ABSTRACT
The aim of this study was to evaluate the in vitro bioactivity of tea tree (Melaleuca alternifolia) essential oil against larvae and adult forms of lesser mealworms (Alphitobius diaperinus) and its influence on the soil fauna. Tests were performed in triplicate using pure tea tree oil (TTO; 1, 5, 10, 25, 50, and 100%), TTO nanoparticles (1, 3, and 7.5%), or terpinen-4-ol, the main compound of the tea tree oil, at the same concentrations of TTO. Larvae and adult mortality occurred at concentrations up to 10 and 50% of TTO, respectively. No larvicidal or insecticidal effect of TTO nanoparticles was observed. Terpinen-4-ol showed insecticidal and larvicidal effect at concentrations higher than 25%. The evaluation of TTO effect on soil organisms was performed by standard ecotoxicological tests (ISO) with the springtail species Folsomia candida. Only TTO was used for ecotoxicological tests in doses of 1, 5, 10, 25, 50, and 100 mg kg-1 of soil. TTO had no negative effects on F. candida survival or reproduction. Therefore, it was concluded that M. alternifolia oil may be a new alternative for control of the lesser mealworm.
Keywords:
Alphitobius diaperinus; essential oil; natural products; nanotechnology; pest management
INTRODUCTION
The lesser mealworm Alphitobius diaperinus , Panzer, is one of the most common pests of poultry industry. Poultry housing provide optimal temperature and humidity conditions for its development, and the lesser mealworm feeds on poultry feed residues, fecal material, and dead birds (Gazoni et al., 2012Gazoni FL, Flores F, Bampi RA, Silveira F, Boufleur R, Lovato M. Avaliação da resistência do cascudinho (Alphitobius diaperinus) Panzer (Coleoptera: Tenebrionidae) a diferentes temperaturas. Arquivos do Instituto Biologico 2012; 79(1):69-74.). A. diaperinus is a potential carrier of several pathogens (viruses, fungi, bacteria, and protozoa) and it can be found everywhere inside poultry houses, hidden in cracks, beneath feeders and other areas (Silva et al., 2005Da Silva AS da, Hoff G, Doyle RL, Santurio JM, Monteiro SG. Ciclo biológico do cascudinho Alphitobius diaperinus em laboratório. Acta Scientiae Veterinariae 2005; 33(2):177-181.). Pest management practices are routinely adopted, and the lesser mealworm control has been based on chemical insecticides applied on wood shavings used as litter. However, some flaws in these sanitary measures leads to litter re-infestation by A. diaperinus (Santos et al., 2009Santos JC, Alves LFA, Opazo MAU, Mertz NR, Marcomini AM, Oliveira DGP, Bonini AK. Eficiência da aplicação de inseticida químico no solo para o controle de Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae) em aviário de frango de corte. Arquivos do Instituto Biologico 2009; 76(3):417-425.). It is known that these chemicals can also cause environmental contamination, affecting soil fauna survival and reproduction (Santos et al. , 2012Santos MJG, Ferreira FML, Cachada A, Duarte AC, Sousa JP. Pesticide application to agricultural fields: effects on the reproduction and avoidance behaviour of Folsomia candida and Eisenia andrei. Ecotoxicology 2012; 21(8):2113-2122.). Natural products may become alternative treatments, and therefore ecotoxicology tests are needed.
