Abstract
There is little information on the nutrition of red-winged tinamous (Rhynchotus rufescens) reared in captivity, and their nutritional requirements still need to be determined. This study aimed at determining dietary crude protein requirements and testing four organic selenium supplementation levels in the diet of red-winged tinamous during the breeding season. Birds were housed in a conventional broiler house divided in 16 boxes with one male and three females each. Iso-energy (2800kcal ME/kg) pelleted feeds, based on corn and soybean meal, were supplied in tube feeders. In the first experiment, treatments consisted of four different diets containing different crude protein (CP) contents (15, 18, 21, or 24%) and in the second experiment, the four diets contained equal protein level (22.5%) and four different organic selenium levels (0, 0.2, 0.4, or 0.8ppm). Data were analyzed by the least square method. The best egg weight and eggshell thickness were obtained with 22.5% dietary CP. Organic selenium did not influence the studied reproductive traits of red-winged tinamous (Rhynchotus rufescens) males or females.
Egg weight; fertility; nutrition; semen quality; shell thickness
Effect of crude protein levels and organic selenium supplementation in the diets fed during the breeding season on reproductive parameters of red-winged tinamous (Rhynchotus rufescens)
Felipe LI; Santos ECII; Tavian AFII; Góes PAAIII; Moraes VMBIV; Tonhati HIV; Boleli ICV; Malheiros EBVI; Barnabé VHIII; Queiroz SAIV
IM.Sc. student of the Post-Graduation Program in Animal Science, FCAV/UNESP-Jaboticabal
IIBiologist
IIIDepartment of Animal Reproduction - FMVZ/USP
IVDepartment of Animal Science - FCAV/UNESP - CNPq grantee
VDepartment of Animal Morphology and Physiology - FCAV/UNESP
VIDepartment of Exact Sciences - FCAV/UNESP
Mail Address Mail Address: Sandra Aidar Queiroz Departamento de Zootecnia FCAV/Unesp 14.884-900. Jaboticabal, SP, Brazil Fone: (16) 3209 2689 E-mail: saquei@fcav.unesp.br
ABSTRACT
There is little information on the nutrition of red-winged tinamous (Rhynchotus rufescens) reared in captivity, and their nutritional requirements still need to be determined. This study aimed at determining dietary crude protein requirements and testing four organic selenium supplementation levels in the diet of red-winged tinamous during the breeding season. Birds were housed in a conventional broiler house divided in 16 boxes with one male and three females each. Iso-energy (2800kcal ME/kg) pelleted feeds, based on corn and soybean meal, were supplied in tube feeders. In the first experiment, treatments consisted of four different diets containing different crude protein (CP) contents (15, 18, 21, or 24%) and in the second experiment, the four diets contained equal protein level (22.5%) and four different organic selenium levels (0, 0.2, 0.4, or 0.8ppm). Data were analyzed by the least square method. The best egg weight and eggshell thickness were obtained with 22.5% dietary CP. Organic selenium did not influence the studied reproductive traits of red-winged tinamous (Rhynchotus rufescens) males or females.
Keywords: Egg weight, fertility, nutrition, semen quality, shell thickness.
INTRODUCTION
The consumption of meat of wild animals has increased in several countries, including Brazil. Research studies carried out with red-winged tinamous (Rhynchotus rufescens) have shown that these birds can be adapted to captivity due to their broad geographic dispersion, and omnivorous feeding habits. It was demonstrated that they can be habituated to the consumption of meal and pelleted feeds (Hoshiba et al., 2003), present good growth rates (Tholon & Queiroz, 2007), and excellent carcass and breast meat yield (Moro et al., 2006) when reared in captivity.
If inadequately fed, birds will shortly suffer nutritional deficiencies demonstrated by impaired productive and reproductive performance. A balanced poultry diet must include macro and trace nutrients in amounts sufficient to allow birds to grow, breed, and maintain their metabolic functions.
Low-protein diets cause reduced body weight gain and feed intake and worse feed conversion ratio (Malheiros et al., 2003), influencing egg production and size. Poultry have limited capacity of storing protein, and egg size is highly dependent on protein intake (Pesti, 1992). Therefore, the success of the domestication of a determined animal species depends on the determination of its nutritional requirements, including protein requirements.
