ABSTRACT
We investigated the egg production, changes in luteinizing hormone (LH), follicle-stimulating hormone (FSH), gonadal hormones, and their mRNA levels in the hypothalamic-pituitary-gonadal axis of White King pigeons submitted to different photoperiods. The treatments consisted of three photoperiods (8 h light (L):16 h dark (D), 12L:12D, and 16L:8D), with three replicates of twelve pairs of adult pigeons. The birds were exposed the photoperiods for 45 days. Egg production performance was recorded daily. Six pigeon pairs per replicate were selected for plasma collection, and six pigeon pairs per replicate for the resection of the hypothalamic-pituitary-gonadal (HPG) axis. Egg production was significantly improved by long-day lighting (16L:8D), while no differences in egg shape index were detected. Higher average egg weight was obtained in 16L:8D group, whereas broken egg percentage was higher in the 8L:16D group. Female LH level was significantly higher in long-day lighting, and the FSH level significantly lower in short-day lighting. The females in the 16L:8D group had higher estrogen level. The photoperiods had a minor effect on plasma LH and testosterone in males, whereas the FSH level was significantly higher in the 16L:8D group. The level of LH mRNA expression was higher in both females and males of the 16L:8D group. Similar trends in FSH mRNA expression observed in both females and males. The 16L:8D photoperiod not only improved egg production, but also stimulated plasma LH, FSH, gonadal hormones, and promoted LH and FSH mRNA expression in pigeons.
Keywords:
Day lighting; expression; hormone; pigeon; reproduction
INTRODUCTION
Light plays an important role on the reproductive performance of poultry. Artificial light is widely applied in the poultry industry to increase production performance. Siopes (2007Siopes TD. Lighting for summer egg production by turkeys: day length and light intensity. Poultry Science 2007;86: 2413-2419.) suggested that increasing day length in the summer improves egg production in turkeys. Wang et al. (2002Wang SD, Jan DF, Yeh LT, Wu GC, Chen LR. Effect of exposure to long photoperiod during the rearing period on the age at first egg and the subsequent reproductive performance in geese. Animal Reproduction Science 2002;73:227-234.) also reported higher egg production in geese submitted to 14 h light (L) and 18L than in those exposed to natural lighting. Short-day lighting may delay egg production in chickens and turkeys (Yang et al., 1999Yang J, Long DW, Bacon WL. Ontogeny of luteinizing hormone and estradiol secretion in turkey hens exposed to different photoperiods from hatch to sixty weeks of age. Poultry Science 1999;78:1611-1618.; Noddegaard et al., 2000Noddegaard F, Talbot RT, Sharp PJ. Effect of delayed step-up lighting on plasma luteinizing hormone and reproductive function in broiler breeders. Poultry Science 2000;79:778-783.). Furthermore, Dobie et al. (1946Dobie JB, Carver JS, Roberts J. Poultry lighting for egg production. Washington Experimental Station Bulletin 1946;471:1-27.) concluded that at least 13 h are needed for maximum egg production. On the other hand, Stokkan & Sharp (1984Stokkan KA, Sharp PJ. The development of photorefractoriness in castrated willow ptarmigan (Lagopus lagopuslagopus). General and Comparative Endocrinology 1984;54:402-408.) indicated that the first day of lay was delayed as the photoperiodic drive progressively declined with increased time of exposure to long days (Follett, 1988Follett BK. Refractoriness in quail leads to a reduction in the photoperiodic drive on LH secretion. The Journal of Endocrinology 1988;116:363-366.). Leighton & Shoffner (1989Leighton AT, Shoffner RN. Effect of light regime and age on reproduction of turkeys. 1. Effect of 15, 24 hour and restricted light treatment. Poultry Science 1989;40:861-870.) found that exposure to light for more than 16 h per day yielded no improvement in production. Furthermore, exposure to 24 h or 22 h of light per day yielded no improvements in egg production of hens (McCartney et al., 1961McCartney MG, Sanger VL, Brown KI, Chamberlin VD. Photoperiodism as a factor in the reproduction of the turkey. Poultry Science 1961;40:368-376.).
Sharp (1993Sharp PJ. Photoperiod control of reproduction in the domestic hen. Poultry Science 1993;72:897-905.) concluded that the photoperiod is the most important factor that affects the concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Dittami et al., 1985Dittami JP, Goldsmith AR, Follett BK. Seasonal changes in follicle-stimulating hormone in a breeding population of barheaded geese, Anser indicus. General and Comparative Endocrinology 1985;57:195-197.; Dawson et al., 2001Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. Journal of Biological Rhythms 2001;16:365-380.). The secretion of FSH and LH controls the secretion of estradiol (E2), which is essential for development of the oviduct and follicle (Bacon et al., 1980Bacon WL, Brown KI, Musser MA. Changes in plasma calcium, phosphorus, lipids and estrogens in turkey hens with reproductive state. Poultry Science 1980;59:444-452.). Photo-induced changes in FSH or LH are indicators of ovarian development (Lewis et al., 1999Lewis PD, Perry GC, Morris TR, Douthwaite JA. Effect of timing and size of photoperiod change on plasma FSH concentration and the correlation between FSH and age at first egg in pullets. British Poultry Science 1999;40:380-384.), and estrogen activates the response of the reproductive neuroendocrine system to photostimulation (Dunn et al., 2003Dunn IC, Lewis PD, Wilson PW and Sharp PJ. Acceleration of maturation of FSH and LH responses to photostimulation in prepubertal domestic hens by oestrogen. Reproduction 2003;126:217-225.). All these hormones are critical for follicular development, and are regulated by the photoperiod. Artificial illumination is widely used in the poultry industry, whereas little is known about its application in the pigeon industry. This study was aimed at determining the optimal photoperiod required to maximize egg production, and at elucidating changes in plasma LH, FSH, gonadal hormones, and LH and FSH expressions in the hypothalamus-pituitary-gonadal (HPG) axis of pigeons submitted to different photoperiods.
