Avilamycin), a prebiotic (Bio Mos), a probiotic (All-Lac XCL5x), and the combination of two last products on (i) salmonella counts in the intestine (cecum) and in the liver of broiler chickens challenged with Salmonella Enteritidis, (ii) anti-Salmonella Enteritidis antibody counts in blood serum, (iii) villi size, and (iv) bird performance.
MATERIALS AND METHODS
Birds and experimental procedures
The broilers used in the present study were reared at "Laboratório de Ensino Zootécnico", Universidade Federal do Rio Grande do Sul, between February and March, 2005. Four-hundred eighty one-day-old, Ross 308 male chicks, vaccinated against Marek's disease, Newcastle disease, avian pox, and infectious bronchitis were housed in 40 cages (10 birds per cage) in a controlled-temperature environment, under 24-h lighting and were offered feed and water ad libitum. Weight gain, feed intake, and feed conversion were weekly evaluated, whereas mortality and temperature were daily measured.
The experimental period lasted from day one to day 28 of the bird's age. Eighty birds were sacrificed on day 15, and the same number on day 28.
Strain and pilot experiment
Birds were challenged with Salmonella Enteritidis (SE) resistant to nalidixic acid and to novobiocin in order to allow the future identification of collected samples. Before the main experiment, a pilot test was conducted with 12 birds in order to establish the SE dose to be adopted, as well as strain sensitivity to the prebiotic used (in vivo agglutination). Initially, 10-5 to 10-10 dilutions were prepared from a NaCl 0.85% solution to determine the concentration in which SE reached the stationary state. The birds used in the pilot experiment were challenged with three SE doses (106, 107, and 108 CFU) at 3 days of age. Seven days later, these birds were sacrificed, and had livers and caeca removed for bacterial isolation and counts. Based on the obtained results, the concentration of 106 CFU was chosen to challenge the birds in the main experiment, since this value allowed better recovery of the inoculated SE strain in the bird intestinal microbiota.
Treatments
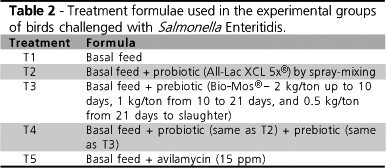
Five treatments were tested, each with eight replicates. All treatments supplied the same basal diet, defined according to Rostagno (2000), according to two different periods (starter: 1 21 days of age; grower: 21 28 days of age) (Table 1).
Treatments differed as to the additives used: probiotic (All-Lac XCL 5x), prebiotic (Bio-Moss), probiotic + prebiotic, and avilamycin (Table 2). The probiotic (T2 and T4) was supplied once during the experiment, shortly after hatching and before chicks were caged, by spray-mixing of a solution at the concentration of 5g/ 400mL/ 2000 chicks in distilled water, to which blue dye was added (Hi-lite tablets, Fort Dodge). Treatment 1 was used as negative control, and Treatment 5 as positive control.
Direct sample inoculation for bacterial counts
On the 3rd day of age, all birds were orally inoculated with 106 CFU of Salmonella Enteritidis. On the 15th day of age, 80 birds were randomly chosen and sacrificed (2 birds per cage) by cervical dislocation, whereupon livers and ceca were collected and stored in sterile Petri dishes at 4 °C overnight. Caeca were removed with their contents, and had both ends tied. Next, caeca were alcohol-flambéed, after which procedure a section was removed to produce a 1-g sample, which was placed in a plastic bag containing 9 mL tetrathionate solution (Merck). Then, each sample was agitated in a Stomacher (Interscience, St. Nom, France) for 60 s. Caeca were used to prepare serial dilutions with tetrathionate (10-1 to 10-4). Each solution provided one 100-µL aliquot that was seeded onto brilliant green agar (BPLS Agar, Merck), containing novobiocin (4 mg/L) and nalidixic acid (25 mg/L) (0.1% each starter solution). All samples were prepared in duplicate, and plates were incubated at 37 °C for 24 to 72 h (according to Seo et al., 2000, with adaptations). After this incubation period, plated bacterial colonies were counted (means calculated for duplicates), and 3 to 5 typical colonies were immediately chosen for confirmation of the Salmonella genus with Poly-O (Probac, São Paulo, Brazil). As for livers, only the qualitative test was performed (presence/absence of the bacterium), using just the 10-1 dilution, which results were also confirmed by serology.