There are very few studies on tinamou nutrition. In an attempt to determine the nutritional requirements of that species, Moro et al. (2002) used the recommendations for pheasants, but did not obtain satisfactory results. On the other hand, the Japanese quail (Coturnix coturnix japonica), which also belongs to the Phasianidae family, could be a better initial model for the determination of protein requirements in redwinged tinamous (Rhynchotus rufescens). Crude protein levels recommended in literature for Japanese quails range between 16 and 25%, and the differences are possibly due to genetic, experimental, or climatic variations that may affect quail performance (Silva et al., 2007).
Minerals are essential for life maintenance, and are involved in several metabolic processes in the body. Minerals can be divided into macro and trace minerals, which classification is based on the amount required for individual metabolic functions.
Most trace minerals metabolically function as enzyme co-factors or components. Among trace minerals, selenium presents flexible oxi-reduction capacity as it is part of the active center of the enzyme glutathione-peroxidase, which protects cell membranes from the oxidative attack by free radicals (Ortolani, 2002). Selenium is also part of the enzyme iodothyronine-deiodinase, responsible for the conversion of thyroxin into its active form (Silva et al., 2007).
According to Hess et al. (2000), inorganic selenium sources (sodium selenite and calcium selenide) are traditionally used in poultry nutrition, starting in 1974, when the FDA approved their inclusion in animal feeds (Echevarria et al., 1988; Leeson & Summers, 1997). However, research has shown that when selenium is incorporated into organic molecules as amino acids and peptides, such as found in plants, grains, and yeasts, its antioxidant action is more effective than that of inorganic selenium forms, promoting higher intestinal absorption, better performance and feathering, and mineral tissue retention. It must also be mentioned that organic trace minerals are more available than their inorganic counterparts. Organic trace minerals can also be biosynthesized, as in the case of selenomethionine and selenocystine, which are produced by adding inorganic selenium and yeast to a culture medium. The yeast incorporates selenium instead of sulfur in the amino acids methionine or cystine (Hynes & Kelly, 1995).
In addition to protecting cells against oxidative processes and to prevent metabolic and infectious diseases, selenium is also important for maintaining sperm integrity. According to Edens (2004), cockerels supplemented with organic selenium presented better semen quality, with a lower number of sperm abnormality, followed by those supplemented with sodium selenite or non-supplemented. The substitution of inorganic by organic selenium improved eggshell quality as shown by its higher weight and thickness (Klecker et al., 2001), as well as egg production (Rutz et al., 2003).
This study aimed at evaluating the effects of crude protein levels and the use of organic selenium in the diets of red-winged tinamous (Rhynchotus rufescens), fed during the breeding season.
MATERIAL AND METHODS
Two experiments were carried out in the Wild Animal Sector of the Animal Science Department of FCAV-UNESP, Jaboticabal, SP, Brazil. The experiment for evaluating dietary crude protein levels during the breeding season was conducted between November 2006 and February 2007 (EXP1). Dietary organic selenium evaluation was carried out between November 2007 and February 2008 (EXP2).
In EXP1, birds were in their third breeding season, and were 28 to 36 months old. In EXP2, birds were 40 to 48 months old, and were in their fourth breeding season.
The following parameters were evaluated in both experiments: egg weight and dimensions, egg production, eggshell thickness, fertility, sperm motility, sperm vigor, semen volume, semen aspect, sperm concentration, sperm abnormalities, hatchability, and hatching rate.
The studied tinamous were housed in a conventional masonry poultry house with concrete floor and asbestos tiles. Birds were housed in 16 pens, with one male and three females each. Pens were separated by a 40-cm wall, and covered with 2.0m x 1.0m x 2.1m wire mesh to isolate the experimental birds from other wild birds in order to minimize disease transmission. The floor was covered with coast-cross (Cynodon dactylon) hay litter. Pens were equipped with tube feeders and bell drinkers. Feed and water were offered ad libitum. Feeds were pelleted and based on corn and soybean meal. A lighting program of 18 hours of light daily, starting in August, was applied in both experiments.
In EXP1, four iso-energy diets (2,800kcal ME/kg feed) were formulated with four different crude protein levels (15, 18, 21, and 24%) based on the studies of Moro et al. (2002). The requirements of all other nutrients followed the recommendations of the NRC (1994), and nutritional ingredient composition was according to Rostagno et al. (2000). In EXP2, iso-energy (2800kcal ME/kg) and iso-protein (22.5% CP) were used, and contained four different concentrations of organic selenium (Sel-Plex®): 0, 0.2, 0.4, and 0.8ppm (Tables 1 and 2).