MATERIALS AND METHODS
Birds and management
Eighty-one pairs of adult White King pigeons were obtained from the Tangshan Cuigu pigeon company, Nanjing Province, China. The experimental treatments consisted of three photoperiods (16 h L:8 h dark (D), 12L:12D, or 8L:16D), with three replicates of 12 pairs each. Photoperiods were provided in three separate compartments, using illuminance of 18-W incandescent tubes of approximately 20 lux. The birds were housed in pairs, and the experiment lasted 45 days. During the experiment, the eggs were collected immediately after lay, and the pigeons entered the next egg-laying period, and the eggs were artificially incubated.
Reproductive performance
Egg production, percentage of broken eggs, average egg weight, and egg shape index (ESI) of the three groups were recorded daily. Egg shape index was calculated using the following equation: ESI = (width/length) × 100 (Anderson et al., 2004Anderson KE, Tharrington JB, Curtis PA, Jones FT. Shell characteristics of eggs from historic strains of single comb white leghorn chickens and the relationship of egg shape to shell strength. International Journal of Poultry Science 2004;3:17-19.).
Plasma samples
Six pigeon pairs (six females and six males) per replicate were selected and their blood was collected from the wing vein between 09:00 and 10:00 h using a 1-mL syringe, after which a dry cotton ball was applied to the site to ensure hemostasis. Blood was collected in tubes containing sodium heparin, and were centrifuged at 4°C for 5 min at 3000 × g. Plasma samples were stored at −20°C for hormone analyses.
Hormones analyses
The plasma concentrations of FSH, LH, estrogen (E2), and testosterone (T)were determined using radioimmunoassay and radioimmunoassay kits (catalog numbers B03PJB, B04PJ, B05PJB, and B10PJB; Beijing North Institute of Biological Technology, Beijing, China). The detection limits for LH, FSH, E2 and T were 200 mIU/mL,100mIU/mL, 4 ng/mL, and 20ng/mL, respectively. Each assay run included four internal and external qualitatively-controlled samples. The mean intra- and inter-assay CV of hormones were between 2% and 6%, respectively. The normal hormone level ranges specified by the respective kits were used as reference (Cao et al., 2008Cao J, Liu W, Wang Z, Xie D, Jia, Chen Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. Journal of Applied Poultry Research 2008;17:211-218.).
Tissue collection
Six bird pairs per treatment (n=36) were randomly selected, anesthetized with sodium pentobarbital, and sacrificed. Their HPG axes were resected, immediately frozen in liquid nitrogen, and stored at −80 °C for subsequent analyses.
Total RNA was extracted and reverse transcribed using the Fast Quant RT Kit (Tiangen Biotech Co., Ltd, Beijing, China). Pigeon glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. The expression levels of the FSH and LH genes were detected using real-time PCR, and primers were designed based on the coding region of the respective target gene (Table 1). Super Real PreMix (Tiangen Biotech Co., Ltd., Beijing, China) was used to detect the expression of FSH and LH according to the manufacturer’s protocol. Furthermore, assays were independently repeated three times. The results are expressed relative to the expression levels of GAPDH in each sample using the 2−ΔΔCT method (Livak & Schmittge, 2001Livak KJ, Schmittge TD. Analysis of relative gene expression data using real-time quantitative PCR and the2-??CT method. Methods 2001;25:402-408.).
Statistical analysis
All data are expressed as mean ± standard deviation and analyzed by one-way analysis of variance (ANOVA) using the software SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The least significant difference post-hoc multiple comparisons test was used to estimate statistical significance of differences, which was set at p<0.05.
RESULTS
Egg production
The egg production of pigeon pairs exposed to the evaluated photoperiods is presented in Table 2. Egg production in pairs submitted to long-day lighting (16L:8D) was higher than that in those of the 12L:12D (p=0.002), whereas egg production in the short-day lighting (8L:16D) was lower than that in the 12L:12D (p=0.021). Average egg weight tended to be higher in the 12L:12D and 16L:8D treatments compared with 8L:16D (p=0.231 and 0.215, respectively). Although the percentage of broken eggs tended to be higher in the 8L:16D group in comparison with the 16L:8D and 12L:12D groups, these differences were not significant (p=0.093 and 0.122, respectively). ESI was similar among the different groups. The long-day photoperiod significantly improved egg production with minor effect on egg quality.
