ABSTRACT
These experiments were performed to determine the chemical composition, coefficients of nutrient and energy metabolizability, amino acid composition, and cytotoxicity of different castor oil meals subjected to different detoxification processes and added to the diet of Japanese quails. In the trial, 180 46-d-old female Japanese quails were distributed according to a completely randomized design into five treatments and with replicates of six bird each. The treatments consisted of following detoxification methods of castor oil meal: Castor oil meal A (CMA) - recovery in alcohol at 80 °C for 20 minutes and drying at 80 °C; castor oil meal B (CMB) and C (CMC) - recovery in alcohol at 80 °C for 6 minutes, neutralization with 5% NaOH, and drying under direct sunlight sun for two days (CMB) or pelleted (CMC); castor oil meal D (CMD) - recovery in alcohol at 110 °C for 15 minutes and drying at 110 °C. Castor oil meal was added replacing 20% of the reference diet. There was slight chemical composition variation (1.21% in crude protein, 6% in dry matter, 2.2% in ether extract and 64 kcal/kg in gross energy) among the castor oil meals submitted to the different treatments. The castor oil meal submitted to treatment C showed the highest amino acid values. In the cytotoxicity test, treatment D presented lower ricin activity. Castor oil meals A, C, and D may be included in Japanese quail diets; however, castor oil meal D is recommended due to the simplicity its industrial process, its low toxicity, and metabolizability coefficients obtained.
Keywords:
Alternative food; poultry; cytotoxicity; digestibility; energy
INTRODUCTION
Japanese quail production stands out in the Brazilian market as an excellent activity because it requires low initial investment costs as well small areas, providing fast return on the capital invested, in addition of presenting low labor costs (Ton et al., 2011Ton APS, Furlan AC, Martins EN, Toledo JB, Scherer C, Conti ACM. Digestible lysine and metabolizable energy requirements of growing meat quails. Revista Brasileira de Zootecnia2011;40(3):593-601.).
Feed represents a major cost in poultry production. Considering the increasing prices of corn and soybean meal, which are typically the main ingredients of poultry diets, alternative feed stuffs have been sought to replace those costly ingredients, which production also competes with human food. Studies on alternative feedstuffs have allowed considerable reduction in production costs (Albuquerque et al., 2014Albuquerque CS, Rabello CBV, Santos MJB, Lima MB, Silva EP, Lima TS, et al . Chemical composition and metabolizable energy values of corn germ meal obtained by wet milling for layers. Revista Brasileira de Ciência Avícola 2014;16(1):107-112.)as the partial or total replacement of expensive ingredients contributes for the economic viability of animal production (Santos et al., 2013Santos MJB, Ludke MCMM, Ludke JV, Torres TR, Lopes LS, Brito MS. Chemical composition and metabolizable energy values of alternative ingredients for broilers. Ciência Animal Brasileira 2013;14(1):32-40.).
The castor oil meal has been the object of research because of its high protein value. However, its protein content may vary due to seed characteristics (Azevedo &Lima, 2007Azevedo DMP, Lima EF. O Agronegócio da mamona no Brasil. Campina Grande: Embrapa Algodão; Brasília, DF: Embrapa Informação Tecnológica; 2007.), as well as its metabolizable energy (ME) level, which is influenced by the industrial methods applied for castor oil extraction for biodiesel production. In Brazil, the National Council for Energy Policy (CNPE) established in Act No. 11.097/2005 the mandatory blending of 2% biodiesel in conventional diesel by2008 and 5% by2013, and therefore, castor bean by-products are highly available in the Brazilian market.