On the 28th day of age, 80 birds were slaughtered, samples were collected, and all experimental procedures described for birds slaughtered at 15 days of age were repeated.
Some Salmonella and Escherichia coli colonies recovered from the inoculated birds were submitted to antibiogram by the agar diffusion method (NCCLS, 1992).
Quantification of anti-SE antibodies
On day 15 (200 birds) and 28 (160 birds), blood samples were collected by cardiac puncture for the quantification of anti-SE antibodies. After serum separation, sera were centrifuged at 8,000 g for 7 min, and stored at -20 °C. Serum pools for sera of each replication were prepared for analyses, yielding eight analyzed pools per treatment.
Anti-SE antibodies were assessed using a commercial ELISA kit (Flockscreen, Guildhay), at a 1:4 dilution. Dilutions were analyzed at 550 nm, with optical density values (OD) interpreted as antibody counts produced by birds under the different treatments.
Intestinal villi measurements
Small intestine samples were collected from the 160 birds sacrificed on days 15 and 28 for intestinal micrometry analysis. One sample of the duodenum flexure linked to the pancreas (approximately 3-cm long) was chosen. Each sample was externally and internally washed with NaCl 0.9% to remove the intestinal contents. Samples were individually transferred to jars containing 10% buffered formalin, for fixation. After a 30-day fixation period, samples were embedded in paraffin, sectioned to a 2-5 µm thickness, mounted on glass slides, and stained with hematoxylin-eosin (Prophet et al., 1992).
Villi height and crypt depth were then measured. Villus height was defined as the length between the villus basal lamina (which coincides with the upper crypt end) and the villus apex. Crypts were measured between the base and the crypt:villus transition zone (Pelicano et al., 2003).
Measurements were carried out using a trinocular stereoscopic microscope (Quimis) under 10× and 15× magnifications. Images were captured by a camera coupled to the microscope and connected to an image analyzer (Leica Software), and measured using the Paint Brush software. Between five and 20 villi and crypts were scored for each bird, and means calculated therefrom were used in the statistical analysis.
Statistical analysis
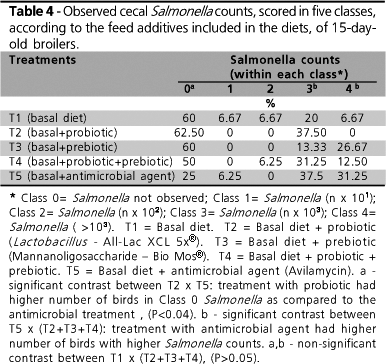
Bird performance and intestinal micrometry data were analyzed using the GLM procedure of the SAS package (1998). When a significant F was observed, LSmeans was used to test the differences among means. Eight replicates per treatment were used to test bird performance, and 16 birds/treatment were used to assess intestinal micrometry and Salmonella counts. Salmonella count data were obtained from 5 classes defined as: Class 0 - "zero" Salmonella counts; Class 1 - n × 101 CFU; Class 2 - n × 102 CFU; Class 3 - n × 103 CFU; Class 4: > n × 104 CFU. Mantel-Haenszel Chi-square test was used to test the results, with contrasts among treatments. Bird livability values were used in the statistical analyses (100% living birds mortality).
Serology data (ELISA OD data obtained on days 15 and 28) did not present normal distribution, therefore were transformed. Tsqsq transformation was used, and allowed ANOVA using the GLM procedure of the SAS package. A non-parametric analysis (Kruskall-Wallis test) was also conducted for confirmation, and produced similar results.
RESULTS AND DISCUSSION
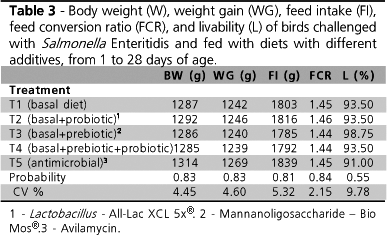
No significant difference was observed between treatments for any of the performance variables assessed throughout the experimental period (Table 3).