Eggs were collected four times daily and identified with a tag per box, laying order, and collection date. Eggs were weighed in a digital scale with decimal precision, and their length and width were measured using a pachymeter. Eggs were submitted to artificial incubation, and eggs that did not hatch were submitted to embryo diagnosis. Three eggs per replicate were collected to measure eggshell thickness, using a digital micrometer (Mitutoyo, 0.001-mm resolution) in fragments removed from the tip, equator, and bottom of the egg, based on which average eggshell thickness was calculated.
Semen was collected six times in both experiments. In EXP2, two additional collections were made before feed adaptation. Before semen collection, cockerels' feathers around the cloaca were removed, and the area was cleaned to prevent semen loss and contamination. Semen was collect by digital pressure of the base of the phallus and of the vas deferens (Cavalcante et al., 2004).
Immediately after collection, semen was analyzed for semen volume, aspect, sperm vigor, and motility. Semen volume was described by direct observation in a capillary tube. Semen aspect was scored as two for milky, one for semi-milky, and zero for transparent (Cavalcante et al., 2004). Sperm motility (% moving sperm) and sperm vigor (progressive movement; 0 to 5 score scale) were evaluated by placing a 5-µL aliquot of the semen diluted in saline solution at 1:10 between a glass slide and coverslip, and observation under optical microscope at 400X magnification (Tiaimin, model TN212B).
After these analyses, a 2-µL aliquot of each semen sample was diluted in 998-µL saline formalin at 37ºC, and stored in a plastic microtube identified with collection date, male number, and pen in a refrigerator at 5ºC for subsequent sperm concentration and morphology analyses.
Sperm concentration was determined by counting in a Neubauer hemocytometer under optical microscope at 400X magnification. The obtained number was used in the sperm concentration formula, according to the criteria recommended by the Brazilian College of Animal Reproduction.
Sperm morphology was described by analyzing 200 cells in wet-mount smear under differential interference phase contrast microscopy at 1000x magnification, according to Barth & Oko (1989). Cells were classified as normal or abnormal as to head, intermediate piece, and tail.
Fertility, hatchability, and hatching rates were calculated as:
Fertility rate = (n. fertile eggs/n. incubated eggs) x 100
Hatchability = (n. hatched eggs/n. fertile eggs) x 100
Hatching rate = (n. hatched eggs/n. incubated eggs) x 100
Using the Guided Data Analysis and Analyst of the Statistical Analysis System Software (SAS 9.1, Institute, Cary, North Carolina, USA), data were tested for residue normality and variance homogeneity. The following traits did not comply with those assumptions: fertility rate, sperm motility, tail defects, intermediate-piece defects, acrosome defects, total defects, and normal sperm. Therefore, data were transformed according to the formula:
YT=ARCSIN (SQRT ((Y+1.5) /100));
where:
YT=transformed trait,
Y = observed trait,
ARCSIN= arcsine,
SQRT= square root.
The traits were submitted to analysis of variance according to a completely randomized experimental design with four treatments (crude protein or selenium levels) of four replicates each, employing the least square method. When traits presented significant differences among treatments, degrees of freedom were detailed in ordinary polynomials, and linear, quadratic, and cubic regression (p=0.10). Means were compared by the test of Duncan (p=0.10).
In EXP2, a split-plot experimental design was applied to evaluate the 4 different treatments (0, 0.2, 0.4, 0.8 ppm organic selenium - Sel-Plex®) and the 8 different semen collection times. Means per treatment and per semen collection times, as well as semen aspect and semen volume and sperm motility, sperm vigor, concentration, and abnormalities (head, intermediate piece, tail, and total defects) were analyzed. Collected data were submitted to analysis of variance by the least square method, according to the following model:
Yijkl = µ + NSi+ Rj(NSi) + TPk + (NS * TP)ijk+ eijkl, em que:
0, 0.2, 0.4, 0.8),
within the ith selenium level,
(k=1,...8)
kth collection time with the ith
selenium level,
as normally and independently
distributed.