Abdalla et al. (2008Abdalla AL, Silva Filho JCD, Godoi ARD, Carmo CDA, Eduardo JLDP. Use of byproducts of the biodiesel industry in ruminant feed. Revista Brasileira de Zootecnia 2008;37(3):260-268.) reported that the crude protein (CP) of castor oil meal ranges between 39 and43%; however, its use is limited because of its toxicity (ricin and ricinin) and allergenicity (2S albumins) (Anandan et al., 2005Anandan S, Anil Kumar GK, Ghosh J, Ramachandra KS. Effect of different physical and chemical treatments on detoxification of ricin in castor cake. Animal Feed Science and Technology, v.120, p.159-168, 2005.).Ricin is the main toxic component of the castor oil plant (Ricinus communis) and it consists of a water-soluble protein found only in the endosperm of the castor oil plant seeds (Severino, 2005Severino LS. What we know about the castor bean pie.; Campina Grande: Embrapa Algodão 2005.). Ricin belongs to the family of lectins A-B, i.e., it is composed of two subunits: one with enzymatic activity and the other with a specific binding site for galactose. Its toxicity mechanism is exerted through the inactivation of ribosomes. The subunit A inactivates specifically and irreversibly the eukaryotic ribosomes, preventing protein synthesis by removing adenine residues in 28S ribosomal RNA (Oliveira et al., 2006). The subunit B is bound to the cell wall and the sub-unit A through the disulfide bonds, allowing the latter to enter the cytosol by endocytosis. Therefore, if the bonds between the two subunits are broken, the resulting parts are not toxic (Audi et al., 2005Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA 2005;294(18):2342-2351.).
After being detoxified, castor oil meal can be provided to animals as protein concentrate in partial replacement of soybean meal. There are different castor oil meal detoxification that allow its inclusion in the feeds of monogastric animals.Anandan et al. (2005Anandan S, Anil Kumar GK, Ghosh J, Ramachandra KS. Effect of different physical and chemical treatments on detoxification of ricin in castor cake. Animal Feed Science and Technology, v.120, p.159-168, 2005.) concluded that submitting the castor bean meal to autoclave (15 psi, 60 min) or treatment with calcium hydroxide (40 g/kg castor oil meal) completely denatured ricin. Other methods, such as autoclave (15 psi for 90 minutes) or calcium hydroxide or calcium oxide diluted in water (1:10) at a dose of 60 g/kg of castor oil meal, have proven effective to denature the ricin (Oliveira et al., 2006Oliveira A S, Oliveira M RS, Campos J MS. Effectiveness of different methods of detoxification of ricin in castor oil meal. Anais do Congresso da Rede Brasileira de Tecnologia e Produção de Biodiesel; 2006 ago 31 a set 1º; Brasília, DF. Brasil. p.1-4 ).
Due to the lack of information on the use of castor oil meal in the diet of Japanese quails, these experiments were performed to determine the chemical composition and coefficients of metabolizability of nutrients and energy, and the cytotoxicity of different castor oil meals submitted to different processing methods.
MATERIAL AND METHODS
The experiment was conducted at the Department of Animal Science, Federal Rural University of Pernambuco (UFRPE), Recife, Pernambuco, Brazil. The project was approved by the Ethics Committee on Animal Use (CEUA-UFRPE).
The castor bean cultivar used was the BRS Nordestina. Oil was extracted from the seeds using the standard method of cooking a batch for 30 minutes at 80 °C and, subsequently, two presses were used for oil extraction, producing the castor bean pie at a pilot scale in the Biodiesel Plan, municipality of Pesqueira, Pernambuco, Brazil.
The castor bean pie was subjected to additional processing at industrial scale for the production of different castor bean meal types, called A, B, C and D. Castor oil meal A (CMA) was subjected to recovery in ethanol at 80 °C for 20 minutes and dried at 80 °C; castor oil meal (CMB) was submitted to the same ethanol recovery and temperature as CMA, but for six minutes, and then to neutralization with 5% sodium hydroxide (NaOH), and drying under direct exposure to sunlight for two days; castor oil meal C was submitted to the same processing procedures as CMB, except for drying, which was replaced by pelleting; castor oil meal D (CMD) was recovered in alcohol at 110 °C for 15 minutes and dried at 110 °C.