It is important to underline the fact that several studies that tested bird performance produced divergent results. This is imputed particularly to bird age and characteristics of the product used. Huang et al. (2004) observed higher weight gain and better feed conversion values (p < 0.05) in broiler chickens after six weeks of treatments with different probiotics (chiefly Lactobacillus casei and L. acidophilus) as compared to the control group (without supplementation). Conversely, in a study with chicks that were orally inoculated with SE (1 × 108 CFU/mL) at 4 days of age, Loddi (2005) did not find any significant difference in bird performance among treatments with flavomycin and phosphorylated mannanoligosaccharides (MOS) used individually or in combination with organic acids, between 1 and 28 days of age which was also observed in the present study.
According to Jin et al. (1997), differences in bacterial strain and form of presentation as used in the probiotic, as well as the concentration of viable cells, may lead to discrepant results. The authors also suggest that probiotic supplementation may produce more satisfactory results when birds are submitted to stress, which did not occur in the present study, though some degree of stress was in fact induced during the inoculation of birds with SE.
No significant difference was observed in livability values among treatments throughout the experiment. Bird age and SE strain used are important factors in the development of SE infections (Smith & Tucker, 1980; Suzuki, 1994), and variations in mortality can be observed in experimentally-induced infections.
Day-old chickens exposed to high paratyphic salmonella doses (109CFU/mL) frequently present significant mortality during the first weeks post-infection (Smith & Tucker, 1980). Nevertheless, lower doses (106 CFU/mL) generally result in lower mortality and in a higher number of birds in which the infection persists for longer periods (Nakamura et al., 1993; Phillips & Optiz, 1995; Gast & Holt, 1998). As in the present experiment the objective was to promote the infection in birds without causing significant increase in mortality, the administered infectious dose (106 CFU) was not expected to affect bird livability to a significant extent.
A significant difference among treatments (P £ 0.02) was observed for cecal Salmonella counts only in the samples collected on day 15 (Table 4). On day 28, no difference was observed (data not shown). It can be observed that the treatment with the antimicrobial agent (T5) led to the lowest occurrence of Class 0 Salmonella counts as compared to the other treatment series (Table 4). In T5, only 25% of samples were ranked as Class 0 as compared to 60% and to 50% of the samples ranked in this class for the other treatments. For Classes 3 and 4 (higher Salmonella counts), the prebiotic (T3) and probiotic (T2) treatments showed a lower number of animals diagnosed as positive for Salmonella, respectively.
In several samples, both on days 15 and 28, it was not possible to identify the presence of Salmonella. Yet, a high number of Escherichia coli colonies was found and confirmed by biochemical assays. In order to assess the possible interference of E. coli in the group of birds treated with prebiotic, E. coli isolates were also tested in vitro for its capacity to attach to the prebiotic (BioMOs). No positive reaction was observed, which indicates no interference. When the sensitivity of isolated E. coli strains was tested against the antimicrobial agents added to the solid medium (novobiocin and nalidixic acid) and to the feed used in T5 (avilamycin), these strains were observed to be resistant to the three tested antimicrobial agents. The detection of E. coli in the ceca of birds treated with avilamycin and the growth of this microorganism in the culture medium is therefore explained. Based on these findings, the fact that Salmonella was not isolated in some samples may be explained by the prevalence of E. coli, and by the inability of the adopted selective media to inhibit the interference of this bacterium.
This expressive growth of E. coli was not observed during the pilot experiment. However, birds in the pilot test, which were also inoculated at 3 days of age were sacrificed on day 10. In the main experiment, bacterial counts were performed on day 15 and 28, which may account for the variation observed, since bird intestinal microbiota considerably changes and becomes more complex as the animals grow older (Lan et al., 2005).
