ABSTRACT
Marek’s disease virus (MDV) has been shown to be evolving to higher virulence. One of the genetic sites involved in virulence which enables such characterization is the 339-amino acid Meq protein encoding gene (meq). The reemergence of clinical Marek’s disease (MD) in vaccinated flocks can be associated to changes in meq. Our studies have shown the presence of very virulent MDV strains in the Brazilian industrial and free-range poultry. We present an overview of MD increasing severity and indicate the necessity of using phylogenetic tools for best accompanying MDV evolution.
Keywords:
Gallid herpesvirus 2; Marek’s disease virus; Gallus gallus domesticus; meq gene; virulence
INTRODUCTION
During the last fifty years, the increased intensification of poultry has provided high numbers of chickens concentrated in industrial farms and specific geographical areas. The proximity of large flocks of chickens, of varied immune and health status, has enabled the circulation of infections, such as Marek’s disease (MD), infectious bronchitis, infectious bursal disease, with the emergence of a large number and diversity of pathogens, as described for MDV. The extensive vaccination of flocks against MDV has additionally provided selective pressure and possibly genetic diversity for the evolutive advantage of immunity-evading strains. After a few decades of MD vaccination, MDV strains have emerged with ever increasing virulence (Eidson et al, 1978Eidson CS, Page RK, Kleven SH. In vivo and in vitro studies on the effect of gentamicin sulfate on the efficacy of the turkey herpesvirus vaccine. Poultry Science 1978;57(6):1519-1525.; Eidson et al., 1981; Imai et al.; 1992Imai K, Yuasa N, Iwakiri H, Nakamura K, Hihara H, Ishita T, et al. Characterization of very virulent Marek's disease viruses isolated in Japan. Avian Pathology 1992;21(1):119-126.; Mckimm-Breschkinn et al., 1990; Powell & Lombardini, 1986Powell PC, Lombardini F. Marek's disease: a worldwide problem. World's Poultry Science 1986;42:205-218.; Sung, 2002Sung HW. Recent increase of Marek's disease in Korea related to the virulence increase of the virus. Avian Diseases 2002;46:517-524.; Venugopal, 1996; Witter, 1997Witter RL. Increased virulence of Marek's disease virus field isolates. Avian Diseases 1997;41:149-163.; Witter et al., 2005). The estimated economic burden of MD may reach US $ 1 to 2 billion annually (Atkins, 2013Atkins KE, Read AF, Savill NJ, Renz KG, Fakhrul Islam AFM,et al. Vaccination and reduced cohort duration can drive virulence evolution:Marek's disease virus and industrialized agriculture. Evolution: International Journal of Organic Evolution 2013;67(3):851-860.).
The breakthrough description of a herpesvirus in MD tumors enabled the differentiation between Marek’s disease and Lymphoid Leukosis (Churchill & Biggs, 1967Churchill AE, Biggs PM. Agent of Marek's disease in tissue culture. Nature 1967;215:528-530.; Nazerian et al., 1968Nazerian K, Solomon JJ, Witter RL, Burmester BR. Studies on the etiology of Marek's disease. II. Finding of a herpesvirus in cell culture. Proceedings of the Society for Experimental Biology and Medicine 1968;127(1):177-182.; Solomon et al., 1968Solomon JJ, Witter RL, Nazerian K, Burmester BR. Studies on the etiology of Marek's disease. I. Propagation of the agent in cell culture. Proceedings of the Society for Experimental Biology and Medicine 1968;127(1):173-177.), formerly considered as part of the avian leukosis complex. Consequently, research rapidly provided the tools for the prevention of MD. The understanding of the transmission mechanism and risk of infection was achieved when experimental infection with cell-free MDV of feather follicle desquamation epithelium was demonstrated (Beasley et al., 1970Beasley JN, Patterson LT, McWade DH. Transmission of Marek's disease by poultry house dust and chicken dander. American Journal of Veterinary Research 1970;31:339-344.; Calnek et al., 1969Calnek BW, Hitchner SB. Localization of viral antigen in chickens infected with Marek's disease herpesvirus. Journal of the National Cancer Institute. 1969;43:935-949.; Calnek et al., 1970a). The virus was serially passaged in primary kidney cell monolayers and successfully attenuated (Churchill et al., 1969), and given to one-day-old chicks induced protection against the challenge (Calnek et al., 1970b), subsequently also acquired by naturally a virulent strains isolated from turkeys (Witter et al., 1970Witter RL, Nazerian K, Purchase HG, Burgoyne GH. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. American Journal of Veterinary Research 1970;31:525-538.b) and chickens (Biggs & Milne, 1972; Cho & Kenzy, 1972Cho BR, Kenzy SG. Isolation and characterization of an isolate (HN) of Marek's disease virus with low pathogenicity. Applied Microbiology 1972;24:299-306.). Among the isolated a virulent low virulence MDV strains, the CVI 988 vaccines became popular or of (Rispens et al., 1972Rispens BH, Van Vloten H, Mastenbroek N, Maas HJ, Schat KA. Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Diseases 1972;16:108-125.) and SB-1 (Schat & Calnek, 1978Schat KA, Calnek BW. Characterization of an apparently nononcogenic Marek's disease virus. Journal of the National Cancer Institute 1978;60:1075-1082.).
Marek’s disease virus
MDV is classified as Gallid Herpesvirus 2, genus Mardivirus, famíly Herpesviridae, subfamíly Alphaherpesvirinae, and divided into three serotypes MDV-1 (RB-1B, Md5 and CVI988), MDV-2 (SB-1 e HPRS24), and the antigenically related Meleagrid Herpesvirus-1 (known as serotype three; herpes vírus of turkeys- strain FC126) (ICTV, 2018; Dunn et al., 2014Dunn JR, Auten K, Heidari M, Buscaglia C. Correlation between Marek's disease virus pathotype and replication. Avian Diseases 2014;58(2):287-292.). Only strains of serotype 1 are capable of causing disease, while MDV-2 e MDV-3 strains are a virulent (Calnek, 2001Calnek BW. Pathogenesis of Marek's disease virus infection. In: Hira K, editor. Current topics in microbiology and immunology. Berlin: Springer-Verlag; 2001. p.25-56.). The classification of MDV according to pathotype was reviewed, including the philosophical and methodological aspects (Witter et al., 2005Witter RL, Calnek BW, Buscaglia C, Gimeno IM, Schat KA. Classification of Marek's disease viruses according to pathotype:philosophy and methodology. Avian Pathology 2005:34(2):75-90.). The correlation between MDV replication and virulence was shown for vMDV and vvMDV strains, although a non-significant difference was found between very virulent (vv) and vv+MDV isolates (Dunn et al., 2014).