The chemical composition of the evaluated meals was analyzed at the Laboratory of Animal Nutrition (LANA), UFRPE. Analyses of dry matter (DM), CP, ash, ether extract (EE) and crude fiber (CF) were carried out according to methods described by Silva & Queiroz (2006Silva DJ, Queiroz A C. Food analysis: chemical and biological. Viçosa: Universidade Federal de Viçosa; 2006.), and neutral detergent insoluble fiber was determined according to the method of Van Soest et al. (1991Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 1991;74(10):3583-3597.). Gross energy (GE) analysis was performed at the Laboratory of Physical and Chemical Analysis of Brazilian Company of Agricultural Research Swine and Poultry (EMBRAPA).
Total amino acids profiles of processed castor oil meals were determined by HPLC (High Performance Liquid Chromatography) at the Laboratory of Applied Technology of EVONIK Animal Nutrition, in Hanau, Germany. The cytotoxicity of processed castor oil meal corresponds to the percentage of specific standardized cells (Vero cells) that are destroyed using a standard quantitative cell counting and measurement of lactate dehydrogenase activity. The limit of detection of the Vero cell assay was 10 ng/mL using a concentration of 1.6 x 10(5) cells/well. The procedure was according to Fernandes et al. (2012) and analysis was performed at the Laboratory of Chemistry and Function of Proteins and Peptides of Bioscience and Biotechnology Center of Universidade Estadual do Norte Fluminense Darcy Ribeiro. Values of cytotoxicity are expressed as percentage comparing with the castor bean cake with no further processing management considered as reference relative 100% cytotoxicity.
The digestibility assay of castor oil meal was performed in the Laboratory of Non-Ruminant Digestibility, UFRPE. One hundred and eighty46-d-old Japanese quails were acquired from Fujikura farm. Birds were distributed in a completely randomized design (CRD) with five treatments and six replicates of six birds each, and housed in two cages. Each cage was divided in five overlapping floors with three divisions each(33 cm x 25 cm x 20 cm), totaling 15 divisions per cage. The cages were equipped with feeders and trough drinkers. Trays lined with plastic canvas were fit under the cages for excreta collection. Birds were distributed according to a completely randomized experimental design (CRD) with five treatments and six replicates of six birds each. The animal use protocol was approved by the institutional animal care guidelines the Federal Rural University of Pernambuco.
The reference diet (Table 1) was formulated using the nutritional values of feedstuffs proposed by Rostagno et al. (2005Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes, DC, et al . Brazilian tables for poultry and pigs: food composition and nutritional requirements. Viçosa: UFV/DZO; 2005.) and the nutritional requirements obtained by Silva & Costa (2009Silva JHV, Costa FGP. Table for Japanese quail and European. Jaboticabal: FUNEP; 2009.).
2 Composition per kg of product: Fe, 20000mg; Co, 200mg, 4000mg Cu, Mn, 75000mg, Zn, 50000mg; If, 250mg; I, 1500mg, Antioxidant, 100000mg; Folic Acid, 200mg, Pantothenic acid, 5350 mg, Niacin, 19,900 mg; - Vit. A, 8000000 IU, Vit. D3, 2 million IU, vit k3, 2000 mg, Vit. B2, 4000 mg, Vit. B6, 1000 mg, B12, 10000 mcg, Vit E, 15000mg.
The treatments included REF - reference feed based on corn and soybean meal, RCMA - 80% of the REF diet + 20% of CMA, RCMB - 80% of the REF diet + 20% of CMB, RCMC - 80% of the REF diet + 20% of CMC and RCMD - 80% of the REF diet + 20% CMD. Feed and water were supplied ad libitum throughout the experimental period, which lasted ten days, with five days of adaptation to the cages and the experimental diet and five days of total excreta collection. Feed intake and excreta output volumes were recorded. In the experiment, on the first and last days of total excreta collection, the external marker ferric oxide (Fe2O3) was added to the diets at2%. Excreta were collected once daily (12:00 hours), placed in duly identified plastic bags, and stored in a freezer at -20 °C until the end of the collection period.