The use of natural products can be an alternative to control the lesser mealworm, as shown by Prado et al. (2013Prado GP, Stefani LM, Silva AS da, Smaniotto LF, Garcia FRM, De Moura NF. Alphitobius diaperinus (Coleoptera: Tenebrionidae) susceptibility to Cunila angustifolia essential oil. Journal of Medical Entomology 2013; 50(5):1040-1045.) using Cunila angustifolia. Plants from the Meliaceae family, particularly Melaleuca alternifolia, can be used for controlling parasites (Mulla & Su 1999Mulla MS, Su T. Activity and biological effects of neem products against arthropods of medical and veterinary importance. Journal American Mosquitoes Control Association 1999; 15(2):133-152., Neves et al., 2012Neves RCSM, Ferraz RHS, Mendonça AJ, Lima SR, Cruz FAS, Rosa JG, Mateus LAF, Ferraz V, Barros LA. Acaricide effect of the Melaleuca alternifolia essential oil on Otodectescynotis. Revista Brasileira de Ciências Veterinária 2012; 19(3):144-148.). The M. alternifolia oil is extracted from leaves and it is rich in terpinen-4-ol, the most likely chemical compound responsible for antifungal and antibacterial activities (Carson & Riley, 1993Carson CF, Riley TV. Antimicrobial activity of the essential oil of Melaleuca alternifolia. Letter Applied Microbiology 1993; 16(1):49-55.), as well as trypanocidal (Baldissera et al., 2014Baldissera MD, Da Silva AS, Oliveira CB, Santos RCV, Vaucher RA, Raffin RP, Gomes P, Dambros MGC, Miletti LC, Boligon AA, Athayde ML, Monteiro SG. Trypanocidal action of tea tree oil (Melaleuca alternifolia) against Trypanosoma evansi in vitro and in vivo used mice as experimental model. Experimental Parasitology 2014; 141(1):21-27.), insecticidal (Klauck et al., 2014Klauck V, Pazinato R, Stefani LM, Santos RC, Vaucher RA, Baldissera MD, Raffin R, Boligon A, Athayde M, Baretta D, Machado G, Da Silva AS. Insecticidal and repellent effects of tea tree and andiroba oils on flies associated with livestock. Medical and Veterinary Entomology 2014; 28(1):33-39.) and acaricidal (Walton et al., 2004Walton SF, McKinnon M, Pizzutto S, Dougall A, Williams E, Currie BJ. Acaricidal activity of Melaleuca alternifolia (tea tree) oil: in vitro sensitivity of Sarcoptes scabiei var hominis to terpinen-4-ol. Archives Dermatology 2004; 140(5):563-566.) effects. On the other hand, the oil has problems due to its high volatility and insolubility in water. In order to manage these problems, increasing its efficacy, nanoparticles have been used quite often (Pazinato et al., 2014Pazinato R, Klauck V, Volpato A, Santos RC, De Souza ME, Vaucher RA, Raffin R, Gomes P, Felippi CC, Stefani LM, Da Silva AS. In?uence of tea tree oil (Melaleuca alternifolia) on the cattle tick Rhipicephalus microplus. Experimental Applied Acarology 2014; 63(1):77-88.). This technology consists of the manipulation of the molecule to a nanometric size, organizing oil atoms into a desirable form, which has several advantages over conventional products, such as slow, gradual and controlled release, increased bioavailability and lower toxicity (Roco, 2001Roco MC. International strategy for nanotechnology research and development. Journal of Nanoparticle Research 2001; 3(5):353-360.).
This study was performed to investigate the bioactivity of pure M. alternifolia oil (TTO), nanoparticles form (TTO nanoparticles) of this same oil, and its major compound (terpinen-4-ol) on larvae and adult forms of A. diaperinus . TTO effects on soil fauna were also assessed by means of ecotoxicity tests.
MATERIALS AND METHODS
Oil forms and compounds
Tea tree oil (TTO), TTO nanoparticles and terpinen-4-ol were evaluated for their larvicidal and insecticidal activites. TTO was purchased from Importadora Química Delaware Ltda, Brazil. Terpinen-4-ol was purchased from Sigma Company. TTO nanoparticles were provided with the company Inventiva (Porto Alegre, Brazil), and the process of nanoparticle preparation was previously described by Pazinato et al. (2014Pazinato R, Klauck V, Volpato A, Santos RC, De Souza ME, Vaucher RA, Raffin R, Gomes P, Felippi CC, Stefani LM, Da Silva AS. In?uence of tea tree oil (Melaleuca alternifolia) on the cattle tick Rhipicephalus microplus. Experimental Applied Acarology 2014; 63(1):77-88.). Solid lipid carriers were prepared with 7.5% of M. alternifolia essential oil using a patented process (Inventiva) based on high-pressure homogenization. Cetyl palmitate was used as the solid lipid and polysorbate 80 as surfactant. The total solid concentration was 18.6%. The particle size and zeta potential were evaluated on diluted samples (500x) using Zetasizer Nanoseries, Malvern. The pH was directly measured by use of a potentiomenter (Digimed, Brazil).