Nevertheless, as this interfering strain was similarly present in all treatments, it is possible to compare Salmonella growth results. Treatment comparison using contrasts allowed the comparison of cecal Salmonella counts, and significant differences were observed (see below, Table 4). Data did not reveal any significant difference when the basal treatment (T1) was compared to the treatments supplemented with probiotic, prebiotic, and prebiotic + probiotic (T2, T3, and T4). These numbers disagree with the results obtained by Spring et al. (2000), who challenged 3-day-old broiler chickens with Salmonella Typhimurium 29E. The researchers observed a significant difference in cecal bacterial counts (5.40 × 4.01 log CFU/g; P < 0.05) between chickens supplemented with mannanoligosaccharides and those without feed supplements, seven days after the challenge. The fact that bacterial counts, in this specific case, were conducted as early as seven days after inoculation may have intensified the effects reported, as the bird's organism is more likely to eliminate the pathogen due to the time elapsed after inoculation.
When the treatment with the antimicrobial agent (T5) is compared to the treatments supplemented with probiotic, prebiotic, and with combinations thereof (T2, T3, and T4), a significantly higher number of birds ranked as Class 3 and 4 (higher Salmonella counts) is observed in T5, along with a lower occurrence of Class 0 birds. With the treatments with probiotic, prebiotic, and with the combination of both, a higher number of birds was grouped in Class 0. These results suggest that the use of antimicrobial agents as growth promoter may have imbalanced the intestinal microflora, thus favoring the development of the inoculated Salmonella strain, which exhibited resistance to the antimicrobial used. Antunes et al. (2003) evaluated the occurrence of different Salmonella serovars in poultry products, as well as their susceptibility to various antimicrobial agents. Salmonella Enteritidis was among the most frequent species detected (44% of the samples), with 75% of the isolated strains being resistant to one or more antimicrobial agents.
When only the probiotic and the antimicrobial agent were compared, a significant difference was found. This is explained by the fact that most birds receiving probiotic was ranked as Class 0.
In the liver, no significant difference was observed among treatments for the presence of Salmonella (P<0.05) on day 15 (Table 5). For the same age, contrast analysis carried out for the different treatments, however, revealed a significant difference (P < 0.03), due to the fact that the prevalence of Salmonella was higher for the antimicrobial treatment, as compared to the prebiotic, probiotic, and combination treatment. Relative to the control treatment (T1), no significant difference was observed as compared to the prebiotic, probiotic, and combination treatments. These results are consistent with those observed in the caecum, once again showing the negative effect of the antimicrobial agent in the experimental setting adopted in the present study. On day 28, no differences were observed.
The results obtained for anti-SE antibodies quantification by ELISA are shown in Table 8, expressed as optical density values (OD). No significant difference was observed among treatments for OD values obtained on days 15 and 28, although a possible stimulus generated by the mannanoligosaccharide present in the intestinal lumen was expected.
In a study that evaluated Bio Mos and the production of antibodies against sheep red blood cells, Cotter et al. (2002) observed an increase in antibody production in layers during the four weeks ensuing immunization with the antigen. Nevertheless, this difference was significant only in the first week. The lower supplementation level (0.05%) yielded higher antibody titers as compared to the other two concentrations tested. In broiler chickens, the difference observed and that showed the benefits of Bio-Mos, was not significant, but the results showed higher antibody titers with higher concentrations of Bio Mos (0.1 and 0.2%).
The mechanisms involved in the effects produced by mannanoligosaccharides on bird immunity have not been fully established. Except for the capacity to attach onto enteric pathogens (Spring et al., 2000), and to adsorb potentially immune-suppressant mycotoxins. Cotter et al. (2002) suggest that mannose, as present on the surface of Bio Mos particles, stimulates the production of lectins that attach to mannose, and plays an important role in assisting phagocytosis, which is essential for the innate immune response against microorganisms. In this case, the highest lectin output could aid the generation and presentation of SE antigens to T cells, which is an indispensable step for the establishment of acquired immunity.
Seo et al. (2000) evaluated the presence of antibodies (IgG) in the plasma of layers inoculated with SE 1.5 × 106 CFU on the 2nd day of age, and treated with or without enrofloxacin and/or normal avian gut flora (NAGF). The authors did not observe increased IgG titers or any significant difference between treatments. Four weeks after infection, only 8% of samples presented detectable anti-SE antibody titers. After 10 weeks post-infection, the number of detectable antibody titers reached peak values, though never exceeding 45% of the samples analyzed.