The samples were thawed, homogenized per experimental unit, weighed, and submitted to the laboratory for analyses. In the lab, samples were pre-dried in a ventilated oven at 65 °C for 72 hours. Dried samples were then weighed, ground to 1-mm particle size, and submitted to the Laboratory of Animal Nutrition (LANA), UFRPE, for DM and nitrogen (N) analyses by the method described by Silva & Queiroz (2006Silva DJ, Queiroz A C. Food analysis: chemical and biological. Viçosa: Universidade Federal de Viçosa; 2006.). The GE analysis was performed at the Laboratory of Physical and Chemical Analysis of Embrapa Swine and Poultry.
The obtained were submitted to analysis of variance and means were compared by the Tukey's test at 5% probability level, using the SAS statistical program (2000).
RESULTS
The processes applied to detoxify castor oil meal resulted in variations in the nutritional composition values assessed (Table 2).
Maximum absolute values differences of 1.6% DM, 1.21% CP, 2.2% EE, 3.9% CF 3%, 0.83% ash, 0.05% calcium, and 0.17% phosphorus were obtained among processed castor oil meal samples.
A GE difference of 64 kcal/kg was determined between CMB and CMA. The lowest GE level, which was obtained in CMB, is compatible with the reduction in EE absolute value (7.21%) in this meal.
The different processed castor oil meal types (Table 3) presented different total amino acid composition, as a function of processing method. The processing method used for CMA resulted in reduced levels of all essential amino acids.
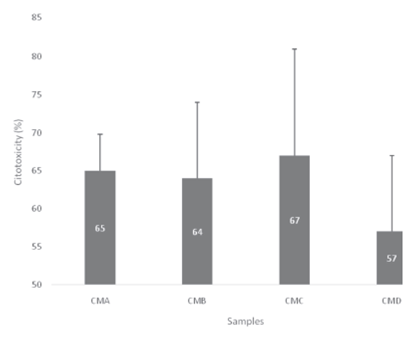
Figure 1 shows the cytotoxicity of processed castor oil meals. The results indicate that the treatment CMD presented the lowest ricin toxic activity. Numbers on the bars represent the relative cytotoxicity when comparing to the castor bean cake with no further processing management (considered the reference relative 100% cytotoxicity).
The digestibility assay results (Table 4) show that the birds fed the diet containing castor oil meal B (CMBF) presented low feed intake, which led to amino acid imbalance, resulting in insufficient N and energy supply to meet the birds requirement. As a consequence, low metabolizability coefficients of DM, N, and GE, and reduced dietary AME and AMEn were determined.
Average values of feed intake (FI), and of metabolizability coefficients of dry matter (MCDM), nitrogen (MCN) and gross energy (MCGE), nitrogen balance (NB), apparent metabolizable energy (AME) and apparent metabolizable energy corrected for nitrogen retention (AMEn) of Japanese quails fed the experimental diets.
The metabolizability coefficients of the different castor oil meals evaluated are shown in Table 5. Castor oil meals C and D promoted greater MCGE and N balance than CMA and CMB. Relative to energy use (AME and AMEn), CMA was not significantly different from CMC and CMD.
Average values of feed intake (FI), and of metabolizability coefficients of dry matter (MCDM), nitrogen (MCN) and gross energy (MCGE), nitrogen balance (NB), apparent metabolizable energy (AME) and apparent metabolizable energy corrected for nitrogen retention (AMEn) castor oil meals submitted to different processing methods
DISCUSSION
Faria Filho et al. (2010Faria Filho DE, Dias AN, Bueno CFD, Matos Junior, JB, Couto, FAP. By-products of castor oil in the diet of birds. Produção Animal - Avicultura 2010;(33):24-16.), evaluating broiler diets with castor oil meal detoxified with 60 g of micro-processed calcium oxide diluted in water at 1:10, obtained CP (33.1%), EE (1.9%), and CF (24.8%) values, which are lower than those found in the present study in treatments A, B, C and D. This may be due to differences in the efficiency of the mechanical press during processing, and in the methods used for castor oil meal analyses.