TTO characterization
Gas chromatography (GC) analyses were carried out using an Agilent Technologies 6890N GC-FID system, equipped with DB-5 capillary column (30mm x 0.25mm; film thickness of 0.25mm) connected to a flame ionization detector (FID). The injector and detector temperatures were set at 280°C. Helium was used as the carrier gas, at a flow rate of 1.3 mL/min. The thermal programmer was 50-300°C at a rate of 5°C/min. Two replicates of oil samples were processed in the same way. Relative component concentration was calculated based on GC peak areas without correction factors. The injection volume of the M. alternifolia (tea tree) essential oil was 1µL (Boligon et al., 2013Boligon AA, Schwanz TG, Piana M, Bandeira RV, Frohlich JK, Brum TF, Zadra M, Athayde ML. Chemical composition and antioxidant activity of the essential oil of Tabernaemontana catharinensis A. DC. leaves. Natural Product Research 2013; 27(1):68-71.).
Gas chromatography mass (GC-MS) analyses were performed on an Agilent Technologies Auto System XL GC-MS system operating in the EI mode at 70 eV, equipped with a split/split less injector (250°C). The transfer line temperature was 280°C. Helium was used as carrier gas (1.5mL/min) and the capillary columns used were a HP 5MS (30m x 0.25mm; film thickness of 0.25mm) and a HP Innowax (30m x 0.32mm i.d., film thickness of 0.5mm). The temperature program and the injected volume of oil were the same as used for GC analyses. The injected volume of the essentials oils was 1 µL. Component identification of both oils was based on the retention index (RI), using as reference of the homologous series of n-alkanes, C7-C30, under identical experimental conditions, and comparing with the mass spectra library search (NIST and Wiley), and with the mass spectra literature data (Adams, 1995Adams RP. Identification of essential oil components by Gas Chromatography/Mass spectroscopy. Illinois; Allured Publishing Corporation; 1995.). The relative amounts of the individual components were calculated based on the CG peak area (FID response).
Lesser mealworms
Lesser mealworms larvae (L8) and adults were obtained from naturally infested poultry farms located in the city of Concordia, State of Santa Catarina, Brazil. On the farms, insects were manually collected, placed in plastic bottles, and transported to the laboratory, where they were reared under controlled conditions (25ºC; 70% R.H.).The insects were kept in plastic pots with poultry litter and wet cottons, and were fed with poultry feed.
Bioassays
In-vitro tests were conducted using test solutions prepared with pure TTO, nanoparticles, and terpinen-4-ol. Pure TTO was first dissolved in Triton solution (1:1) and then diluted in water to the concentrations of 1, 5, 10, 25, 50, and 100%. TTO nanoparticles were dissolved in water to the concentrations of 1, 3, and 7.5%. Terpinen-4-ol was dissolved only in water to the same concentrations as TTO. The solutions (300 µL) were added to filter paper-lined Petri dishes according to a completely randomized design and three replicates per treatment at 25 °C (±3) and 70% (±10) relative humidity, and submitted to a photoperiod of 12:12 (L:D). Three controls were used, and included water-treated mealworms, Triton-treated mealworms and nanoparticle-treated mealworms (only the capsule of cetyl palmitate and polysorbate 80).
Larvae and adults
Ten 8th instar larvae or adults were placed in a Petri dish. Solutions were then added and the dishes were transferred to an incubator with controlled environment (25ºC; 70% R.H.). The percentage of live larvae was monitored daily for 13 days. The percentage of live lesser mealworm adults on each disk was monitored daily for 24 or 31 days.
Ecotoxicity tests
The evaluation of the effect of TTO on soil organisms was performed by standard ecotoxicological tests (ISO 1999, ISO 2010) with the springtail species Folsomia candida. The springtails were obtained from cultures of the Laboratory of Soil and Sustainability at the University of the State of Santa Catarina (UDESC) and kept under controlled conditions at 20±2 °C, and a photoperiod of 16:8 (L:D).
