Abstract
Two metabolism assays were carried out to determine corn and soybean meal metabolizable energy when enzymes were added. In the first trial, 35 cockerels per studied feedstuff (corn and soybean meal) were distributed in a completely randomized experimental design with four treatments of seven replicates of one bird each. The evaluated treatments were: ingredient (corn and soybean meal) with no enzyme addition, with the addition of an enzyme complex (xylanase, amylase, protease - XAP), xylanase, or phytase. Precise feeding method was used to determine true metabolizable energy corrected for nitrogen balance (TMEn). The use of enzymes did not result in any differences (p>0.05) in soybean meal TMEn, but phytase improved corn TMEn in 2.3% (p=0.004). In the second trial, 280 seven-day-old broiler chicks were distributed in a completely randomized experimental design with seven treatments of five replicates of eight birds each. Treatments consisted of corn with no enzyme addition or with the addition of amylase, xylanase, phytase, XAP complex, XAP+phytase combination, or xylanase/ pectinase/β-glucanase complex (XPBG). Corn was supplemented with macro and trace minerals. Total excreta collection was used to determine apparent metabolizable energy corrected for nitrogen balance (AMEn). Differences were observed (p=0.08) in AMEn and dry matter metabolizability coefficient (p=0.03). The combination of the XAP complex with phytase promoted a 2.11% increase in corn AMEn values, and the remaining enzymes allowed increased between 0.86% and 1.66%.
Amylase; forced feeding; phytase; protease; xylanase
Corn and soybean meal metabolizable energy with the addition of exogenous enzymes for poultry
Dourado LRBI,II; Sakomura NKII; Barbosa NAAII; Bonato MAII; Kawuauchi IMII; Fernandes JBKIII; Costa FGPIV
IUniversidade Federal do Piauí - Campus: Profª Cinobelina Elvas
IIDepartamento de Zootecnia Faculdade de Ciências Agrárias and Veterinárias -UNESP. Jaboticabal, SP, Brasil. E-mail: sakomura@fcav.unesp.br
IIICentro de Aqüicultura da UNESP. Jaboticabal, SP, Brasil
IVUniversidade Federal da Paraíba - Convênio PROCAD UNESP. Jaboticabal-UFPB. Areia, PB, Brasil
Mail Address Mail Address: Leilane Barros Dourado Universidade Federal do Piauí Campus Profa. Cinobelina Elvas BR 135, Km 03, Planalto Horizonte 64.900-000 Bom Jesus, PI, Brasil E-mail: leilane@ufpi.br
ABSTRACT
Two metabolism assays were carried out to determine corn and soybean meal metabolizable energy when enzymes were added. In the first trial, 35 cockerels per studied feedstuff (corn and soybean meal) were distributed in a completely randomized experimental design with four treatments of seven replicates of one bird each. The evaluated treatments were: ingredient (corn and soybean meal) with no enzyme addition, with the addition of an enzyme complex (xylanase, amylase, protease - XAP), xylanase, or phytase. Precise feeding method was used to determine true metabolizable energy corrected for nitrogen balance (TMEn). The use of enzymes did not result in any differences (p>0.05) in soybean meal TMEn, but phytase improved corn TMEn in 2.3% (p=0.004). In the second trial, 280 seven-day-old broiler chicks were distributed in a completely randomized experimental design with seven treatments of five replicates of eight birds each. Treatments consisted of corn with no enzyme addition or with the addition of amylase, xylanase, phytase, XAP complex, XAP+phytase combination, or xylanase/ pectinase/β-glucanase complex (XPBG). Corn was supplemented with macro and trace minerals. Total excreta collection was used to determine apparent metabolizable energy corrected for nitrogen balance (AMEn). Differences were observed (p=0.08) in AMEn and dry matter metabolizability coefficient (p=0.03). The combination of the XAP complex with phytase promoted a 2.11% increase in corn AMEn values, and the remaining enzymes allowed increased between 0.86% and 1.66%.
Keywords: Amylase, forced feeding, phytase, protease, xylanase.
INTRODUCTION
The use of enzymes has been proposed to improve nutrient digestibility in poultry diets. Most studies have determined the effect of enzymes on dietary energy (Zanella et al., 1999; Douglas et al., 2000; Kocher et al., 2003; Brito et al., 2006; Olukosi et al., 2007), but just a few were carried out to determine the effect of enzymes on ingredients. The knowledge of the nutritional value of ingredients with the addition exogenous enzymes is essential to establish their nutritional matrix.