MDV genome is large and encodes for more than 200 genes, including genes that are involved in pathogenicity, such as meq (Jones et al., 1992Jones D, Lee L, Liu JL, Kung HJ, Tillotson JK. Marek's disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. Proceedings of the National Academy of Sciences of the United States of America 1992;89(9):4042-4046.; Lupiani et al., 2004Lupiani, B, Lee LF, Cui X, Gimeno I, Anderson A, Morgan RW, et al. Marek's disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proceedings of the National Academy of Sciences 2004;32:11815-11820.; Nair, 2013Nair V. Latency and tumorigenesis in Marek's disease. Avian Diseases 2013;57:360-365.). MDV genomic integration was demonstrated in host cells (Nikura et al., 2006Nikura M, Dodgson J, Cheng H. Direct evidence of host genome acquisition by the alphaherpesvirus Marek's disease virus. Archives Virology 2006;151:537-549.). MDV encodes a basic-leucine zipper protein (MDV EcoRI-Q), similar to the fos/jun oncogenes products, that is highly expressed in tumors (Jones et al., 1992). Meq is involved in the transformation of T-lymphocytes but not needed for replication (Lupiani et al., 2004). Meq protein is a 339-phosphoprotein expressed abundantly by meq during the lytic and latent phases of cellular interaction (Gennart et al., 2015Gennart I, Coupeau D, Pejakovic´ S, Laurent S, Rasschaert D, Muylkens B. Marek's disease: genetic regulation of Gallid herpesvirus 2 infection and latency. The Veterinary Journal 2015;205:339-348.), activating transcription and involved in the transformation of T lymphocytes (Gennart et al., 2015; Brown et al., 2009Brown AC, Smith LP, Kgosana L, Baigent SJ, Nair V, Allday MJ. Homodimerization of the meq viral oncoprotein is necessary for induction of t-cell lymphoma by Marek's disease virus. Journal of Virology 2009;83(21):11142-11151.). Although meq is consistently associated to pathogenicity, other genes were shown to be involved (Wozniakowski et al., 2010Wozniakowski G, Samorek-Salamonowicz E, Kozdrun W. Sequence analysis of meq oncogene among Polish strains of Marek's disease. Polish Journal of Veterinary Sciences 2010;13:263.; Jarosinski et al., 2006Jarosinski KW, Karsten Tischer B, Trapp S, Osterrieder N. Marek's disease virus: lytic replication, oncogenesis and control. Expert Review of Vaccines 2006;5(6):761-762.), such as vTR (Fragnet et al., 2005Fragnet L, Kut E, Rasschaert D. Comparative functional study of the viral telomerase RNA based on natural mutations. Journal of Biological Chemistry 2005;280(25):23502-23515.; Trapp et al., 2006Trapp S. A virus-encoded telomerase RNA promotes malignant T cell lymphomagenesis. The Journal of Experimental Medicine 2006;203:1307-1317.) and vil-8 e pp38. However, the oncogenicity was retained by a MDV mutant (RB1BD4.5lac) lacking unique short region genes (Parcells et al., 1995Parcells MS, Anderson AS, Morgan TW. Retention of oncogenicity by a Marek's disease virus mutant lacking six unique short region genes. Journal of Virology 1995;69(12):7888-7898.).
The meq encoded oncogenic protein Meq is detected in all MD tumors (Ross, 1999Ross LJN. Recombinant vaccines against Marek's disease. Avian Pathology 1999;27:65-73.). Meq interferes negatively with the expression (down-regulates) of cellular apoptosis genes, and up-regulates viral genes involved in cellular transformation (Liu & Kung, 1999Liu JL, Kung HJ. Marek's disease herpesvirus transforming protein MEQ:a c-Jun analogue with an alternative life style. Virus Genes 1999;21:51-64.), as well as its own expression. MDV lacking meq is not oncogenic, as for serotypes 2 and 3, and its deletion of pathogenic strains will result in loss of oncogenicity (Silva et al., 2010Silva RF, Dunn JR, Cheng HH, Niikura M. A MEQ-deleted Marek's disease virus cloned as a bacterial artificial chromosome is a highly efficacious vaccine. Avian Diseases 2010;54(2):862-869.; McPherson & Delany, 2016McPherson MC, Delany ME. Virus and host genomic, molecular, and cellular interactions during Marek's disease pathogenesis and oncogenesis. Poultry Science 2016;95(2):412-429.). The equilibrium of cell-virus interaction during persistence is genetically determined in Herpes simplex virus (HSV) persistently infected cells, and gradual increase in virulence as opposed to cellular resistance would result in tumorigenesis (Cummings et al., 1989Cummings PJ, Rinaldo Jr CR. Coevolution of virulent virus and resistant cells as a mechanism of persistence of herpes simplex virus type 1 in a human T lymphoblastoid cell line. Journal of General Virology 1989;70:97-106.).
Latency starts approximately within one week of infection, mainly in T lymphocytes CD4+, although the transition from cytolytic to latent infection is not entirely understood (Nair, 2013Nair V. Latency and tumorigenesis in Marek's disease. Avian Diseases 2013;57:360-365.). Latently infected T CD4+ cells in genetically susceptible unvaccinated chickens are transformed and originate tumors (McPherson & Delany, 2016McPherson MC, Delany ME. Virus and host genomic, molecular, and cellular interactions during Marek's disease pathogenesis and oncogenesis. Poultry Science 2016;95(2):412-429.), and may systemically disseminate MDV through the feather follicle epithelium, where the productive replication may resume, disseminating MDV to the environment and housing in desquamating epithelial cells (Baigent & Davison, 2004Baigent SJ, Davison F. Marek's disease virus: biology and life cycle. In: Marek's disease, an evolving problem. Amsterdam: Elsevier Academic Press; 2004. p.62-77.; Baigent et al., 2013). During latency in T CD4+, the productive (lytic) infection is suppressed and apoptosis is blocked (Baigent et al., 1998). MDV reactivation in latently infected lymphocytes will result in elevated genetic expression (McPherson & Delany, 2016). The mechanisms of latency and transformation are not well understood (Nair, 2013), although both are associated to genomic integration (NAIR, 2005Nair V. Evolution of Marek's disease- A paradigm for incessant race between the pathogen and the host. Veterinary Journal 2005;170:175-183.), and a few T CD4+ lymphocytes will undergo transformation and give rise of T-cell tumor lineages (Calnek, 2001Calnek BW. Pathogenesis of Marek's disease virus infection. In: Hira K, editor. Current topics in microbiology and immunology. Berlin: Springer-Verlag; 2001. p.25-56.). Latency is mediated by the Meq protein by blocking apoptosis and gene expression is transactivated and reactivation is dependable on meq repression and expression of phosphoprotein 38 (pp38), Hep and Mys encoding open reading frames (Parcells et al., 2003Parcells MS, Arumugaswami V, Prigge JT, Pandya K, Dienglewicz RL. Marek's disease virus reactivation from latency:changes in gene expression at the origin of replication. Poultry Science 2003;82(6):893-898.), and the susceptibility is determined by higher numbers of pp38+ lymphocytes (Baigent et al., 1998).
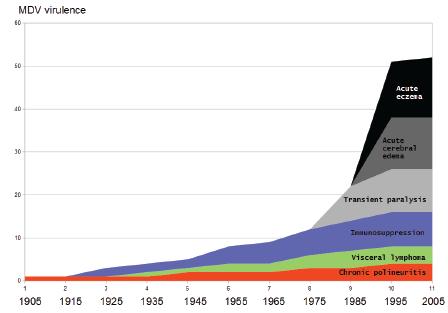
Marek’s disease chronological increase in severity and acuteness. (Adapted from Osterrieder et al., 2006Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek's disease virus:from miasma to model. Nature Reviews Microbiology 2006;4(4):283.).