RESULTS
Dietary protein levels
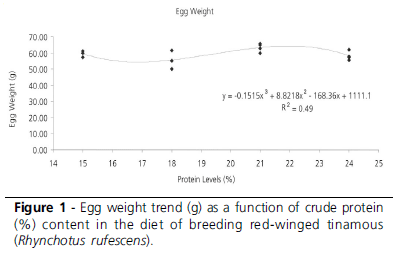
In EXP1, egg weight and eggshell thickness were influenced (p<0.1) by dietary crude protein levels (Table 3). The experimental design explained 52% of the variation observed in these traits, which coefficients of variation were 5.7 and 3.9%, respectively. The heaviest eggs were obtained with 21% crude protein level; however, they were not significantly different (p>0.1) from those from birds fed 15 and 24% crude protein. Eggshell thickness was different between the eggs produced by birds fed 15 and 18% crude protein (p<0.1), but these were not different from those fed 21 and 24% crude protein (Table 3).
The decomposition of the degrees of freedom showed significant effect of the cubic regression (p<0.1) for egg weight and eggshell thickness. Figures 1 and 2 present the regression curves and their respective equations.
Dietary selenium levels
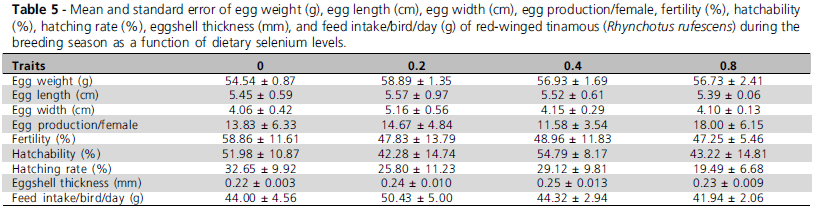
The different dietary selenium levels did not statistically influence (p>0.1) none of the evaluated traits. However, as this is a pioneer study, mean values obtained for red-winged tinamous (Table 4) per treatment (Tables 5 and 6) are presented.
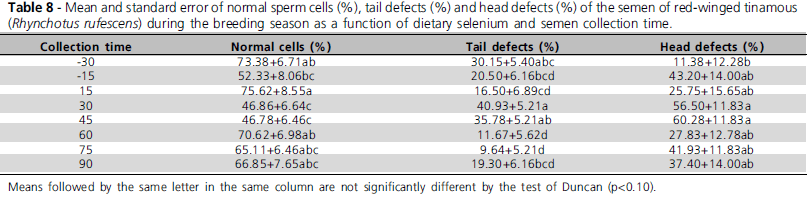
There was no significant effect of dietary selenium levels (p>0.1) on male or semen traits. However, collection time significantly affected (p<0.1) sperm motility, sperm vigor, sperm concentration, semen volume, normal sperm cells, head defects, and tail defects (Tables 7 and 8). There selenium levels and semen collection time only on sperm head defects (p<0.1).
DISCUSSION
Dietary crude protein levels significantly affected egg weight and eggshell thickness (p<0.1), with mean and standard deviation as 59.35±3.38g and 0.27±0.01mm, respectively. The coefficients of determination, expressing the fitness of the model to the parameters, were 50% and 52%, respectively, indicating that the statistical models explained most of the variation. The coefficients of variation were low (5.7% and 3.9%), demonstrating that there was little variation among individuals for these parameters.
In the study of Moro et al. (2002), red-winged tinamous were fed meal diets containing 15% CP and 2800kcal ME/kg, and their egg weights ranged between 47.5 and 54.6g, but were not statistically different. Bruneli et al. (2005) obtained an average weight of red-winged tinamous (Rhynchotus rufescens) eggs of 52.6g during the breeding season (October to January), which is the phase of highest egg production, and therefore, of lighter eggs. Nakage et al. (2002), also working with red-winged tinamous, observed that egg with weights of 51 to 55g presented 0.22mm eggshell thickness, as well as those weighing, in average, between 56 and 60g, value that are similar to those obtained in the present study. In the studies of Nakage et al. (2002) and Bruneli et al. (2005), birds were fed diets containing the same crude protein and energy content as that of Moro et al. (2002), but in the pelleted form, instead of meal. Feed physical form influenced egg weight in those studies, which were carried out after that of Moro et al. (2002), because tinamous present a very selective feeding behavior. When fed meal diets, tinamous tend to select larger particles, leaving the smaller one, which usually contain trace minerals. Pelleted feeds prevent particle selection, promoting the ingestion of all feed nutrients, which results in better reproductive performance of these birds. In addition, when there is particle selection, the true levels of crude protein (CP) and energy intake are not known, interfering with the determination of the birds' nutritional requirements.