Bird immune response against different antigens presents, among other factors, considerable variation in terms of strain. In studies on different commercial strains, highly variable responses are expected. In the present study, IgG could have increased near the day of infection (day 3), but birds had not reached the peak of anti-SE immunoglobulin production when the measurements were done. According to Holt et al. (1999), chicks exposed to Salmonella soon after hatching may remain infected upon maturity, without the development of significant immunity against the pathogen inoculated.
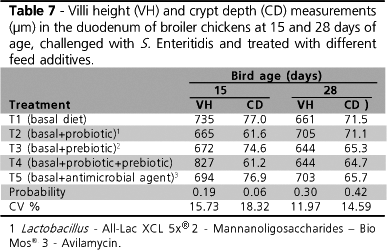
No significant difference was observed in intestinal villi height and crypt depth on days 15 or 28. It is interesting to observe that villi dimensions remained virtually the same, in spite of the growing age of chicks.
Prebiotics may affect intestinal microbiota by supplying nutrients to beneficial bacteria, or by lowering colonization rates by harmful bacteria and infection rates, and by improving intestinal integrity (Iji & Tivey, 1998). When harmful colonization is reduced, improved intestinal integrity may be expected. Such improvement may be represented by higher villi and shallower crypts, as compared to the event of pathogenic colonization, with consequent sloughing and renewal of the intestinal epithelium.
Loddi (2003) included mannanoligosaccharides (MOS) and one organic acidifier in broiler chicken diets, and observed an increase in villi height and perimeter when MOS was administered on day 21 (1.485,89µm × 960.44µm) (P<0.05). Nevertheless, at 42 days of age, the authors did not report any significant effect of the used promoters on intestinal morphology, as likewise observed in the present study.
Pelicano et al. (2003) studied birds sacrificed at 42 days of age, and observed that those belonging to the control group presented shorter duodenal villi as compared to birds that received probiotics (794.21µm × 1.109,86µm). The authors also verified a significant interaction between the probiotics added to water and feed, and concluded that the addition of Lactobacillus reuteri and L. johnsonii in drinking water were essential to the occurrence of deeper crypts.
It is important to stress the fact that the villi height and crypt depth values observed in the present experiment were lower than those verified in the mentioned studies. This is possibly due to the experimental Salmonella challenge to which birds were submitted, which was not used in the other reviewed papers. Intestinal colonization by Salmonella causes intestinal mucosa lesions and the consequent reduction in villi height, which, in turn, leads to deeper crypts. Yet, this last characteristic was not observed in the present study.
CONCLUSIONS
-
The use of prebiotics and probiotics did not influence the performance of the birds challenged with
Salmonella Enteritidis, neither the production of anti-
Salmonella Enteritidis antibodies.
-
The use of an antimicrobial agent allowed higher colonization of the ceca by
Salmonella Enteritidis as compared to the prebiotic and to the probiotic, which diminished colonization.
-
Intestinal morphometry was not influenced by the supplementation with prebiotic, probiotic, or antimicrobial agent.
-
The early intestinal infection of broiler chickens with
Salmonella Enteritidis did not overtly impair bird performance, mortality, intestinal health, or immune competence.
Arrived: May / 2007
Approved: August / 2007
- Antunes P, Réu C, Souza JC, Peixe L, Pestana N. Incidence of Salmonella from poultry products and their susceptibility to antimicrobial agents. International Journal of Food Microbiology 2003; 82(2):97-103.
- Collet S. Nutrição, imunidade e produtividade. 10ş Ronda Latino-Americana: o futuro da alimentação; 2000; Campinas, São Paulo. Brasil. p. 20-30.
- Cotter PF, Sefton AE, Lilburns MS. Manipulating the immune system of layers and breeders: novel applications of mannan oligosaccharides. In: Lyons TP, Jacques KA, editors. Nutritional biotechnology in the feed and food industries. Proceedings of 18th Alltech's Annual Symposium; 2002; Lexington, Kentuky, USA. p. 21-27.