MD in Brazil
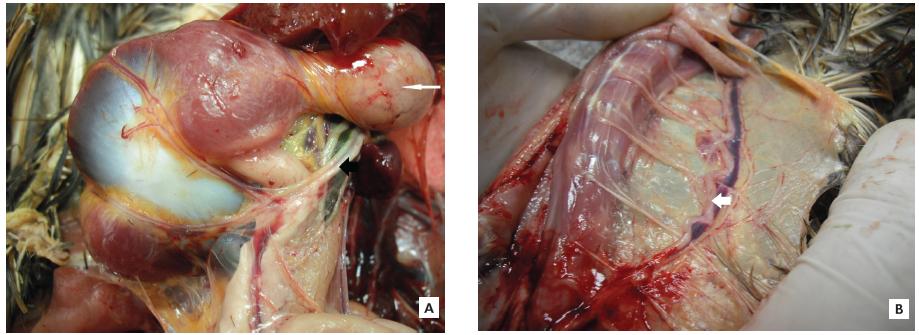
Although MD has been studied in the poultry producing countries all over the world, studies in Brazil are scarce, especially regarding the characterization of MDV strains virulence. Research in our laboratory (in press) has revealed the widespread occurrence of pathogenic and very pathogenic MDV in free-range and industrial chickens, with also the detection of vv+MDV (Fig. 2). Natural outbreaks, for instance in free-range chickens and involving the classical MD, include enlarged peripheral nerves, such as vagus at the proventricular/ventricular region (Fig. 3A) and at the cervical region (Fig. 3B). Preliminary findings suggest that the eventual future reemergence of MD in Brazil could be principally associated to genetic changes in meq and resulting in insufficient protection through single HVT vaccination, as described elsewhere (Wozniakowsi & Samorek-Salamonowicz, 2014).
Brazilian industrial or free-range chickens MDV strains were evaluated as based on meq gene sequences. The strain 157 (Accession number KY322682) was grouped with GXY2 (EF546430.1), a very virulent MDV strain from China which caused acute tumors in CVI988 (Rispens) or HVT vaccinated chickens. Strains 500 (KY322683), 573 (KY322684), 578 (KY322685), 590 (KY322689), 754b (KY322689) and 755 (KT768121.1) had identity with virulent MDV (vMDV). Strains 500 (KY322683) (Fig. 2), 754b (KY322689) and 755 (KT768121.1) caused severe peripheral nerve inflammatory disease in free-range chickens. Strains 1042 (KY352470), 924-3 (KY322691) and 155 (KY322681) had substitutions in the meq oncogene compatible with highly virulent MDV strains (vvMDV), and grouped separately. Herpesvirus of turkeys (HVT) vaccine strain was added as a heterologous herpesvirus. The evolutionary history was inferred using the Neighbor-Joining method (Saitou N. and Nei M., 1987Saitou N, Nei N. The neighbor-joining method:A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 1987;4:406-425.). The optimal tree with the sum of branch length = 5.66821085 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura, Nei and Kumar, 2004) and are in the units of the number of base substitutions per site. The analysis involved 13 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 353 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar, Stecher & Tamura, 2016).
An additional preoccupation has come to light in Brazil, with the description of MD in peafowl (Pavo cristatus) at a Zoological Park, characterized by visceral lymphomatosis and the detection of a virulent MDV (serotype 1) strain, through PCR and partial sequencing of the Meq protein encoding gene (Blume et al., 2016Blume GR, Cardoso SP, Oliveira ML, Matiolli MP, Gómez SY, Reis Júnior JL, et al. Visceral Marek's disease in white-peafowl (Pavo cristatus). Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2016;68(6):1602-1608.). Tumoral disease in the liver, spleen, kidneys and skin, although with mild clinical expression, was characterized by histopathology as MD in free-range chickens in Rio de Janeiro (Abreu et al., 2016Abreu DLC, Santos FF, José DS, Tortelly R, Nascimento ER, Pereira VLA. Pathological aspects of a subclinical marek's disease case in free-range chickens. Revista Brasileira de Ciência Avícola 2016;18(1):197-200.). Thickened feather follicles and focal whitish tumors suggestive of MD in liver, heart, kidneys, spleen, ovary, proventriculus and peripheral nerves of broilers and layers of the poultry industry were described during 1999 to 2003 (Sousa, 2010Sousa E. Registro da doença de Marek, Leucose aviária e doença Infecciosa da bolsa na Região do Triângulo Mineiro, no período de 1999 a 2003. PUBVET 2010;4:27.) in Minas Gerais, Brazil.
MD vaccination
MD control through vaccination was paramount for the growth of the poultry industry. However, the increasing virulence of MDV strains may however compromise the success of control through vaccination (Witter, 1997Witter RL. Increased virulence of Marek's disease virus field isolates. Avian Diseases 1997;41:149-163.) and reports of vvMDV are spread worldwide (López-Osório et al., 2017). The pioneering experiments with vaccines were developed almost immediately after the description of MD etiology (Churchill & Biggs, 1967Churchill AE, Biggs PM. Agent of Marek's disease in tissue culture. Nature 1967;215:528-530.), with its attenuation by the end of the 1960’s (Churchill et al., 1969) and the discovery of a virulent strains in turkeys (Witter et al., 1970) and chickens (Biggs & Milne, 1972; Cho & Kenzy, 1972Cho BR, Kenzy SG. Isolation and characterization of an isolate (HN) of Marek's disease virus with low pathogenicity. Applied Microbiology 1972;24:299-306.).
The vaccination of chickens against MD is mandatory in the Brazilian poultry industry and must be given at the 18th day of incubation or at the day of hatching (Brasil, 2007). MD vaccines in use in Brazil should contain 1,500 plaque forming units per dose and are prepared with live Meleagrid Herpesvirus 1 (FC-126) and/or CVI988/Rispens (Gallid Herpesvirus 2) or SB1 (Gallid Herpesvirus 3) and may be monovalent or polyvalent (OIE, 2018). However, imperfect vaccination would enhance transmission of highly virulent MDV strains and other pathogens (Read et al., 2015Read AF, Baigent SJ, Powers C, Kgosana LB, Blackwell L, Smith LP, et al. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLOS Biology 2015;13(7):198-200.).
Increased virulence
A comprehensive review was previously published, indicating the advancement of knowledge regarding MDV interaction with host cells and virulence (Osterrieder et al., 2006Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek's disease virus:from miasma to model. Nature Reviews Microbiology 2006;4(4):283.) (Fig. 1).
One of the driving forces involved in MDV selection to higher virulence is the immune response, possibly more relevant if vaccine derived (Davison & Nair, 2005Nair V. Evolution of Marek's disease- A paradigm for incessant race between the pathogen and the host. Veterinary Journal 2005;170:175-183.). The pioneering description of potential evolution to evading vaccination protection was proposed very early after adoption of HVT vaccine (Okazaki et al., 1973Okazaki W, Purchase HG, Burmester BR. Vaccination against Marek's disease: possible causes of failure of herpesvirus of turkeys (Strain FC-126) to protect chickens against Marek's disease. American Journal of Veterinary Research 1973;34:813-817.). Virulence may evolve partially due to a compromise loss between damage and infection, and although pathogenicity might evolve from competition with the host, bacterial virulence would evolve from within the host pathogen competition (Smith et al., 2011Smith J. Superinfection drives virulence evolution in experimental populations of bacteria and plasmids. Evolution 2011;65(3):831-841.).