The castor oil meal composition (89.40% DM, 39.20% CP, 1.55% EE, 18.50%, on natural matter basis) in the Brazilian Tables for Poultry and Swine (Rostagno et al., 2011) presents similar DM and as hand lower in CF and EE values than those found in the present study. However, as previously mentioned, this may result from differences in the methods of oil extraction. Gomes et al. (2009Gomes FHT, Cândido MJD, Pereira ES, Lopes JWB, Feitosa JV, Pompeu RCFF. Chemical composition and in situ degradation of chain byproducts of biodiesel. Revista Científica de Produção Animal 2009;11(2):144-156.) analyzed castor oil meal submitted to the detoxification process proposed by Anandan et al. (2005Anandan S, Anil Kumar GK, Ghosh J, Ramachandra KS. Effect of different physical and chemical treatments on detoxification of ricin in castor cake. Animal Feed Science and Technology, v.120, p.159-168, 2005.), obtaining 89.8% DM, 56.8%CP, 3.67% EE, 12.80% ash, 47.32% NDF levels on natural matter basis, which are different from the values observed in the current study.
Variations in temperature, pressure, and chemical agents used for the extraction of castor bean oil at industrial scale account for differences in castor oil meal protein levels and quality (Silva et al., 2010Silva DC, Alves AA, Vasconcelos VR, Nascimento HTS do, Filho MM, Oliveira ME de. Nitrogen metabolism in sheep fed diets containing detoxified castor bean meal. Acta Scientiarum. Animal Sciences 2010;32(2):219-224.), directly influencing the efficiency of nutrient utilization by poultry. In the present study, essential amino acid contents differed as a function of processing method. In particular, CMA presented lower concentrations of essential amino acids compared to the other evaluated meals. This effect was probably due to long time (20 minutes) of recovery in ethanol, which may have caused protein denaturation, thereby affecting amino acid availability. The best total amino acids results were obtained in CMB and CMC, both of which were submitted to rapid recovery in ethanol at 80°C for 6 minutes, neutralization, and differed only as to drying method, with CMB dried by sun exposure and CMC by pelleting. The average essential amino acid contents obtained were lower than those reported by Rostagno et al.(2011)Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes DC, et al .. Brazilian tables for poultry and pigs: food composition and nutritional requirements Viçosa: UFV/DZO; 2011. and Benesi (1979)Benesi F J. Influência do farelo de mamona (Ricinus comunis L.) destoxicado sobre o proteinograma sangüíneo e desempenho de suínos [dissertação]. Belo Horizonte: Universidade Federal de Minas Gerais; 1979. , probably due to the detoxification methods of castor bean meal. When compared with the commercial castor oil meal evaluated by Vilhjalmsdottir & Fisher (1971Vilhjalmsdottir L, Fisher H. Castor bean meal as a protein source for chickens: detoxification and determination of limiting amino acids. Journal of Nutrition 1971;101(9):1185-1192.), which was submitted to crushing for oil extraction, followed by4-5 washings with solvent. The resulting meal was boiled for four hours, dried and ground for detoxification process, the values were higher than those found in the present study.
Diets with amino acid imbalance may induce poultry to increase their feed intake to meet their physiological needs. On the other hand, birds may reduce their feed intake in an attempt to protect themselves from possible pathological lesions caused by amino acid excess in the body. Japanese quails fed the diets containing CMC and CMD were possibly able to reduce the effect of unbalanced diets, as shown by their better N and energy utilization compared with those fed the CMB diet.
The lowest GE levels obtained in CMB is compatible with its lowest absolute EE value (7.21%) relative to the other meals. This reduction EE content was possibly due to the drying method applied (two days of sun exposure), allowing the longest exposure time to the catalyst, which in turn promoted a continuation of the saponification reaction or the transesterification of residual ethanol with the catalyst. These reactions occur simultaneously.