This study was conducted using the tropical artificial soil (TAS) method, with humidity set at 65% of retention capacity and pH adjusted to 6.0±0.5. The treatments consisted of TAS enriched with TTO in the laboratory. Similarly to the in-vitro tests, the TTO was previously mixed with Triton solution (1:1), and the final doses were obtained by diluting this mixture with distilled water.
For survival tests, the soil was exposed to six concentrations of TTO (1, 5, 10, 25, 50 and 100 mg kg-1) using four replicates per concentration. Each plastic recipient contained 50g of soil, to which 10 springtails were added, along with 2mg of dried granular baker's yeast. The containers were opened to allow air contact for three seconds three times weekly, and 2mg of dry yeast granules were added again after 14 days of incubation. At the end of 28 days of testing, the contents of each test vessel was poured into a beaker of water. A few drops of blue ink were gently added and homogenized. The floating springtails were counted and missing adults were considered dead.
TTO concentrations used in the reproduction test were the same as those applied in the survival test. This test followed the procedures described by ISO (1999), and the methodology was based on the survival test, i.e., larvae that floated in the water were photographed for better counting.
Statistical analysis
The normality test was applied for the results of the bioassays with the lesser mealworm, and data showed normal distribution. Data were then submitted to analysis of variance (ANOVA) and Duncan test (p<0.05). The data on springtail survival and reproduction were subjected to ANOVA followed by Dunnett test (p<0.05). Untreated lesser mealworms were used as control group.
RESULTS
Components present in the essential oil of tea tree
The results of the components found in the TTO oil are shown in Table 1. The three main compounds were identified as terpinen-4-ol, g-terpinene, and a-terpineol.
TTO nanoparticles
The TTO nanoparticles were evaluated for their physical and chemical properties. The particle size was 287 (±2nm) and polydispersity index of 0.2 (±0.022) with a zeta potential of-14,2 ± 1,7 mV.
In-vitro tests
The three controls were used this study (water-treated mealworms, triton-treated mealworms and nanocapsule-treated mealworms) validated in vitro tests, i.e., water, triton and nanocapsule are not harmful to lesser mealworm larvae or adults. The controls are described below each Figure.
TTO oil
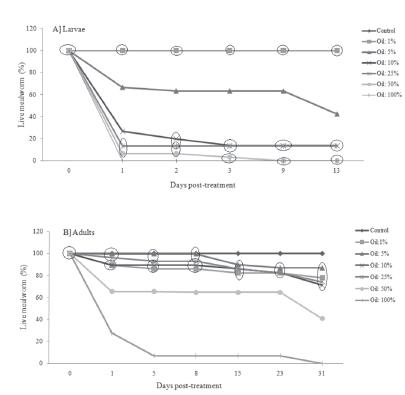
The results of the percentage of live adults and larvae are presented in Figure 1. The tests lasted 13 days for larvae, and 31 days for adults. The larvae were not affected by TTO at 1%, similarly to what was observed in the control group (Fig. 1a). A significant percentage of larval mortality (over 60%) was observed with 10% of TTO. TTO was efficient to eliminate all larvae at 25, 50, and 100%, demonstrating great larvicidal potential. The mortality rate was lower in adults when compared with the larvae, i.e., 40% adult mortality was observed with TTO at 50% (Fig. 1b). Adult mortality was not significantly different among lower TTO concentrations (1, 5, 10, and 25%); however, TTO concentrations of 50 and 100% demonstrated high insecticidal effect. In adults, observations were carried out until 50 days, but no changes were observed after 31 days; therefore, only data until 31 days were included in the analyses.
Percentage of live larvae (A) and adults (B) of Alphitobius diaperinus after treatment with Melaleuca alternifolia essential oil (1, 5, 10, 25, 50, and 100%). Means within a circle are similar by the Duncan's test (p>0.05). "Control" used to triton-treated lesser mealworms.