Since the first description of MD in 1907, up to 1915, the principal form of clinical presentation was chronic polyneuritis. Starting from 1915 to approximately 1925, increasing number of cases of immunosuppression were registered. From 1925 up to 1975, visceral lymphoma, immuno-suppression and chronic polyneuritis were the clinical forms described in ever increasing incidence. From 1975, transient paralysis began to be encountered in the field. In 1985, cases of acute cerebral edema and acute eczema were additionally described. The classical form of MD was characterized as a paralytic syndrome of relatively low occurrence involving peripheral nerve inflammation, involving more commonly the sciatic, brachial, trigeminal, and vagus nerves, but rarely exceeding 10-15% mortality (OIE, 2018). By the end of the 1950s, with the intensification of the poultry production, more virulent forms of MD were described (Benton & Cover, 1957Benton WJ, Cover MS. The increased incidence of visceral lymphomatosis in broiler and replacement birds. Avian Diseases 1957;1:320-327.), characterized by up to 40% mortality in layers and the occurrence of up to 10% of broilers with visceral lymphomatosis.
During the 1960s the more aggressive forms of MD were also described in the United Kingdom (Biggs, 1965Biggs PM. Avian transmissible tumours. Proceedings 3rd Congress of the World Veterinary Poultry Association; 1965; Paris. p.61-67.), and United States, characterized as more acute and precocious disease with early onset of visceral tumors (Biggs, 1966).
After the generalized use of day-old chicks vaccination against MD, in the beginning of the 1970s, the clinical disease was nearly eradicated, with punctual problems associated to the administration or titer of vaccines, or too early exposure to field MDV (Buscaglia & Crosetti, 1993Buscaglia C, Crosetti C. Studies on Marek's disease vaccination failures in Argentina. Proceedings of the 4th International Symposium on Marek's Disease and 2nd Meeting on the Cooperative Research Project in the Area of Veterinary Sciences between National University of La Plata and Japan International Cooperation Agency, 1993; La Plata, Argentina, p.19-21, 1993.). However, by the end of the 1970’s, visceral and early disease outbreaks were described, and associated to variant MDV strains, including in HVT (Herpesvirus of turkeys) vaccinated flocks, in the US and elsewhere (Eidson et al., 1978Eidson CS, Page RK, Kleven SH. In vivo and in vitro studies on the effect of gentamicin sulfate on the efficacy of the turkey herpesvirus vaccine. Poultry Science 1978;57(6):1519-1525.; Eidson et al., 1981; Schat et al., 1981Schat KA, Calnek BW, Fabricant J, Abplanalp H. Influence of oncogenicity of Marek's disease virus on evaluation of genetic resistance. Poultry Science 1981;60(12):2559-2566.; Powell & Lombardini, 1986Powell PC, Lombardini F. Marek's disease: a worldwide problem. World's Poultry Science 1986;42:205-218.; McKimm-Breschkinn et al., 1990McKimm-Breschkinn JL, Faragher JT, Withell J, Forsyth WM. Isolation of very virulent Marek's disease viruses from vaccinated chickens in Australia. Australian Veterinary Journal 1990;67:205-209.; Imai et al.; 1992Imai K, Yuasa N, Iwakiri H, Nakamura K, Hihara H, Ishita T, et al. Characterization of very virulent Marek's disease viruses isolated in Japan. Avian Pathology 1992;21(1):119-126.; Sung, 2002Sung HW. Recent increase of Marek's disease in Korea related to the virulence increase of the virus. Avian Diseases 2002;46:517-524.).
Several very virulent strains of MDV (vvMDV) have been described since the 1980s, with the pathogenicity determined by the capacity of oncogenesis in HVT vaccinated chickens (Eidson et al., 1981). According to the capacity of evading the specific immunity derived from HVT vaccination, the more virulent MDV strains have been classified as mildly virulent (mMDV), virulent MDV (vMDV), very virulent MDV (vvMDV), and very virulent plus MDV (vv+MDV) (Witter, 1997Witter RL. Increased virulence of Marek's disease virus field isolates. Avian Diseases 1997;41:149-163.), being all strains above virulent capable of breaking the vaccinal protection (Wozniakowsi & Samorek-Salamonowicz, 2014).
The increased occurrence of vvMDV strains has also been reported in vaccinated flocks in Germany, France, the Mediterranean countries, Japan and the UK (Boer et al., 1985). The description of naturally occurring vvMDV and vv+MDV has also been reported in Argentina in the early 1990s, but initially associated to vaccination error (Buscaglia & Crosetti, 1993Buscaglia C, Crosetti C. Studies on Marek's disease vaccination failures in Argentina. Proceedings of the 4th International Symposium on Marek's Disease and 2nd Meeting on the Cooperative Research Project in the Area of Veterinary Sciences between National University of La Plata and Japan International Cooperation Agency, 1993; La Plata, Argentina, p.19-21, 1993.). The outbreaks in Argentina were associated to four varying prototypes (Buscaglia et al., 1995), the first vvMDV strains of disease in vaccinated flocks in South America. In Colombia (López-Osório et al., 2017) the strain UDEACO-2013, isolated from an outbreak in chickens, was genetically related to hypervirulent strains of the United States, with the oligonucleotide position substitutions 176 (P/A), 217(P/A) and P233 (P/L) considered as indicative of vvMDV, although not related to other strains around the world, although the amino acid substitution at residue 77 (E/K) was suggestive of mMDV.
The emergence of higher virulence of MDV is associated to the selective pressure induced by the immunity derived from vaccination (Witter, 1997Witter RL. Increased virulence of Marek's disease virus field isolates. Avian Diseases 1997;41:149-163.), in addition to the increased genetic resistence by chickens, and has resulted in the widespread description of higher virulence strains, such as in Argentina, India and China (Buscaglia et al., 2004Buscaglia C, Nervi P, Risso M. Characterization of four very virulent Argentinian strains of Marek's disease virus and the influence of one of those isolates on synergism between Marek's disease vaccine viruses. Avian Pathology 2004;33(2):190-195.; Zhang et al., 2011). In addition, the interaction of MDV with chicken anemia virus might result in evolving advantage (Zanella et al., 2001Zanella A, Dall'ara P, Lavazza A, Marchi R, Morena MA, Rampin T. Interaction between Marek's disease vírus and chicken infectious anemia virus. In: Schat KA, Morgan RM, Parcells MS, Spencer JL., editors. Current progress on Marek's disease research. Kennett Square: American Association of Avian Pathologists; 2001. p.1119.).
Within the last 15 years, vv+MDV strains have been described principally in vaccinated flocks (Zhang et al., 2011), indicating a lack of protective spectrum for the HVT strain. The molecular analysis of 1020 nucleotídes which encode 339 amino acids of the Meq protein of Chinese MDV strains obtained from 2006 to 2008 has shown that all isolates possessed two substitutions of amino acids at residues 139 (T/A) and 176 (P/R), similar to sequences of the atenuated strain CVI988. However, six isolates have shown substitutions at positions 176 or 177 (P/T). Results have suggested a specific clade for the Chinese strains.