The metabolizability coefficient of gross energy is influenced by several factors, including the quality of the feedstuff, because nutrients such as carbohydrates, proteins, and lipids are not used efficiently by poultry, when the efficiency of energy is reduced as a function of diet(Sakomura & Rostagno, 2007Sakomura NK, Rostagno HS. Research methods in nutrition of monogastric.São Paulo: Funep; 2007.). Other factors may influence energy value of feedstuffs, such as feedstuff processing, bird age and species, and experimental procedures (Penz Jr. et al., 1999Penz Jr. AM, Kessler AM, Brugalli I. New concepts of energy for birds. Anais do Simpósio Internacional sobre Nutrição de Aves; 1999; Campinas, São Paulo. Brasil: Facta, 1999. cap. 1, p.1-24.). The efficiency of energy utilization by the Japanese quails in the present study was reduced by the high CF content of 38.73% (average of all treatments). The castor oil meal C and D promoted greater MCGE and nitrogen balance than CMA and CMB. Relative to energy utilization (AME and AMEn), CMA was not significantly difference from CMC or CMD.
CMB presented the lowest metabolizability coefficients of DM, N, GE, and the lowest AME and AMEn values, as well as the lowest feed intake when added to the reference diet. These results may also be attributed to processing method (ethanol, recovery at 80 °C for 6 min, neutralization with NaOH, and drying by exposure to the sun for two days), which caused long exposure of the meal to NaOH, possibly resulting in undesirable reactions, and therefore compromising the availability of nutrients for poultry. It is should be noted that CMB also presented the lowest EE value (7.21%), and consequently lower GE value (4684 kcal/kg).
Faria Filho et al. (2010Faria Filho DE, Dias AN, Bueno CFD, Matos Junior, JB, Couto, FAP. By-products of castor oil in the diet of birds. Produção Animal - Avicultura 2010;(33):24-16.), evaluating broiler diets containing detoxified castor oil meal with 60g of micro-processed calcium oxide diluted in water at the ratio of 1:10, observed an AME value of 1,829 kcal/kg), which was lower than those obtained with treatments CMB and CMC and similar to CMD. The AME (2032 kcal/kg), AMEn (1829 kcal/kg), and MCDM (57.8%) values obtained by Junior et al. (2011Júnior JBM, Dias NA, Bueno CFD, Rodrigues PA, Veloso ALC, Faria Filho DE. Metabolizable energy and nutrient digestibility of detoxified castor meal and castor cake for Poultry.. Revista Brasileira de Zootecnia 2011;40 (11); 2439-2442.) with broilers fed detoxified castor bean meal compared with those obtained with Japanese quails in the present experiment demonstrate that there are species differences.
CONCLUSION
The castor oil meal submitted to treatments A, C, and D showed satisfactory results for Japanese quails. However, considering the simplicity its industrial process, its low toxicity, and metabolizability coefficients obtained, the castor oil meal obtained by recovery in ethanol at 110 °C for 15 minutes (CMD) is the most recommended for inclusion in Japanese quail diets.
ACKNOWLEDGEMENTS
The authors thank FACEPE (Fundo de Amparo a Pesquisa do Estado de Pernambuco) for providing the MSc scholarship, Usina de Biodiesel de Pesqueira, Pernambuco, Brazil, for donating the detoxified castor bean meal, Fujikura farm for donating Japanese quails, and Evonik Degussa Brazil for analyzing the amino acid contents of the evaluated castor bean meals, the Laboratory of Chemistry and Function of Proteins and Peptides of Bioscience and Biotechnology Center of Universidade Estadual do Norte Fluminense Darcy Ribeiro for cytotoxicity analyse.
REFERENCES
- Abdalla AL, Silva Filho JCD, Godoi ARD, Carmo CDA, Eduardo JLDP. Use of byproducts of the biodiesel industry in ruminant feed. Revista Brasileira de Zootecnia 2008;37(3):260-268.
- Albuquerque CS, Rabello CBV, Santos MJB, Lima MB, Silva EP, Lima TS, et al . Chemical composition and metabolizable energy values of corn germ meal obtained by wet milling for layers. Revista Brasileira de Ciência Avícola 2014;16(1):107-112.