- Furlan RL. Avaliação e uso de pré e probióticos. 6° Simpósio Brasil Sul de Avicultura; 2005; Chapecó, Santa Catarina. Brasil. p. 58-76.
- Gast RK, Holt PS. Persistence of Salmonella enteritidis from one day of age until maturity in experimentally infected layers chickens. Poultry Science 1998; 77(12):1759-1762.
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concepts of prebiotics. Journal of Nutrition 1995; 125(6):1401-1412.
- Holt PS, Gast RK, Porter Jr RE, Stone HD. Hyporesponsiveness of the systemic and mucosal humoral immune systems in chickens infected with Salmonella enterica serovar Enteritidis at one day of age. Poultry Science 1999; 78(11):1510-1517.
- Huang MK, Choi YJ, Houde R, Lee JW, Lee B, Zhao X. Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poultry Science 2004; 83(5):788-795.
- Iji PA, Tivey DR. Natural and synthetic oligossaccharides in broiler chickens diets. World's Poultry Science Journal 1998; 54:129-143.
- Jin LZ, Ho YW, Abdullah N, Jalaludin S. Probiotics in poultry: modes of action. World's Poultry Science Journal 1997; 53:351-368.
- Lan Y, Verstegen MWA, Tamminga S, Williams BA. The role of the commensal gut microbial community in broiler chickens. World's Poultry Science Journal 2005; 61:95-104.
- Loddi MM. Probióticos, prebióticos e acidificantes orgânicos em dietas para frangos de corte [dissertação]. Jaboticabal (SP): Universidade Estadual Paulista; 2003.
- Loddi MM. Mananoligossacarídeo fosforilado e flavomicina: respostas em frangos de corte desafiados com salmonella. Ave World 2005; 3(17 supl especial): 7.
- Nakamura M, Nagamine N, Suzuki S, Norimatsu M, Oishi K, Kijima M, Tamura Y, Sato S. Long-term shedding of Salmonella enteritidis in chickens which received a contact exposure within 24 hrs of hatching. Journal of Veterinary Medicine Science 1993; 55:649-653.
- NCCLS- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. M2A2. Villanova, Pa; 1992.
- Pelicano ERL, Souza PA, Souza HBA, Oba A, Norkus EA, Kodawara LM, Lima TMA. Morfometria e ultra-estrutura da mucosa intestinal de frangos de corte alimentados com dietas contendo diferentes probióticos. Revista Portuguesa de Ciências Veterinárias 2003; 98 (547):125-134.
- Phillips RA, Opitz HM. Pathogenicity and persistence of Salmonella enteritidis and egg contamination in normal and infectious bursal disease virus-infected Leghorn chicks. Avian Diseases 1995; 39: 778-787.
- Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory methods in histotechnology. London (UK): Armed Forces Institute of Pathology;1992.
- Saif YM, Barnes HJ, Fadly AM, Clisson JR, Mcdougald LR, Swayne DE. Diseases of Poultry. 11th ed. Ames: Iowa State University Press; 2003. p.407-465.
- Silva ENda. Probióticos e prebióticos na alimentação de aves. Anais da Conferencia Apinco 2000 de Ciencia e Tecnologia Avícolas; 2000; Campinas, SP, Brasil: FACTA; 2000; v.2. p.241-251.
- Seo KH, Holt PS, Gast RK, Hofacret CL. Elimination of early Salmonella enteritidis infection after treatment with competitive-exclusion culture and enrofloxacin in experimentally infected chicks. Poultry Science 2000; 79:1408-1413.
- Smith HW, Tucker JF. The virulence of Salmonella strains for chickens: their excretion by infected chickens. Journal of Hygiene 1980; 84:479-488.
- Spring P, Wenk C, Dawson KA, Newman KE. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poultry Science 2000; 79:205-211.
- Suzuki S. Pathogenicity of Salmonella enteritidis in poultry. International Journal of Food Microbiology 1994; 21:89-105.
Mail Address
Publication Dates
-
Publication in this collection
08 Jan 2008 -
Date of issue
Sept 2007
History
-
Accepted
Aug 2007 -
Received
May 2007