It was shown that higher dietary protein (Parsons et al.,1993; Leeson, 1989), lipid (Grobas et al., 1999; Wu et al., 2005), methionine (Keshavarz, 1995), lysine (Zimmerman, 1997), and linoleic acid (Smith & Pourezza, 1989) increases egg weight. On the other hand, when the diet is deficient in protein, egg size tends to be reduced, while egg production level is maintained because of the bird's drive to perpetuate its species. Indeed, in the present experiment, birds fed 21% CP produced heavier eggs than those fed 18% CP; however, egg weight decreased when 24% CP was fed (Table 3). These results are partially consistent with the findings of Perly (1979), Murakami & Furlan (2002), and Pinto et al. (2002), who mentioned that egg weight is highly dependent on daily protein intake in layer chickens, and can be used as a reference to ensure amino acid requirements. Calderon & Jensen (1990) verified that methionine significantly increased egg production when commercial layers were fed 13, 16, or 19% CP. Sulfur amino acid requirements were estimated as 0.48-0.55% in feeds containing 13% crude protein, 0.59-0.61% in diets with 16% CP, and 0.67-0.70% in diets with 19% CP. It should be mentioned that lysine and methionine+cystine requirements were met in the present study.
Red-winged tinamous fed 15% CP produced eggs with thicker eggshells as compared to those fed the 18% CP diets (Table 3). This result is different from literature findings, such as those of Harms (1983) and Koelkebeck et al. (1991), who did not observe any differences in the specific gravity of eggs laid by birds fed different protein levels. Aiming at evaluating the effect of increasing dietary protein levels on the morphometrical characteristics of the oviduct of Japanese quails, Artoni et al. (2001) showed that 24% crude protein increased the thickness of the glandular layer of the magnum, isthmus, and shell gland, which may result in heavier and thicker eggs.
The decomposition of the degrees of freedom of treatments showed a cubic effect (P<0.1) on egg weight and eggshell thickness, with the highest values for these parameters obtained at 22.5% CP (Figures 1 and 2). Heavier eggs are related to heavier hatchling weight, and thicker eggshells to higher egg resistance to environmental harsh conditions (Nakagi et al., 2002). Egg production and egg size are highly dependent on daily protein intake, as birds can only store this nutrient for a limited period of time (Pesti, 1992). Similar results were reported by Singh & Narayan (2002), who recommended 22% crude protein (CP) for quails during lay, and by Pinto et al. (2002), who suggested 22.42% CP dietary level. Murakami et al. (1993) found higher egg weight in quails fed 20% crude protein (CP).
In EXP2, the different organic selenium supplementation levels did not significantly influence any of the evaluated parameters (P>0.1), perhaps because bird requirements were already supplied by the selenium contained in the trace mineral premix. Also, this result may be explained by the fact that birds were not submitted to any challenge that may have required higher dietary selenium levels. Similar results were reported by Pan et al. (2004), Xavier et al. (2004), De Lange & Elferink (2004), and Klecker et al. (2001) who also found that egg production was not affected by dietary selenium addition.
Klecker et al. (2001) observed that the replacement of inorganic selenium by organic selenium in the diet increased eggshell thickness as compared to the controls. Xavier et al. (2004) and Pan et al. (2004) despite reporting better eggshell quality, did not find significant differences in their results, which is consistent with the findings of the present study, where eggshell thickness was not statistically different among treatments, but numerically increased in 5 to 14% with increasing organic selenium levels relative to the control treatment not fed organic selenium.
Dietary organic selenium levels did not influence any trait of the semen of red-winged tinamous (P>0.10). Different results were obtained by Tavian et al. (2008), who reported higher sperm cell (52.31% vs. 38.96%), lower total defect (47.69% vs. 61.04%), and lower intermediate-piece defect (10.54% vs. 17.47%) percentages (P<0.001) in the semen of red-winged tinamous fed liquid selenium and vitamin E.