Among the methods used to determine ingredient metabolizable energy (ME), the total excreta collection, with the use of a test diet and a reference diet were the most used (Sakomura Rostagno, 2007). However, this technique does not allow the determination of the effect of enzymes exclusively on feed ME, as not only the tested feedstuff, but the entire feed may suffer the action of the enzyme, making it difficult to establish the changes caused in the feedstuff only.
When evaluating individual ingredients using total excreta collection technique with chicks, it is difficult to obtain correct estimations, as the deficiency or excess of some nutrients could be limiting. On the other hand, the technique of precise feeding of cockerels, supplying an exact amount of the ingredient directly in the crop of fasted birds, allows energy determination. Also, the supply of small amounts of a pure ingredient for a short period of time allows isolating the effect of enzymes on the specific substrates of each ingredient. Therefore, the determination of feedstuff energy by the addition of enzymes may be used to improve poultry diet formulation. Bedford (2002) reported the importance of adjusting information relative to the effect of enzymes on nutrient availability or performance, allowing building models that can predict animal responses and a more economical feed formulation.
The objective of this study was to evaluate the effect of the addition of exogenous enzymes on corn and soybean meal metabolizable energy for chickens.
MATERIAL AND METHODS
Two metabolic trials were carried out at the Poultry Sector of the Animal Science Department of the School of Agrarian and Veterinary Sciences (FCAV), UNESP, Jaboticabal, SP, Brazil. The first trial was conducted with cockerels using precise feeding technique, and the second one with broilers chicks, using total excreta collection.
In the cockerel trial, 35 intact Hy-line cockerels were used for each studied ingredient (corn and soybean meal). Birds were distributed in a completely randomized experimental design, with four treatments of seven replicates of one cockerel each. A group of seven cockerels was fasted to determine endogenous losses. Each food constituted an independent assay.In the beginning of the trial, cockerels were individually weighed and distributed to allow similar replicate average weight (2.007±0.043kg). Corn and soybean meal were supplied with or without addition of exogenous enzymes. The following treatments were applied: T1- Ingredient (corn or soybean meal) with no enzyme addition; T2- Ingredient + XAP complex (xylanase, amylase, and protease - 200g/ton); T3-Ingredient + xylanase (100g/ton); T4- Ingredient + phytase (100g/ton).
Birds were submitted to forced feeding by placing a funnel directly in the crop. Ingredients were supplied twice daily: 20g at 07:00am and 20g at 05:00pm. Excreta collecting bags were coupled to a plastic ring fixed in the cloaca after the first supply. Excreta were collected twice daily, and for 48 hours after the last supply. Excreta were weighed, frozen, freeze-dried, ground, and had their nitrogen, gross energy, and dry matter analyzed to calculate true metabolizable energy corrected for nitrogen balance (TMEn), according to Sakomura & Rostagno (2007).
In the chick trial, seven-day-old male Cobb® broilers were distributed in a completely randomized experimental design with seven treatments of five replicates of eight birds each, and housed in metabolic cages. In the beginning of the trial, chicks were individually weighed and distributed to allow similar replicate average weight (138.77±0.51g).
At the corn evaluated corn was evaluated, dicalcium phosphate (1.20%), limestone (1.18%), salt (0.405%), coccidiostat (0.05%), and vitamin (0.10%) and mineral (0.05%) supplements were added in order to supply minimum available phosphorus (0.300%), calcium (0.780%), and sodium (0.180%) levels, as well as adequate vitamins and minerals. Corn was the only energy source with specific substrates for enzymes. The evaluated enzymes were purchased from Danisco Animal Nutrition and were added to corn at the following ratios: T1- Corn with no enzyme addition; T2- Corn with amylase (500g/ton); T3- Corn with xylanase (500g/ton); T4- Corn with phytase (100g/ton); T5- Corn with XAP (xylanase, amylase, and protease - 500g/ton); T6- Corn with XAP (500g/ton)+ phytase (100g/ton); T7- Corn with XPBG (xylanase, pectinase and β-glucanase, 500g/ton).
The method used was total excreta collection. Diets were offered ad libitum for eight days (7 to 14 days of age), with the first four days for diet adaptation, and four for excreta collection. Feeds were weighed in the beginning of the experiment, and feed residues were weighed at the end in order to determine feed intake.