TTO nanoparticles
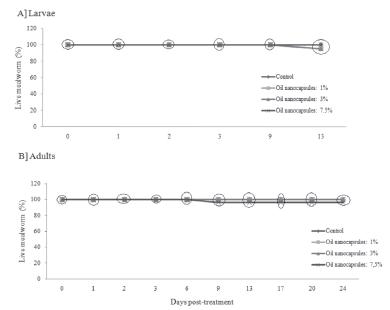
TTO nanoparticles had no effect on A. diaperinus larvae and adult when compared with the control group, independent of the concentration (Figure 2). The experimental periods were 13 days for the larvae and 24 days for the adults. Thus, TTO nanoparticles had neither larvicidal nor insecticidal effect at these concentrations.
Percentage of live larvae (A) and adults (B) of Alphitobius diaperinus after treatment with Melaleuca alternifolia nanoparticles (1, 3, and 7.5%). Means within a circle are similar by the Duncan's test (p>0.05). "Control" used refers to nanoparticle-treated lesser mealworms (only the capsule of cetyl palmitate and polysorbate 80).
Terpinen-4-ol
The results obtained with terpinen-4-ol were similar to those verified with pure TTO (Figure 3). Terpinen-4-ol at 1, 5, and 10% promoted similar results as those of the control group (Fig. 3a), whereas concentrations of 25, 50 and 100% had both larvicidal and insecticidal effects (Fig. 3b).
Percentage of live larvae (A) and adults (B) of Alphitobius diaperinus after treatment with terpinen-4-ol (1, 5, 10, 25, 50, and 100%). Means within a circle are similar by the Duncan's test (p>0.05). "Control" refers to water-treated lesser mealworms.
Ecotoxicity tests
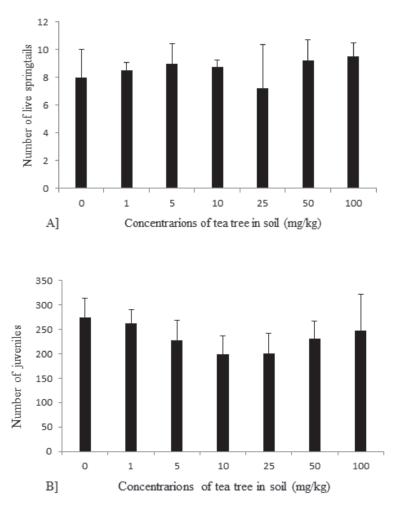
The results of springtail survival and reproduction tests are reported in Figure 4. TTO had no lethal effect on the adult springtails submitted to the survival test, which indicates it is not toxic to the soil fauna. The reproduction test showed no statistical differences among the treatments. TTO doses of 10 and 25 mg kg-1 resulted in the lowest number of juveniles, despite the lack of statistical differences (p>0.05).
Survival (A) and reproduction (B) tests with Folsomia candida exposed to tropical artificial soil (TAS) with different concentrations of Melaleuca alternifolia essential oil (TTO - tea tree oil). Treatments were similar by the Dunnet's test (p>0.05).
DISCUSSION
The lesser mealworm control in poultry houses is typically controlled by an intense use of chemicals; however, these insects eventually become resistant to these products (Chernaki-Leffer et al., 2011Chernaki-Leffer AM, Sosa-Goméz DR, Almeida LM, Lopes ION. Susceptibility of Alphitobius diaperinus (Panzer) (Coleoptera, Tenebrionidae) tocypermethrin, dichlorvos and triflumuron in southern Brazil. Revista Brasileira de Entomologia 2011; 55(1):125-128.). This has motivated the search for efficient alternative control methods and that also have lower environmental impact. These requirements can be met using natural products, including essential oils, which have attracted the interest of many researchers (Prado et al., 2013Prado GP, Stefani LM, Silva AS da, Smaniotto LF, Garcia FRM, De Moura NF. Alphitobius diaperinus (Coleoptera: Tenebrionidae) susceptibility to Cunila angustifolia essential oil. Journal of Medical Entomology 2013; 50(5):1040-1045.; Pazinato et al., 2014Pazinato R, Klauck V, Volpato A, Santos RC, De Souza ME, Vaucher RA, Raffin R, Gomes P, Felippi CC, Stefani LM, Da Silva AS. In?uence of tea tree oil (Melaleuca alternifolia) on the cattle tick Rhipicephalus microplus. Experimental Applied Acarology 2014; 63(1):77-88.; Klauck et al., 2014Klauck V, Pazinato R, Stefani LM, Santos RC, Vaucher RA, Baldissera MD, Raffin R, Boligon A, Athayde M, Baretta D, Machado G, Da Silva AS. Insecticidal and repellent effects of tea tree and andiroba oils on flies associated with livestock. Medical and Veterinary Entomology 2014; 28(1):33-39.).