Cavalcante et al. (2004), feeding red-winged tinamous (Rhynchotus rufescens) pelleted feeds with 15% CP, obtained lower mean semen volume (14.13uL), sperm motility (66%), sperm vigor (2.30) and sperm concentration (0.93 x 109cells/mL) as compared to the present study. Those authors also reported higher rates of sperm morphological changes than here. However, the birds used in their study were in their first breeding season (12 to 18 months old), which may have increased the percentage of sperm defects as, according to Correa & Arceo (1995), animals with immature testicles may present higher levels of sperm abnormalities. Moreover, this difference could be also explained by the longer conditioning time of the birds in the present experiment as compared to that described by Cavalcante et al. (2004), allowing better adaptation of birds to handling, that is, reducing their stress. Stress may compromise reproductive performance due to the physiological and behavioral changes it causes.
The morphological changes found by Cavalcante et al. (2004) in red-winged tinamous were defects of the acrosome (0.79%), head (10.45%), intermediate piece (16.06%), tails (14.99%), and others (1.28%). In the present study, the most common defects were folded intermediate piece, folded and coiled tail, and strongly coiled tail. A lower percentage of acrosomal and intermediate-piece defects were also observed as compared to Cavalcante et al. (2004), but higher rates of head defects, and intact sperm heads are essential for fertilization.
Silva et al. (2003) studied the supplementation of cockerel diets containing inorganic selenium with organic selenium or not, and reported that, when organic selenium was supplied, sperm abnormality rates tended to decrease, particularly intermediate-piece defects, and normal sperm cell rates tended to increase in the ejacultate. Similar to our results, those authors also observed higher head defect values.
Collection time significantly influenced (P<0.1) sperm motility, sperm vigor, concentration, semen volume, normal sperm cells, head defects, and tail defects, which improved along the breeding season (Tables 7 and 8) and presented the best performance during the summer (December and January). The improvement of semen traits along the breeding season was expected, as red-winged tinamous present breeding seasonality, which extends from September to April, and fertile egg production peak between December and February, according to Bruneli et al. (2005).
Conclusions
A crude protein content of 22.5% must be provided in the diet of breeding red-winged tinamous for the production of the heaviest eggs and thickest eggshell.
The supplementation of organic selenium in the diet of male and female red-winged tinamous is not required during the breeding season.
The semen quality of red-winged tinamous improved along the breeding season, and presented the best results during the summer.
Arrived: September/2009
Approved: January/2010
- Artoni SMB, Carneiro APM, Giacomini G, Moraes VMB, Araújo CSS, Araújo LF. Avaliação macroscópica e morfométrica do oviduto de codornas (Coturnix coturnix japonica), quando tratadas com diferentes níveis de proteína. Revista Brasileira de Ciência Avícola 2001; 3(3):211-217.
- Bruneli FAT, Tholon P, Isaac FL, Damasceno PR, Tonhati H, Queiroz SA. Caracterização da reprodução de perdizes (Rhynchotus rufescens) em cativeiro. Ars Veterinária 2005; 21(2):272-280.
- Barth AD, Oko RJ. Anormal morphology of bovine spermatozoa. Ames: lowa State University Press; 1989. 285p.
- Cavalcante AK, Goes PAA, Nichi M, Hatamoto NM, Mantovani AP, Bertola RP, Lilla MP, Tholon P, Bruneli FAT, Gabriel JM, Queiroz SA, Tonhatti H Barnabe VH. Evaluation of seminal characteristics in captive male partridges (Rhynchotus rufescens) raised in São Paulo State. Revista Brasileira de Reprodução Animal 2004; 1(1):225.
- Calderon VM, Jensen LS. The requirement for sulfur amino acid by laying hens as influenced by the protein concentration. Poultry Science 1990; 69(6):934-944.
- Correa JCS, Arceo AMA. Edad a la pubertad y características seminalles de gallos Rhodes Island e Criollo Cuello Desnudos bajo condiciones tropicales. Veterinária México 1995; 26(4):375-379.
- De Lange LLM, Elferink GO. Produção de ovos enriquecidos com selênio através da inclusão de fontes orgânicas e inorgânicas do mineral na ração. Anais da 14Ş Ronda Latinoamerica da Alltech Simpósio da Indústria de Alimentos Animal; 2004; Curitiba, Paraná. Brasil. p.58.
- Echevarria MG, Henry PR, Ammerman CB, Roa PV, Miles RD. Estimation of the relative bioavailability of inorganic selenium sources for poultry: 1. Effect of time and high dietary selenium on tissue selenium uptake. Poultry Science 1988; 69(11):1295-1301.