The excreta collected from each experimental unit were weighed, frozen, dried in a force-ventilation oven at 55ºC for 76 hours, and subsequently submitted to nitrogen content, gross energy, and dry matter analyses to calculated apparent metabolizable energy corrected for nitrogen balance (AMEn) and dry matter metabolizability (DMM), according to Sakomura & Rostagno (2007). Ingredient chemical composition and fiber content were also determined.
The data obtained in both trials were submitted to homogeneity and normality analyses. Identified outliers were removed, and data were then submitted to analysis of variance using GLM procedures of SAS, and means were compared by the Duncan's Test at 10% significance level.
RESULTS
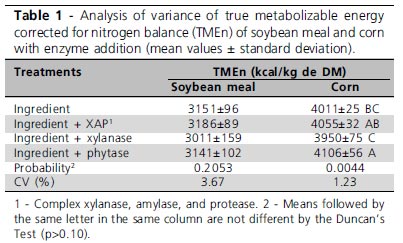
Soybean meal and corn TMEn results obtained in the cockerel trial are shown in Table 1. There was no effect (p>0.05) of enzyme addition on soybean meal TMEn. In corn, a significant increase of 95kcal/kg in TMEn was observed with phytase addition as compared to corn with no supplementation. The addition of XAP or xylanase did not influence corn TMEn.
AMEn values and dry matter metabolizability coefficients (DMMC) obtained in the chick trial are presented in Table 2.
All enzymes improved (p<0.05) dry matter metabolizability coefficient, indicating better nutrient utilization by the birds. For AMEn, the addition of the combination XAP + phytase, XAP, phytase, xylanase, and amylase promoted improvements (p<0.10) of 2.11%, 1.66%, 1.51%, 1.51%, and 1.26% in corn AMEn, representing increases of 74, 58, 53, 53, and 44 kcal/kg, respectively. The addition of XPBG improved corn AMEn in 0.86% (30kcal/kg); however, this result was not statistically different from the corn with no supplementation or the other supplemented diets.
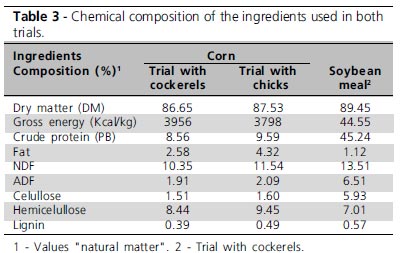
The chemical analysis of the ingredients used in both trials is shown in Table 3.
DISCUSSION
Phytase improves energy availability of feedstuffs and feeds, as demonstrated in several studies (Kornegay, 2001; Cowieson Adeola, 2005; Olukosi et al., 2007; Barbosa et al., 2008; Santos et al., 2008). This enzyme acts on the molecule phytate, which may complex with cations, carbohydrates, enzymes and amino acids, and inhibits the activity of several digestive enzymes, such as pepsin, α-amylase, and trypsin mainly due to the chelation of phytate with calcium ions (Ca++), which are essential for the activity of these enzymes (Lima, 2005).
The action of phytase on phytate may release starch, enzymes, enzyme co-factors, proteins, and minerals, which will be better digested and absorbed by the birds, consequently improving the use of energy by the birds.
Phytase addition improved only corn TMEn. Managi Coon (2006), working with semi-purified diets and soybean meal as the only vegetable ingredient, and did not find significant differences in energy retention when adding phytase.
Olukosi et al. (2007) found that phytase effect on energy availability when dietary metabolizable energy is limiting, suggesting that the lower energy availability of soybean meal as compared to corn in the present study may have interfered with phytase action. Cowieson et al. (2006) reported that phytase addition in absence of substrate reduced dry matter and nitrogen digestilibility probably because it stimulated endogenous losses and nitrogen catabolism.
The addition of the XAP complex in the cockerel trial increased corn TMEn in 1.1%, but it was not sufficient to promote significant improvements. On the other hand, in the chick trial, AMEn increased when this enzyme complex was added. Olukosi et al. (2007) reported that phosphorus deficiency can limit energy utilization when carbohydrases (xylanase) are added. This may explain the response to the addition of the enzyme complex only in the trial with chicks, which diet was supplemented with dicalcium phosphate. According to Ávila et al. (2006), the addition of vitamin and mineral supplements in assays to determine feedstuff energy must be adjusted to obtain more precise values.