Very virulent (vv) MDV strains were first reported in vaccinated chickens in Europe (Powell & Lombardini, 1986Powell PC, Lombardini F. Marek's disease: a worldwide problem. World's Poultry Science 1986;42:205-218.) and subsequently in Australia (McKimm-Breschkin et al., 1990), and Japan (IMAI et al., 1992Imai K, Yuasa N, Iwakiri H, Nakamura K, Hihara H, Ishita T, et al. Characterization of very virulent Marek's disease viruses isolated in Japan. Avian Pathology 1992;21(1):119-126.). In Korea (Sung, 2002Sung HW. Recent increase of Marek's disease in Korea related to the virulence increase of the virus. Avian Diseases 2002;46:517-524.), five strains of vvMDV were described in layers and broilers with tumours, with one strain resulting in severe immunosuppression and high incidence of tumors (93,3%) in inoculated SPF chickens. Strains obtained of broiler flocks with visceral tumors in China were not adequately protected with the CVI988 vaccine strain (Zhang et al., 2015Zhang YP, Li ZJ, Bao KY, Lv HC, Gao YL, Gao HL, et al. Pathogenic characteristics of Marek's disease virus field strains prevalent in China and the effectiveness of existing vaccines against them. Veterinary Microbiology 2015;177(1-2):62-68.), protecting only 66% after the challenge with the LTS strain. MDV phosphoprotein pp38 and meq transformation protein encoding genes were evaluated, and meq mutations were associated to higher virulence (Shamblin et al., 2004Shamblin CE, Greene N, Arumugaswami V, Dienglewicz RL, Parcells MS. Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Veterinary Microbiology 2004;102:147-167.).
The meq oncogene sequences of MDV strains of 2006-2008 were analyzed in China (Zhang et al., 2011), revealing 19 strains of broilers with sanitary problems. In Guangxi, the vvMDV strains in vaccinated flocks were genetically distinct of the CV1988/Rispens vaccine strain, in use for 14 years (Teng et al., 2011Teng LQ, Wei P, Song ZB, He JJ, Cui ZZ. Molecular epidemiological investigation of Marek's disease virus from Guangxi, China. Archives of Virology 2011;156:203-206.). The characterization of MDV strains (2007-2010) of vaccinated flocks in Poland (Wozniakowski et al., 2011Wozniakowski G, Samorek-Salamonowicz E, Kozdrun W. Molecular characteristics of Polish field strains of Marek's disease herpesvirus isolated from vaccinated chickens. Acta Veterinaria Scandinavica 2011;53(1):10.), revealed the recombination of MDV and REV (reticuloendotheliosis) viruses. Twelve out of 24 isolates had 68 bp insertions in the meq gene, and 0.78, 0.8, 0.82, 1.6 kb and other random LTR-REV insertions in 28 of 29 evaluated strains, although the insertions could influence MDV replication, were not associated to virulence. MDV field strains (n=85) isolated in Poland within the years 1974-2012 were compared, evaluating 85 sequences of MDV076 (RLORF7) region of meq, 60 sequences of MDV077 encoding a 23 kDa protein which binds alpha-enolase and 58 sequences of MDV077.5 (RLORF6) genes. Although the 23 kDa and LORF6 sequences were related to low pathogenic MDV, the RLORF7 sequences were similar to vMDV and vvMDV strains. However, specific motifs within the three genes could be associated to virulence, indicated an increased virulence since 2006 and strains obtained in 2012 were similar to vvMDV+ strains (Wozniakowski et al., 2014).
In Egypt, vaccinated chickens showing neurological and tumoral lesions were investigated. Lesions were mostly observed in the liver, spleen and gonads, as localized or diffuse tumors, although the meq oncogene was detected in five out of the 30 chickens, with substitutions in positions 77(E/K), 80(Y/D), 88(T/A), 112(F/S), 139(A/T) and 176(R/P), although with deducted amino acid sequences showing five strains with identity (≥ 98%) with the vvMDV strains ATE (Hungary), C12/130 (UK) and Chinese LMS, YA, WS03 and GX070060 (Hassanin et al., 2013Hassanin O, Abdallah F, El-Araby IE. Molecular characterization and phylogenetic analysis of Marek's disease virus from clinical cases of Marek's disease in Egypt. Avian Diseases 2013;57:555-561.).
MDV meq sequences of strains were evaluated in Japan (Murata et al., 2013Murata S, Hashiguchi T, Hayashi Y, Yamamoto Y, Matsuyama-Kato Y, Takasaki S, et al. Characterization of Meq proteins from field isolates of Marek's disease virus in Japan. Infection Genetics and Evolution 2013;16:137-143.) and China (Yu et al., 2013Yu ZH, Teng M, Luo J, Wang XW, Ding K, Yu LL, et al. Molecular characteristics and evolutionary analysis of field Marek's disease virus prevalent in vaccinated chicken flocks in recent years in China. Virus Genes 2013;47:282-291.), revealing point mutations and diversity potentially associated to higher oncogenicity. In Iraq (Wajid et al., 2013Wajid SJ, Katz ME, Renz KG, Walkden-Brown SW. Prevalence of Marek's disease virus in different chicken populations in Iraq and indicative virulence based on sequence variation in the EcoRI-Q (meq) gene. Avian Diseases 2013;57(2s1):562-568.), MDV was detected in 49.5% of provinces, and based on meq sequences, with similar occurrence and identity of vaccinated and non-vaccinated broiler flock’s strains.
The genetic diversity of MDV in Saudi Arabia (Mohamed et al., 2016Mohamed MHA, El-Sabagh IM, Al-Habeeb MA, Al-Hammady YM. Diversity of Meq gene from clinical Marek's disease virus infection in Saudi Arabia. Veterinary World 2016;9(6):572-578.), as based on the meq gene, has shown that the strains of chickens with visceral tumors were similar to strains described in Poland, and indicated that the international trade or migratory birds might have a role in the transportation of virus. Although vaccination was implemented for commercial chickens in Colombia (López-Osório et al., 2017), with CVI988/Rispens + HVT vaccine strains in the first day, sporadic cases of MD continue to occur, with mortality reaching up to 30% by 50 weeks of age, and no visible lymphomas were observed. Although MD outbreaks in vaccinated flocks in Argentina (Buscaglia & Crosetti, 1993Buscaglia C, Crosetti C. Studies on Marek's disease vaccination failures in Argentina. Proceedings of the 4th International Symposium on Marek's Disease and 2nd Meeting on the Cooperative Research Project in the Area of Veterinary Sciences between National University of La Plata and Japan International Cooperation Agency, 1993; La Plata, Argentina, p.19-21, 1993.) during the early 1990s were associated to vaccination failure, MDV strains of higher virulence were detected in later outbreaks (Buscaglia et al., 1995).