- Anandan S, Anil Kumar GK, Ghosh J, Ramachandra KS. Effect of different physical and chemical treatments on detoxification of ricin in castor cake. Animal Feed Science and Technology, v.120, p.159-168, 2005.
- Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA 2005;294(18):2342-2351.
- Azevedo DMP, Lima EF. O Agronegócio da mamona no Brasil. Campina Grande: Embrapa Algodão; Brasília, DF: Embrapa Informação Tecnológica; 2007.
- Benesi F J. Influência do farelo de mamona (Ricinus comunis L.) destoxicado sobre o proteinograma sangüíneo e desempenho de suínos [dissertação]. Belo Horizonte: Universidade Federal de Minas Gerais; 1979.
- Faria Filho DE, Dias AN, Bueno CFD, Matos Junior, JB, Couto, FAP. By-products of castor oil in the diet of birds. Produção Animal - Avicultura 2010;(33):24-16.
- Gomes FHT, Cândido MJD, Pereira ES, Lopes JWB, Feitosa JV, Pompeu RCFF. Chemical composition and in situ degradation of chain byproducts of biodiesel. Revista Científica de Produção Animal 2009;11(2):144-156.
- Júnior JBM, Dias NA, Bueno CFD, Rodrigues PA, Veloso ALC, Faria Filho DE. Metabolizable energy and nutrient digestibility of detoxified castor meal and castor cake for Poultry.. Revista Brasileira de Zootecnia 2011;40 (11); 2439-2442.
- Oliveira A S, Oliveira M RS, Campos J MS. Effectiveness of different methods of detoxification of ricin in castor oil meal. Anais do Congresso da Rede Brasileira de Tecnologia e Produção de Biodiesel; 2006 ago 31 a set 1º; Brasília, DF. Brasil. p.1-4
- Penz Jr. AM, Kessler AM, Brugalli I. New concepts of energy for birds. Anais do Simpósio Internacional sobre Nutrição de Aves; 1999; Campinas, São Paulo. Brasil: Facta, 1999. cap. 1, p.1-24.
- Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes, DC, et al . Brazilian tables for poultry and pigs: food composition and nutritional requirements. Viçosa: UFV/DZO; 2005.
- Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes DC, et al .. Brazilian tables for poultry and pigs: food composition and nutritional requirements Viçosa: UFV/DZO; 2011.
- Sakomura NK, Rostagno HS. Research methods in nutrition of monogastric.São Paulo: Funep; 2007.
- Santos MJB, Ludke MCMM, Ludke JV, Torres TR, Lopes LS, Brito MS. Chemical composition and metabolizable energy values of alternative ingredients for broilers. Ciência Animal Brasileira 2013;14(1):32-40.
- Severino LS. What we know about the castor bean pie.; Campina Grande: Embrapa Algodão 2005.
- Silva DC, Alves AA, Vasconcelos VR, Nascimento HTS do, Filho MM, Oliveira ME de. Nitrogen metabolism in sheep fed diets containing detoxified castor bean meal. Acta Scientiarum. Animal Sciences 2010;32(2):219-224.
- Silva DJ, Queiroz A C. Food analysis: chemical and biological. Viçosa: Universidade Federal de Viçosa; 2006.
- Silva JHV, Costa FGP. Table for Japanese quail and European. Jaboticabal: FUNEP; 2009.
- Ton APS, Furlan AC, Martins EN, Toledo JB, Scherer C, Conti ACM. Digestible lysine and metabolizable energy requirements of growing meat quails. Revista Brasileira de Zootecnia2011;40(3):593-601.
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 1991;74(10):3583-3597.
- Vilhjalmsdottir L, Fisher H. Castor bean meal as a protein source for chickens: detoxification and determination of limiting amino acids. Journal of Nutrition 1971;101(9):1185-1192.
Publication Dates
-
Publication in this collection
Oct-Dec 2015
History
-
Received
Nov 2014 -
Accepted
June 2015