However, the differences in the responses between the two trials may be related to the employed method and bird physiology, as the enzyme system of young birds is still immature, and therefore use exogenous enzymes to better utilize dietary nutrients, resulting in lower energy cost to activate endogenous enzymes, thereby enhancing feedstuff ME.
According to the results obtained in the total excreta collection trial with chicks, enzyme addition significantly increased corn AMEn. The best relative increase (2.11%) was obtained with XAP and phytase combination, which may be explained by the release of nutrients that are embedded in the cell wall by the action of xylanase and consequent better utilization of the other compounds released by the action of phytase, amylase, and protease. According to Olukosi et al. (2007), glycosidases are capable of breaking down the layer of non-starch polysaccharides (NSPs) of the cell membrane, allowing the access of phytase to the phytate stored in the cell wall membrane. However, when phytase is used alone, its capacity is limited due to the lack of access to its substrate, which is embedded inside the NSP matrix. In addition, some soluble fiber-phosphorus bonds may be hydrolyzed by glycosidases (xylanase), releasing that mineral for animal utilization, thereby improving animal energy metabolism. Some studies have shown nutrient digestibility and broiler performance improvement with the dietary addition of XAP + phytase (Cowieson & Adeola, 2005; Olukosi et al., 2007; Barbosa et al., 2008).
The use of xylanase may have beneficial effects as it promotes changes in the cell wall architecture by hydrolyzing the structural arabinoxylans that encapsulate nutrients, enhancing their utilization (Sheppy, 2001; Gracia et al., 2003; Yu & Chung, 2004; Hruby & Pierson, 2005; Lima, 2005; Olukosi et al., 2007). Cowieson (2005) stressed that the effect of xylanase can be better evidenced when associated with other exogenous enzymes, such as protease, amylases, and phytase.
The addition of amylase to diets aids the hydrolysis of starch amylose and amylopectin, improving starch digestion in the small intestine and enhancing nutrient use, with consequent higher growth rates (Sheppy, 2001), as it complements the activity of endogenous amylase, reducing its endogenous synthesis by the pancreas (Gracia et al., 2003), and therefore saving energy, which becomes more available for growth.
The absence of a significant response to the addition of the complex xylanase, pectinase, and β-glucanase may be related to the low availability of their substrate, as corn has low pectin levels (Malathi & Devegowda, 2001) and corn β-glucan levels have not been reported or are negligible (Knudsen, 1997; Choct, 2006).
In the present study, it was observed that the energy values obtained for corn were different between the two trials. This may be related to the applied methods and to bird age. ME determination techniques have changed throughout the years (Avila et al., 2006). The most commonly used methods to determine ME in poultry feedstuffs are total excreta collection and forced feeding, and each has its advantages and disadvantages (Sakomura & Rostagno, 2007).
Due to the problems in isolating enzyme effects, particularly on feedstuffs such as corn or soybean meal, the methods applied in the present study produced results that may contribute to define a nutritional matrix of these ingredients when exogenous enzymes are used. Therefore, the choice of the method must be based on the purpose of the enzymes and the birds' needs for those enzymes, as young animals have different digestive systems and different requirements as compared to adults, and therefore the use of exogenous enzymes may also be different. Therefore, the methods used in the present study can be applied to evaluate metabolizable energy increase in the diets supplemented with exogenous enzymes.
CONCLUSIONS
The addition of phytase, xylanase, amylase, and protease was efficient to increase corn apparent metabolizable energy. No increase in true metabolizable energy of soybean meal was evidenced with the addition of the tested enzymes.
Acknowledgements
Danisco Animal Nutrition for supplying the enzymes, and for funding the study. Granja Planalto for donating the cockerels. Nutron for supplying mineral and vitamin supplements.
Arrived: December/2008
Approved: March/2009
Parts of this article were published in the proceedings of Reunião Anual da Sociedade Brasileira de Zootecnia 2007 and in supplement 9 of Brazilian Journal of Poultry Science, presented during Apinco 2007.
- Avila VS, Paula A, Brum PAR, Barioni Jr W, Maier JC. Uso da metodologia de coleta total de excretas na determinação da energia metabolizável em rações para frangos de corte ajustadas ou não quanto aos níveis de vitaminas e minerais. Revista Brasileira de Zootecnia 2006; 35 (4):1691-1695.