In the present study, we showed for the first time the efficacy of M. alternifolia oil against both larval and adult forms of the lesser mealworm, with promising results. TTO showed potent larvicidal effect at concentrations greater than 10%, as well as against adults at concentrations greater than 25%. It is likely that the mortality of the lesser mealworm with the use of pure oil was related to the presence of its main compound, known as terpinen-4-ol, which promoted results similar to TTO when tested alone. However, we cannot exclude the application of other compounds, which may act in synergism for the control of this pest. Other oil compounds, such as thymol and carvacrol, have been shown to have strong toxic properties on some parasites (Szczepanik et al., 2012Szczepanik M, Zawitowska B, Szumny A. Insecticidal activities of Thymus vulgaris essential oil and its components (thymol and carvacrol) against larvae of lesser mealworm, Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae). Allelopathy Journal 2012; 30(1):129-142).
Studies have demonstrated that M. alternifolia oil has toxic effects against horn flies (Haematobia irritans), and repelent effect at concentration of 5% (Klauck et al., 2014Klauck V, Pazinato R, Stefani LM, Santos RC, Vaucher RA, Baldissera MD, Raffin R, Boligon A, Athayde M, Baretta D, Machado G, Da Silva AS. Insecticidal and repellent effects of tea tree and andiroba oils on flies associated with livestock. Medical and Veterinary Entomology 2014; 28(1):33-39.). In addition, TTO has acaricidal effect on cattle ticks (Rhipicephalus microplus), and damages their reproductive system, both in its pure and nanoparticle forms, but the nanoparticles potentiated its lethal effect (Pazinato et al., 2014Pazinato R, Klauck V, Volpato A, Santos RC, De Souza ME, Vaucher RA, Raffin R, Gomes P, Felippi CC, Stefani LM, Da Silva AS. In?uence of tea tree oil (Melaleuca alternifolia) on the cattle tick Rhipicephalus microplus. Experimental Applied Acarology 2014; 63(1):77-88.), which was not observed in this study with A. diaperinus. Nanotechnology has been used to improve the efficiency of essential oils and to reduce the need for large oil volumes (Martinez et al., 2011Martinez HR. Aplicación de la alternifolia a em alternifolia: nano-odontología. CES Odontology 2011; 24(2):87-91.).
Other studies have shown insecticidal effect of different essential oils on some A. diaperinus life stages. Tests demonstrated that thyme essential oil (Thymus vulgaris) was harmful to this insects, as well as when two compounds (carvacrol and thymol) of this oil were individually tested (Szczepanik et al., 2012Szczepanik M, Zawitowska B, Szumny A. Insecticidal activities of Thymus vulgaris essential oil and its components (thymol and carvacrol) against larvae of lesser mealworm, Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae). Allelopathy Journal 2012; 30(1):129-142). Anise oil (Illiciumverum) showed 100% efficacy against A. diaperinus larvae and adults at the concentrations of 3.12, 6.25, 12.5, and 25.0 mg ml-1 (Szczepanik & Szumny, 2011), similarly to the effects obtained with the higher TTO concentrations used in the present study. Our findings suggest that other formulations of the oil, in addition of nanoparticles, may be useful and need to be tested both in-vitro and under field conditions, since no toxicity to the soil fauna was detected.