- Edens FW. Efeito da suplementação de selênio orgânico (Sel Plex) sobre a integridade dos espermatozóides em frangos de corte machos. Anais da 14Ş Ronda Latinoamericana da Alltech, Simpósio da Indústria de Alimentos Animal; 2004; Lexington, Kentucky. p.54.
- Grobas S, Mendez J, Blas C, Mateos GG. Laying hen productivity as affected by energy, supplemental fat, and linoleic acid concentration of the diet. Poultry Science 1991; 78:1542-1551.
- Harms, RH. Influence of protein level in the resting diet upon performance of force rested hens. Poultry Science 1983; 62:273-276.
- Hess JB, Dows KM, Bilgili SF. Selenium nutrition and poultry meat quality. Poultry Science 2000; 79(2):107-112.
- Hoshiba MA, Tanaka ALR, Rodrigues GA, Figueiredo G, Tholon P, Junqueira OM, Tonhati H, Queiroz SA. Resultados preliminares do consumo e desperdício de ração com diferentes tamanhos de péletes de perdizes (Rhynchotus rufescens) em cativeiro. Anais. da 40Ş Reunião Anual da Sociedade Brasileira de Zootecnia; 2003; Santa Maria, Rio Grande do Sul. Brasil. CD-Rom.
- Hynes MJ, Kelly P. Metal ions, chelates and proteinates. Annual of the 11th Symposium of Biotechnology in the Feed Industry; 1995; Nottingham, United Kingdom. p. 233-48.
- Keshavarz K. Further investigations on the effect of dietary manipulations of nutrients on early egg weight. Poultry Science 1995; 74:62-74.
- Klecker RD, ZatloukaL M, Zeman L. Effect of organic selenium, zinc and manganese on reproductive traits of laying hens and cockerels. European Symposium on Poultry Nutrition; 2001; Blankenberge. p. 258-262.
- Koelkebeck KW, Parsons CM, Leeper RW, Moshtaghian J. Effect of protein and methionine levels in molt diets and posmolt performance of laying hens. Poultry Science 1991; 70: 2063-2073.
- Leeson S, Summers JD. Commercial poultry nutrition. 2nd ed. New York (NY): State Manual Book & Periodical Services; 1997.
- Leeson S. Energy intake and layer performance. Proceedings of the Californian Nutrition Conference; 1989; Grain. California. p. 72-79.
- Malheiros RD, Moraes, VMB, Collin A, Janssens GPJ, Decuypere E, Buyse, J. Dietary macronutrients, endocrine functioning and intermrdiary metabolism in broiler chinckens pair wise substitutions between protein, fat and carbohydrate. Nutrition Research 2003; 23(4):567-578.
- Moro EG, Ariki J, Souza PA, Souza HB, Moraes VMB. Rendimento de carcaça e composição química da carne de perdiz nativa. Ciência Rural 2006; 36(1):258-262.
- Moro EG, Ariki J, Malheiros EB. Avaliação dos níveis de proteína da dieta sobre a idade à maturidade sexual e produção de ovos de perdiz (Rhynchotus rufescens Temminek). Acta Scientiarum 2002; 24 (4):997-1000.
- Murakami AE, Moraes VMB, Ariki J, Junqueira OM, Kronka SN. Níveis de proteína e energia em rações para codornas japonesas (Coturnix coturnix japonica) em postura. Revista da Sociedade Brasileira de Zootecnia 1993; 22(4):541-551.
- Murakami AE, Furlan AC. Pesquisa na nutrição e alimentação de codornas em postura no Brasil. Anais do 1ş Simpósio Internacional de Coturnicultura; 2003; Lavras, Minas Gerais. Brasil. p.113-120.
- Nakage ES, Cardozo JP, Pereira GT, Queiroz AS, Boleli IC. Efeito da forma física da ração sobre a porosidade, espessura da casca, perda de água e eclodibilidade em ovos de perdiz (Rhynchotus rufescens). Revista Brasileira de Ciência Avícola 2002; 4(3):227-234.
- National Research Council. Nutrient requirements of poultry. 9th ed. Washington: National Academy Press; 1994.
- Ortolani EL. Macro e micronutrientes. In: Spinosa HS, Górniak SL, Bernardi MM. Farmacologia aplicada à medicina veterinária. Rio de Janeiro: Guanabara Koogan; 2002. p.641-651.