The emergence of vvMDV strains in Brazil strains might result of similar mechanisms as described in Germany, France, Mediterranean countries, Japan and the UK (Boer et al., 1985), Argentina (Buscaglia et al., 1995Buscaglia C, Nervi P, Garbi JL, Piscopo M. Isolation of very virulent strains of Marek's disease virus from vaccinated chickens in Argentina. Proceedings of the Western Poultry Disease Conference; 1995; Sacramento; CA; 1995.) and Colombia (López-Osório et al., 2017). Here, the selective pressure induced by vaccination of chicks with HVT strain FC126, might have similarly enabled varying strains with evolving advantage, as detected by the end of the 1970’s in vaccinated flocks, in the US and elsewhere (Eidson et al., 1978Eidson CS, Page RK, Kleven SH. In vivo and in vitro studies on the effect of gentamicin sulfate on the efficacy of the turkey herpesvirus vaccine. Poultry Science 1978;57(6):1519-1525.; Eidson et al., 1981; Schat et al., 1981Schat KA, Calnek BW, Fabricant J, Abplanalp H. Influence of oncogenicity of Marek's disease virus on evaluation of genetic resistance. Poultry Science 1981;60(12):2559-2566.; Powell & Lombardini, 1986Powell PC, Lombardini F. Marek's disease: a worldwide problem. World's Poultry Science 1986;42:205-218.; McKimm-Breschkinn et al., 1990McKimm-Breschkinn JL, Faragher JT, Withell J, Forsyth WM. Isolation of very virulent Marek's disease viruses from vaccinated chickens in Australia. Australian Veterinary Journal 1990;67:205-209.; Imai et al.; 1992Imai K, Yuasa N, Iwakiri H, Nakamura K, Hihara H, Ishita T, et al. Characterization of very virulent Marek's disease viruses isolated in Japan. Avian Pathology 1992;21(1):119-126.; Baigent et al., 1998Baigent SJ, Ross LJN, Davison TF. Differential susceptibility to Marek's disease is associated with differences in number, but not phenotype or location, of pp38+ lymphocytes." Journal of General Virology 1998;79(11):2795-2802.; Sung, 2002Sung HW. Recent increase of Marek's disease in Korea related to the virulence increase of the virus. Avian Diseases 2002;46:517-524.). However, differently to other countries, the emergence of clinical disease is still negligible. Different environmental condition for build up and challenge, as observed for infected cells in dust (Baigent et al., 2013) might play a role and may provide additional information regarding risk. In Brazil, most commonly, new chick flocks are housed in carefully cleaned and disinfected houses, which might have been providing reduced challenge, at least with clinically significant doses of field MDV. The occurrence of vvMDV in free-range flocks might arise from the eventual proximity of industrial and free-range flocks, common in certain regions, and might also result in continuous spill-over and spill-back mechanisms, although strains could also have emerged independently..
MDV strains detected in Brazil were evaluated for meq gene sequences (Fig. 2). Strain 157 (Accession number KY322682) was grouped with GXY2 (EF546430.1), a very virulent MDV strain which caused acute tumors in CVI988 (Rispens) or HVT vaccinated chickens in China. Strains 500 (KY322683), 573 (KY322684), 578 (KY322685), 590 (KY322689), 754b (KY322689) and 755 (KT768121.1) were considered virulent MDV (vMDV). Strains 500 (KY322683) (Fig. 3), 754b (KY322689) and 755 (KT768121.1) were detected in chickens with severe peripheral nerve inflammatory disease. Strains 1042 (KY352470), 924-3 (KY322691) and 155 (KY322681) had identity with highly virulent MDV strains (vvMDV) (Fig. 2).
Natural Marek’s disease case by strain 500. (A)Note the enlarged left vagus (black arrow) below the proventriculus (thin arrow). (B)Enlarged left vagus (cervical region) along with the jugular vein (white arrow), with enlarged branches.
CONCLUSION
The increasing virulence of MDV may pose as a threat to the standard MD prevention strategy, progressively reducing the success of vaccine protection, especially for programs based on HVT strain vaccines. Research and continuing surveys may provide answers regarding the epidemiology of MD, the evolving virulence of circulating MDV strains, and might enable determining the best fit vaccination protocols and strategy.
ACKNOWLEDGEMENTS
The authors are indebted to CAPES, CNPq and FAPEMIG for financial support and scholarships.
REFERENCES
- Abreu DLC, Santos FF, José DS, Tortelly R, Nascimento ER, Pereira VLA. Pathological aspects of a subclinical marek's disease case in free-range chickens. Revista Brasileira de Ciência Avícola 2016;18(1):197-200.
- Atkins KE, Read AF, Savill NJ, Renz KG, Fakhrul Islam AFM,et al. Vaccination and reduced cohort duration can drive virulence evolution:Marek's disease virus and industrialized agriculture. Evolution: International Journal of Organic Evolution 2013;67(3):851-860.
- Baigent SJ, Davison F. Marek's disease virus: biology and life cycle. In: Marek's disease, an evolving problem. Amsterdam: Elsevier Academic Press; 2004. p.62-77.
- Baigent SJ, Kgosana LB, Gamawa AA, Smith LP, Read AF, Nair VK. Relationship between levels of very virulent MDV in poultry dust and in feather tips from vaccinated chickens. Avian Diseases 2013;52 (2-1):440-447.
- Baigent SJ, Ross LJN, Davison TF. Differential susceptibility to Marek's disease is associated with differences in number, but not phenotype or location, of pp38+ lymphocytes." Journal of General Virology 1998;79(11):2795-2802.
- Beasley JN, Patterson LT, McWade DH. Transmission of Marek's disease by poultry house dust and chicken dander. American Journal of Veterinary Research 1970;31:339-344.
- Benton WJ, Cover MS. The increased incidence of visceral lymphomatosis in broiler and replacement birds. Avian Diseases 1957;1:320-327.
- Biggs PM, Milne BS. Biological properties of a number of Marek's disease virus isolates. In: Biggs PM, Thé GF, Payne LN, editors. Oncogenesis and herpesviruses. Lyon: IARC; 1972. p.88-94.
- Biggs PM. Avian leukosis and Marek's disease. Proceedings of the Thirteenth World's Poultry Congress Symposium; 1966; Kiev, Russia; 1966. p.91-118.
- Biggs PM. Avian transmissible tumours. Proceedings 3rd Congress of the World Veterinary Poultry Association; 1965; Paris. p.61-67.
- Blume GR, Cardoso SP, Oliveira ML, Matiolli MP, Gómez SY, Reis Júnior JL, et al. Visceral Marek's disease in white-peafowl (Pavo cristatus). Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2016;68(6):1602-1608.
- Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology 1990;28(3):495-503.
- Brasil. Manual de Legislação. Programas Nacionais de Saúde Animal do Brasil. Secretaria de Defesa Agropecuária. Brasília (DF): Departamento de Saúde Animal. Ministério da Agricultura, Pecuária e Abastecimento; 2009.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução normativa nº 44 de 23 de agosto de 2001. Aprova as normas técnicas para o controle e a certificação de núcleos e estabelecimentos avícolas para a Micoplasmose Aviária (Mycoplasma gallisepticum, synoviae e melleagridis). Diário Oficial da União. Brasília, DF, p.68-70, de 24 de ago. de 2001, Seção I.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento, Gabinete do Ministro. Instrução Normativa nº 56, 4 dez. 2007.
- Brown AC, Smith LP, Kgosana L, Baigent SJ, Nair V, Allday MJ. Homodimerization of the meq viral oncoprotein is necessary for induction of t-cell lymphoma by Marek's disease virus. Journal of Virology 2009;83(21):11142-11151.