Cypermethrin is the main insecticide used to control A. diaperinus; however, this drug may be toxic to the soil fauna (Styrishave et al., 2010Styrishave B, Hartnik T, Christensen P, Andersen O, Jensen J. Influence of soil type and organic matter content on the bioavailability, accumulation, and toxicity of a-cypermethrin in the springtail F. candida. Environmental Toxicology and Chemistry 2010; 29(5):1084-1090; Zortéa et al., 2015Zortéa T, Baretta D, Maccari AP, Segat JC, Boiago ES, Sousa JP, Da Silva AS. Influence of cypermethrin on avoidance behavior, survival and reproduction of Folsomia candida in soil. Chemosphere 2015; 122(1):94-98.), and consequently cause environmental damage. Therefore, it is important to evaluate the environmental impact of new treatments because contamination may affect many ecology systems. In the present study, the M. alternifolia oil had no significant toxic effect on the soil fauna, differently from the essential oil of E. globulus, which resulted in a 76% reduction in reproduction of F. candida at 60 mg kg-1(Martins et al., 2013Martins C, Natal-da-Luz T, Sousa JP, Gonçalves MJ, Salgueiro L, Canhoto C. Effects of essential oils from Eucalyptus globulus leaves on soil organisms involved in leaf degradation. PLoS ONE 2013; 8(4):e61233). The overall results of the present study show that poultry litter can be used as organic fertilizer after TTO application to control A. diaperinus.
CONCLUSIONS
Both TTO and terpinen-4-ol have insecticidal and larvicidal effect against the lesser mealworm A. diaperinus. In addition, it was demonstrated that TTO does not negatively affect the soil fauna, and consequently, there is no environmental impact when this essential oil is mixed with soil. Therefore, the tea tree oil is an option to control of A. diaperinus, particularly its larval stage.
REFERENCES
- Adams RP. Identification of essential oil components by Gas Chromatography/Mass spectroscopy. Illinois; Allured Publishing Corporation; 1995.
- Baldissera MD, Da Silva AS, Oliveira CB, Santos RCV, Vaucher RA, Raffin RP, Gomes P, Dambros MGC, Miletti LC, Boligon AA, Athayde ML, Monteiro SG. Trypanocidal action of tea tree oil (Melaleuca alternifolia) against Trypanosoma evansi in vitro and in vivo used mice as experimental model. Experimental Parasitology 2014; 141(1):21-27.
- Boligon AA, Schwanz TG, Piana M, Bandeira RV, Frohlich JK, Brum TF, Zadra M, Athayde ML. Chemical composition and antioxidant activity of the essential oil of Tabernaemontana catharinensis A. DC. leaves. Natural Product Research 2013; 27(1):68-71.
- Carson CF, Riley TV. Antimicrobial activity of the essential oil of Melaleuca alternifolia. Letter Applied Microbiology 1993; 16(1):49-55.
- Chernaki-Leffer AM, Sosa-Goméz DR, Almeida LM, Lopes ION. Susceptibility of Alphitobius diaperinus (Panzer) (Coleoptera, Tenebrionidae) tocypermethrin, dichlorvos and triflumuron in southern Brazil. Revista Brasileira de Entomologia 2011; 55(1):125-128.
- Da Silva AS da, Hoff G, Doyle RL, Santurio JM, Monteiro SG. Ciclo biológico do cascudinho Alphitobius diaperinus em laboratório. Acta Scientiae Veterinariae 2005; 33(2):177-181.
- Gazoni FL, Flores F, Bampi RA, Silveira F, Boufleur R, Lovato M. Avaliação da resistência do cascudinho (Alphitobius diaperinus) Panzer (Coleoptera: Tenebrionidae) a diferentes temperaturas. Arquivos do Instituto Biologico 2012; 79(1):69-74.
- ISO. Soil quality-inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants [ISO 11267]. Geneva; International Organization for Standardization; 1999.
- ISO. Soil quality-avoidance test for determining the quality of soils and effects of chemicals on behavior - Part 2: Test with collembolans (Folsomia candida) [ISO 17512-2]. Geneva; International Organization for Standardization; 2010.