- Barbosa NAA, Sakomura NK, Fernandes JBK, Dourado LRB. Enzimas exógenas no desempenho e na digestibilidade ileal de nutrientes em frangos de corte. Pesquisa Agropecuária Brasileira 2008; 43(6): 755-762.
- Bedford MR. The foundation of conducting feed enzyme research and the challenge of explaining the results. Journal Applied Poultry Research 2002; 11(4):464-470.
- Brito CO, Albino LFT, Rostagno HS, Gomes PC, Dionizio MA, Carvalho DCO. Adição de complexo multienzimático em dietas à base de soja extrusada: valores energéticos e digestibilidade de nutrientes em pintos de corte. Revista Brasileira de Zootecnia 2006; 35(3):1047-1055.
- Choct M. Enzymes for the feed industry: past, present and future. World's Poultry Science Journal 2006; 62(2):5-15.
- Cowieson AJ. Factors that affect the nutritional value of maize for broilers. Animal Feed Science and Technology 2005; 119(3-4):293-305.
- Cowieson AJ, Acamovic T, Bedford MR. Phytic acid and phytase: implications for protein utilization by poultry. Poultry Science 2006; 85(5):878-885.
- Cowieson AJ, Adeola O. Carbohydrases, protease, and phytase have an additive beneficial effect in nutritionally marginal diets for broiler chicks. Poultry Science 2005; 84(12):1860-1867.
- Douglas MW, Parsons CM, Bedford MR. Effect of various soybean meal sources and Avizyme on chick growth performance and ileal digestible energy. Journal Applied Poultry Research 2000; 9(1):74-80.
- Garcia MI, Araníbar MJ, Lázaro R. α-Amylase supplementation of broiler diets based on corn. Poultry Science 2003; 82(3):436-442.
- Hruby M, Pierson EEM. The use of enzymes in broiler nutrition. Anais do 1ş Fórum Internacional de Avicultura; 2005; Foz do Iguaçu, Paraná. Brasil. p. 142-147.
- Knudsen KEB. Carbohydrate and lignin contents of plant materials used in animal feeding. Animal Feed Science Technology 1997; 67(4):319-338.
- Kocher A, Choct M, Ross G, Broz J, Chung TK. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. Journal Applied Poultry Research 2003; 12(3):275-283.
- Kornegay ET. Digestion of phosphorus and other nutrients: the role of phytases and factors influencing their activity. In: Bedford MR, Partridge GG. Enzymes in farm nutrition. Londres: Cab International; 2001. p. 237-271.
- Lima FR. Aditivos zootécnicos: enzimas. In: Palermo Neto J, Spinosa HS, Górniak S. Farmacologia aplicada à avicultura. São Paulo: Roca; 2005. p. 239-248.
- Malathi V, Devegowda G. In vitro evaluation of nonstarch polysaccharide digestibility of feed ingredients by enzymes. Poultry Science 2001; 80(3):302-305.
- Managi MK, Coon CN. Evaluation of phytase with chicks fed basal diets contain different soybean meal samples. Journal Applied Poultry Research 2006; 15(2):292-306.
- Olukosi OA, Cowieson AJ, Adeola O. Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poultry Science 2007; 86(1):77-86.
- Sakomura NK, Rostagno HS. Métodos de pesquisa em nutrição de monogástricos. Jaboticabal: FUNEP; 2007. 283 p.
- Santos FR, Hruby M, Pierson EEM, Remus JC, Sakomura NK. Effect of Phytase Supplementation in Diets on Nutrient Digestibility and Performance in Broiler Chicks. Journal Applied Poultry Research 2008; 17(2):191-201.
- Sheppy C. The current feed enzyme market and likely trends. In: Bedford MR, Partridge GG. Enzymes in farm nutrition. Londres: Cab International; 2001. p. 1-10.
- Yu B, Chung TK. Effects of multiple-enzyme mixtures on growth performance of broilers fed corn-soybean meal diets. Journal Applied Poultry Research 2004; 13(2):178-182.
- Zanella I, Sakomura NK, Silversides FG, Fiqueirdo A, Pack M. Effect of enzyme supplementation of broiler diets based on corn and soybeans. Poultry Science 1999; 78(4):561-568.
Publication Dates
-
Publication in this collection
17 June 2009 -
Date of issue
Mar 2009
History
-
Accepted
Mar 2009 -
Received
Dec 2008