- Pan EA, Rutz F, Dionello NJL, Anciuti MA, DA Silva ERR. Desempenho de poedeiras semipesadas alimentadas com dietas contendo selênio orgânico (Sel-Plex). Anais da 14Ş Ronda Latinoamericana da Alltech Simpósio da Indústria de Alimentos Animal; 2004; Curitiba, Paraná. Brasil. Cd-Rom
- Parsons CM, Koelkebeck KW, Zhang YX, Wang, Leeper RW. Effect of dietary protein and added fat levels on performance of young laying hens. Journal of Applied Poultry Research 1993; 2:214-220.
- Perly L. Correlação entre índice morfológico, peso do ovo e peso vivo ao final da fase de crescimento em codorna doméstica (Coturnix coturnix japonica). Revista do Setor de Ciências Agrárias 1979; 1(1):41-53,
- Pesti GM. Temperatura ambiente e exigências de proteína e aminoácidos para poedeiras. Anais Simpósio Internacional de Não Ruminantes; 1992; Lavras, Minas gerais. Brasil. p. 208-219.
- Pinto R, Ferreira AS, Albino LFT, Gomes PC, Vargas JG. Níveis de proteína e energia para codornas japonesas em postura. Revista Brasileira de Zootecnia 2002; 31(4):1761-1770.
- Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Ferreira AS, Oliveira RFM, Lopes DC Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. Viçosa: UFV; 2000. 124 p.
- Rutz F, Pan EA, Xavier GB, Anciutti MA. Meeting selenium demands o modern poultry: responses to Sel Plex organic selenium in broiler and breeder diets. Nutritional Biotehnology the Feed and Food Industries. Proceedings of Alltech's 19th Annual Symposium, 2003; Nottingham; 2003. p.147-161.
- Silva LA, Silva RR, Zauk NHF, Dionello NJ, Poposki M, Rovatti E, Ziguer EA, Rutz F. Características seminais qualitativas em galos recebendo dietas contendo selênio inorgânico suplementado ou não com orgânico. Anais do 12ş Congresso de Iniciação Científica, 6ş Encontro de Pós-Graduação; 2003; Pelotas, Rio Grande do Sul. Brasil. Cd-Rom.
- Silva JHV, Costa FGP, Silva EL, Filho JJ, Goulart CC, Neto RCL, Ribeiro LGR. Exigências nutricionais de codornas. Anais do 3ş Simpósio Internacional de Coturnicultura; 2007; Lavras, Minas Gerais. Brasil. p.44-64.
- Singh RV, Narayan R. Produção de codornas nos trópicos. Anais do 1ş Simpósio Internacional de Coturnicultura, 2002; Lavras, Minas Gerais. Brasil, p. 27-35.
- Smith WK, Pourezza J. Supplemental lipids and sulphur amino acid utilization in laying hens [abstract]. British Poultry Science 1989; 30:977-978.
- Tavian AF, Cavalcante AKS, Góes PAA, Tonhati H, Queiroz SA. Description of the semen of red-winged tinamous (Rhynchotus rufescens) treated with vitamin E and selenium. Proceedings of 23th World's Poultry Congress; 2008; Brisbane, Queensland. Australia. CD-ROM.
- Tholon P, Queiroz SA. Models for the analysis of growth curves for rearing tinamous (Rhynchotus rufescens) in captivity. Brazilian Journal of Poultry Science 2007; 9(1):23-31.
- Wu G, Bryant MM, Voitle RA, Roland DA. 2005. Effect of dietary energy on performance and egg composition of Bovans White and Dekalb White hens during phase 1. Poultry Science 2005; 84:1610-1615.
- Xavier GB, Rutz F, Dionello NJL, Duarte AD, Gonçalves FM, Zauk NHF, Ribeiro CLG. Desempenho de poedeiras alimentadas com dietas contendo selênio, zinco e manganês orgânico durante o segundo ciclo de produção. Anais do 14ş Ronda Latino-Americana da Alltech, Simpósio da Indústria de Alimentos Animal; 2004; Rio Grande do Sul, Brasil. p.8.
- Zimmerman RA. Management of egg size through precise nutrient delivery. Journal of Applied Poultry Research 1997; 6:478-482.
Publication Dates
-
Publication in this collection
17 May 2010 -
Date of issue
Mar 2010
History
-
Received
Sept 2009 -
Accepted
Jan 2010