- Buscaglia C, Crosetti C. Studies on Marek's disease vaccination failures in Argentina. Proceedings of the 4th International Symposium on Marek's Disease and 2nd Meeting on the Cooperative Research Project in the Area of Veterinary Sciences between National University of La Plata and Japan International Cooperation Agency, 1993; La Plata, Argentina, p.19-21, 1993.
- Buscaglia C, Nervi P, Garbi JL, Piscopo M. Isolation of very virulent strains of Marek's disease virus from vaccinated chickens in Argentina. Proceedings of the Western Poultry Disease Conference; 1995; Sacramento; CA; 1995.
- Buscaglia C, Nervi P, Risso M. Characterization of four very virulent Argentinian strains of Marek's disease virus and the influence of one of those isolates on synergism between Marek's disease vaccine viruses. Avian Pathology 2004;33(2):190-195.
- Calnek BW, Hitchner SB. Localization of viral antigen in chickens infected with Marek's disease herpesvirus. Journal of the National Cancer Institute. 1969;43:935-949.
- Calnek BW, Alexander AM, Kahn DE. Feather follicle epithelium:a source of enveloped and infectious cell-free herpesvirus from Marek's disease. Avian Diseases 1970a;14:219-233.
- Calnek BW, Ubertini T, Adldinger HK. Viral antigen, virus particles, and infectivity of tissues from chickens with Marek's disease. Journal of the National Cancer Institute1970b;45:341-351.
- Calnek BW. Pathogenesis of Marek's disease virus infection. In: Hira K, editor. Current topics in microbiology and immunology. Berlin: Springer-Verlag; 2001. p.25-56.
- Cho BR, Kenzy SG. Isolation and characterization of an isolate (HN) of Marek's disease virus with low pathogenicity. Applied Microbiology 1972;24:299-306.
- Churchill AE, Biggs PM. Agent of Marek's disease in tissue culture. Nature 1967;215:528-530.
- Churchill AE, Chubb RC, Baxendale W. The attenuation, with loss of oncogenicity, of the herpes-type virus of Marek's disease (strain HPRS-16) on passage in cell culture. Journal of General Virology 1969;4(4):557-564.
- Cummings PJ, Rinaldo Jr CR. Coevolution of virulent virus and resistant cells as a mechanism of persistence of herpes simplex virus type 1 in a human T lymphoblastoid cell line. Journal of General Virology 1989;70:97-106.
- Davison F, Nair V. Use of Marek's disease vaccines:could they be driving the virus to increasing virulence? Expert Review of Vaccines 2005;4(1):77-88.
- De Boer GF, Groenendal JE, Oei HL, Pol JM. Protective efficacy of clones of Marek's disease virus strain CVI-988. Proceedings of the International Symposium on Marek's Disease; 1985; Kennett Square, PA: American Association of Avian Pathologists; 1985. p.531-544.
- Dunn JR, Auten K, Heidari M, Buscaglia C. Correlation between Marek's disease virus pathotype and replication. Avian Diseases 2014;58(2):287-292.
- Eidson CS, Page PK, Kleven SH. Effectiveness of cell-free or cell-associated turkey herpesvirus vaccine against Marek's disease in chickens as influenced by maternal antibody, vaccine dose and time of exposure to Marek's disease virus. Avian Diseases 1978:22:583-587.
- Eidson CS, Page RK, Kleven SH. In vivo and in vitro studies on the effect of gentamicin sulfate on the efficacy of the turkey herpesvirus vaccine. Poultry Science 1978;57(6):1519-1525.
- Fragnet L, Kut E, Rasschaert D. Comparative functional study of the viral telomerase RNA based on natural mutations. Journal of Biological Chemistry 2005;280(25):23502-23515.
- Gennart I, Coupeau D, Pejakovic´ S, Laurent S, Rasschaert D, Muylkens B. Marek's disease: genetic regulation of Gallid herpesvirus 2 infection and latency. The Veterinary Journal 2015;205:339-348.
- Hassanin O, Abdallah F, El-Araby IE. Molecular characterization and phylogenetic analysis of Marek's disease virus from clinical cases of Marek's disease in Egypt. Avian Diseases 2013;57:555-561.
- ICTV- International Committee on Taxonomy of Viruses. ICTV 9th report. Herpesviridae [cited 2018 Feb 16]. 2011. Available from: https://talk.ictvonline.org/ictv-reports/ictv_9th_report/dsdna-viruses-2011/w/dsdna_viruses/91/herpesviridae
» https://talk.ictvonline.org/ictv-reports/ictv_9th_report/dsdna-viruses-2011/w/dsdna_viruses/91/herpesviridae - Imai K, Yuasa N, Iwakiri H, Nakamura K, Hihara H, Ishita T, et al. Characterization of very virulent Marek's disease viruses isolated in Japan. Avian Pathology 1992;21(1):119-126.
- Jarosinski KW, Karsten Tischer B, Trapp S, Osterrieder N. Marek's disease virus: lytic replication, oncogenesis and control. Expert Review of Vaccines 2006;5(6):761-762.
- Jones D, Lee L, Liu JL, Kung HJ, Tillotson JK. Marek's disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. Proceedings of the National Academy of Sciences of the United States of America 1992;89(9):4042-4046.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 2016;33:1870-1874.
- Liu JL, Kung HJ. Marek's disease herpesvirus transforming protein MEQ:a c-Jun analogue with an alternative life style. Virus Genes 1999;21:51-64.
- López-Osorio S, Piedrahita D, Espinal-Restrepo MA, Ramírez-Nieto GC, Nair V, Williams SM, et al. Molecular characterization of Marek's disease virus in a poultry layer farm from Colombia. Poultry Science 2017;96(6):1598-1608.
- Lupiani, B, Lee LF, Cui X, Gimeno I, Anderson A, Morgan RW, et al. Marek's disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proceedings of the National Academy of Sciences 2004;32:11815-11820.
- Mohamed MHA, El-Sabagh IM, Al-Habeeb MA, Al-Hammady YM. Diversity of Meq gene from clinical Marek's disease virus infection in Saudi Arabia. Veterinary World 2016;9(6):572-578.
- McKimm-Breschkinn JL, Faragher JT, Withell J, Forsyth WM. Isolation of very virulent Marek's disease viruses from vaccinated chickens in Australia. Australian Veterinary Journal 1990;67:205-209.
- McPherson MC, Delany ME. Virus and host genomic, molecular, and cellular interactions during Marek's disease pathogenesis and oncogenesis. Poultry Science 2016;95(2):412-429.
- Murata S, Hashiguchi T, Hayashi Y, Yamamoto Y, Matsuyama-Kato Y, Takasaki S, et al. Characterization of Meq proteins from field isolates of Marek's disease virus in Japan. Infection Genetics and Evolution 2013;16:137-143.
- Nair V. Evolution of Marek's disease- A paradigm for incessant race between the pathogen and the host. Veterinary Journal 2005;170:175-183.
- Nair V. Latency and tumorigenesis in Marek's disease. Avian Diseases 2013;57:360-365.
- Nazerian K, Solomon JJ, Witter RL, Burmester BR. Studies on the etiology of Marek's disease. II. Finding of a herpesvirus in cell culture. Proceedings of the Society for Experimental Biology and Medicine 1968;127(1):177-182.