- Klauck V, Pazinato R, Stefani LM, Santos RC, Vaucher RA, Baldissera MD, Raffin R, Boligon A, Athayde M, Baretta D, Machado G, Da Silva AS. Insecticidal and repellent effects of tea tree and andiroba oils on flies associated with livestock. Medical and Veterinary Entomology 2014; 28(1):33-39.
- Martinez HR. Aplicación de la alternifolia a em alternifolia: nano-odontología. CES Odontology 2011; 24(2):87-91.
- Martins C, Natal-da-Luz T, Sousa JP, Gonçalves MJ, Salgueiro L, Canhoto C. Effects of essential oils from Eucalyptus globulus leaves on soil organisms involved in leaf degradation. PLoS ONE 2013; 8(4):e61233
- Mulla MS, Su T. Activity and biological effects of neem products against arthropods of medical and veterinary importance. Journal American Mosquitoes Control Association 1999; 15(2):133-152.
- Neves RCSM, Ferraz RHS, Mendonça AJ, Lima SR, Cruz FAS, Rosa JG, Mateus LAF, Ferraz V, Barros LA. Acaricide effect of the Melaleuca alternifolia essential oil on Otodectescynotis. Revista Brasileira de Ciências Veterinária 2012; 19(3):144-148.
- Pazinato R, Klauck V, Volpato A, Santos RC, De Souza ME, Vaucher RA, Raffin R, Gomes P, Felippi CC, Stefani LM, Da Silva AS. In?uence of tea tree oil (Melaleuca alternifolia) on the cattle tick Rhipicephalus microplus. Experimental Applied Acarology 2014; 63(1):77-88.
- Prado GP, Stefani LM, Silva AS da, Smaniotto LF, Garcia FRM, De Moura NF. Alphitobius diaperinus (Coleoptera: Tenebrionidae) susceptibility to Cunila angustifolia essential oil. Journal of Medical Entomology 2013; 50(5):1040-1045.
- Roco MC. International strategy for nanotechnology research and development. Journal of Nanoparticle Research 2001; 3(5):353-360.
- Santos MJG, Ferreira FML, Cachada A, Duarte AC, Sousa JP. Pesticide application to agricultural fields: effects on the reproduction and avoidance behaviour of Folsomia candida and Eisenia andrei. Ecotoxicology 2012; 21(8):2113-2122.
- Santos JC, Alves LFA, Opazo MAU, Mertz NR, Marcomini AM, Oliveira DGP, Bonini AK. Eficiência da aplicação de inseticida químico no solo para o controle de Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae) em aviário de frango de corte. Arquivos do Instituto Biologico 2009; 76(3):417-425.
- Styrishave B, Hartnik T, Christensen P, Andersen O, Jensen J. Influence of soil type and organic matter content on the bioavailability, accumulation, and toxicity of a-cypermethrin in the springtail F. candida. Environmental Toxicology and Chemistry 2010; 29(5):1084-1090
- Szczepanik M, Szumny A. Insecticidal activity of star anise (Illicum verum Hook. F.) fruits extracts against lesser mealworm, Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae). Allelopathy Journal 2011; 27(2):277-288.
- Szczepanik M, Zawitowska B, Szumny A. Insecticidal activities of Thymus vulgaris essential oil and its components (thymol and carvacrol) against larvae of lesser mealworm, Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae). Allelopathy Journal 2012; 30(1):129-142
- Walton SF, McKinnon M, Pizzutto S, Dougall A, Williams E, Currie BJ. Acaricidal activity of Melaleuca alternifolia (tea tree) oil: in vitro sensitivity of Sarcoptes scabiei var hominis to terpinen-4-ol. Archives Dermatology 2004; 140(5):563-566.
- Zortéa T, Baretta D, Maccari AP, Segat JC, Boiago ES, Sousa JP, Da Silva AS. Influence of cypermethrin on avoidance behavior, survival and reproduction of Folsomia candida in soil. Chemosphere 2015; 122(1):94-98.
Publication Dates
-
Publication in this collection
Jan-Mar 2016
History
-
Received
Apr 2015 -
Accepted
Oct 2015