- Nikura M, Dodgson J, Cheng H. Direct evidence of host genome acquisition by the alphaherpesvirus Marek's disease virus. Archives Virology 2006;151:537-549.
- OIE. Marek's disease in OIE terrestrial manual [cited 2018 Ago 12]. Paris; 2016. Available from: http://www.oie.int/fileadmin/Home/eng/Healthstandards/tahm/2.03.13MAREK DIS.pdf
» http://www.oie.int/fileadmin/Home/eng/Healthstandards/tahm/2.03.13MAREK DIS.pdf - Okazaki W, Purchase HG, Burmester BR. Vaccination against Marek's disease: possible causes of failure of herpesvirus of turkeys (Strain FC-126) to protect chickens against Marek's disease. American Journal of Veterinary Research 1973;34:813-817.
- Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek's disease virus:from miasma to model. Nature Reviews Microbiology 2006;4(4):283.
- Parcells MS, Anderson AS, Morgan TW. Retention of oncogenicity by a Marek's disease virus mutant lacking six unique short region genes. Journal of Virology 1995;69(12):7888-7898.
- Parcells MS, Arumugaswami V, Prigge JT, Pandya K, Dienglewicz RL. Marek's disease virus reactivation from latency:changes in gene expression at the origin of replication. Poultry Science 2003;82(6):893-898.
- Powell PC, Lombardini F. Marek's disease: a worldwide problem. World's Poultry Science 1986;42:205-218.
- Read AF, Baigent SJ, Powers C, Kgosana LB, Blackwell L, Smith LP, et al. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLOS Biology 2015;13(7):198-200.
- Rispens BH, Van Vloten H, Mastenbroek N, Maas HJ, Schat KA. Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Diseases 1972;16:108-125.
- Ross LJN. Recombinant vaccines against Marek's disease. Avian Pathology 1999;27:65-73.
- Saitou N, Nei N. The neighbor-joining method:A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 1987;4:406-425.
- Schat KA, Calnek BW. Characterization of an apparently nononcogenic Marek's disease virus. Journal of the National Cancer Institute 1978;60:1075-1082.
- Schat KA, Calnek BW, Fabricant J, Abplanalp H. Influence of oncogenicity of Marek's disease virus on evaluation of genetic resistance. Poultry Science 1981;60(12):2559-2566.
- Shamblin CE, Greene N, Arumugaswami V, Dienglewicz RL, Parcells MS. Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Veterinary Microbiology 2004;102:147-167.
- Silva RF, Dunn JR, Cheng HH, Niikura M. A MEQ-deleted Marek's disease virus cloned as a bacterial artificial chromosome is a highly efficacious vaccine. Avian Diseases 2010;54(2):862-869.
- Silveira P, Marin SY, Moreira PA, Tocantins BB, Lacorte G, Paixão TA, et al. Interactions of Plasmodium juxtanucleare and chicken anaemia virus: establishing a model. Parasitology 2013;1:1-12.
- Smith J. Superinfection drives virulence evolution in experimental populations of bacteria and plasmids. Evolution 2011;65(3):831-841.
- Solomon JJ, Witter RL, Nazerian K, Burmester BR. Studies on the etiology of Marek's disease. I. Propagation of the agent in cell culture. Proceedings of the Society for Experimental Biology and Medicine 1968;127(1):173-177.
- Sousa E. Registro da doença de Marek, Leucose aviária e doença Infecciosa da bolsa na Região do Triângulo Mineiro, no período de 1999 a 2003. PUBVET 2010;4:27.
- Sung HW. Recent increase of Marek's disease in Korea related to the virulence increase of the virus. Avian Diseases 2002;46:517-524.
- Teng LQ, Wei P, Song ZB, He JJ, Cui ZZ. Molecular epidemiological investigation of Marek's disease virus from Guangxi, China. Archives of Virology 2011;156:203-206.
- Trapp S. A virus-encoded telomerase RNA promotes malignant T cell lymphomagenesis. The Journal of Experimental Medicine 2006;203:1307-1317.
- Wajid SJ, Katz ME, Renz KG, Walkden-Brown SW. Prevalence of Marek's disease virus in different chicken populations in Iraq and indicative virulence based on sequence variation in the EcoRI-Q (meq) gene. Avian Diseases 2013;57(2s1):562-568.
- Witter RL, Schat KA. Marek's disease. In: Saif Y M, Barnes H J, Fadly A M, Glisson J R, Mcdougald L R, Swayne E, editors. Diseases of poultry. 11th ed. Ames: Iowa State University Press; 2003. p.407-465.
- Witter RL. Increased virulence of Marek's disease virus field isolates. Avian Diseases 1997;41:149-163.
- Witter RL, Nazerian K, Purchase HG, Burgoyne GH. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. American Journal of Veterinary Research 1970;31:525-538.
- Witter RL, Calnek BW, Buscaglia C, Gimeno IM, Schat KA. Classification of Marek's disease viruses according to pathotype:philosophy and methodology. Avian Pathology 2005:34(2):75-90.
- Witter RL, Schat KA. Marek's diseases. In: Saif YM, editor. Diseases of poultry. Ames: Blackwell Publishing; 2003. chapter 15, p.407-465.
- Wozniakowski G, Samorek-Salamonowicz E, Kozdrun W. Sequence analysis of meq oncogene among Polish strains of Marek's disease. Polish Journal of Veterinary Sciences 2010;13:263.
- Wozniakowski G, Samorek-Salamonowicz E, Kozdrun W. Molecular characteristics of Polish field strains of Marek's disease herpesvirus isolated from vaccinated chickens. Acta Veterinaria Scandinavica 2011;53(1):10.
- Wozniakowski G, Samorek-Salamonowicz E. Molecular evolution of Marek's disease virus (MDV) field strains in a 40-year time period. Avian Diseases 2014;58(4):550-557.
- Yu ZH, Teng M, Luo J, Wang XW, Ding K, Yu LL, et al. Molecular characteristics and evolutionary analysis of field Marek's disease virus prevalent in vaccinated chicken flocks in recent years in China. Virus Genes 2013;47:282-291.
- Zanella A, Dall'ara P, Lavazza A, Marchi R, Morena MA, Rampin T. Interaction between Marek's disease vírus and chicken infectious anemia virus. In: Schat KA, Morgan RM, Parcells MS, Spencer JL., editors. Current progress on Marek's disease research. Kennett Square: American Association of Avian Pathologists; 2001. p.1119.
- Zhang Y-p, Chang-jun L, Feng Z, Weisong S, Jingmei L. Sequence analysis of the Meq gene in the predominant Marek's disease virus strains isolated in China during 2006-2008. Virus Genes 2011;43(3):353.
- Zhang YP, Li ZJ, Bao KY, Lv HC, Gao YL, Gao HL, et al. Pathogenic characteristics of Marek's disease virus field strains prevalent in China and the effectiveness of existing vaccines against them. Veterinary Microbiology 2015;177(1-2):62-68.
Publication Dates
-
Publication in this collection
09 May 2019 -
Date of issue
Jan-Mar 2019
History
-
Received
27 Aug 2018 -
Accepted
05 Nov 2018