Hormone analyses
The plasma concentrations of FSH, LH, and E2 in females (Table 3) were markedly increased in the long-day photoperiod group (16L:8D). In the 16L:8D group, LH level was almost 2-fold compared with the 8L:16D and 12L:12D groups (p<0.001 and p<0.001, respectively). The concentrations of FSH were similar between the 12L:12D and 16L:8D (p=0.522), but both were significantly higher than that in the short-day photoperiod group (p=0.001 and 0.001, respectively). No significant differences in E2 concentration were observed between the 8L:16D and 12L:12D groups (p=0.233); however, the 16L:8D group showed higher E2 level than the 8L:16D group (p=0.043). The evaluated photoperiods did not influence LH and T levels measured in males (Table 4). However, higher FSH level was observed in the 16L:8D group compared with the 8L:16D and 12L:12D groups (p=0.003 and 0.005, respectively).
LH and FSH expression analysis
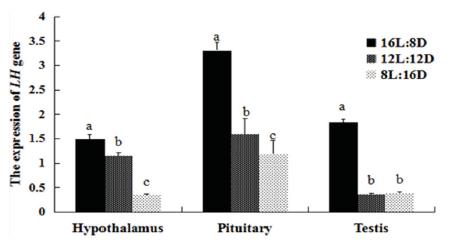
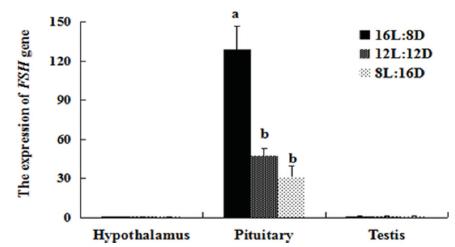
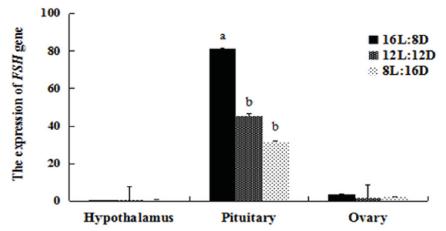
The expression of LH in the HPG axis was affected by the photoperiods (Figs. 1 and 2). The LH mRNA level was highest in the HPG axis of both females and males of the 16L:8D group. The LH mRNA levels in the hypothalamus of females were lower in the 12L:12D group than in the 16L:8D group (p=0.001), showed an almost 2-fold increase in the 8L:12D group, while no differences were detected among treatments in males. Similar LH expression in the pituitary gland of females were detected in the 12L:12D and 8L:12D groups (p=0.459); whereas in males, lower expression was determined in the 8L:12D group compared with the 12L:12D group (p=0.004). In both the ovary and testis, higher LH expression was determined in the 16L:8D group than in the other groups. However, in the gonads, the 8L:12D photoperiod determined higher LH expression than 12L:12D (p=0.022), but no significant differences in the testis of these groups were detected (p=0.581). We also examined the expression of FSH (Figs. 3 and 4), and found that FSH rhythms were similar in both females and males. In the hypothalamus and gonads, FSH expression was not significantly different among treatments. However, in the pituitary gland, FSH mRNA levels were higher in the 16L:8D group, but similar in the 12L:12D and 8L:12D groups (p=0.073 and 0.162, respectively). The 16L:8D photoperiod evidently promoted higher LH and FSH expression than the other photoperiods under investigation.
Relative (2-ΔΔCT) expression levels of LH in the HPG axis of females under various photoperiods, obtained after normalization to GAPDH. Values marked with different letters on the bars are significantly different (p<0.05).
Relative (2-ΔΔCT) expression levels of LH in the HPG axis of males under various photoperiods, obtained after normalization to GAPDH. Values marked with different letters on the bars are significantly different (p<0.05).
Relative (2-ΔΔCT) expression levels of FSH in the HPG axis of females under various photoperiods, obtained after normalization to GAPDH. Values marked with different letters on the bars are significantly different (p<0.05).
Relative (2-ΔΔCT) of expression levels of FSH in the HPG axis of males under various photoperiods, obtained after normalization to GAPDH. Values marked with different letters on the bars are significantly different (p<0.05).
DISCUSSION
Our results suggest that the longer daily photoperiod significantly improved egg production in pigeons, in agreement with Siopes (2007Siopes TD. Lighting for summer egg production by turkeys: day length and light intensity. Poultry Science 2007;86: 2413-2419.) and Wang et al. (2002Wang SD, Jan DF, Yeh LT, Wu GC, Chen LR. Effect of exposure to long photoperiod during the rearing period on the age at first egg and the subsequent reproductive performance in geese. Animal Reproduction Science 2002;73:227-234.). However, McCartney et al. (1961McCartney MG, Sanger VL, Brown KI, Chamberlin VD. Photoperiodism as a factor in the reproduction of the turkey. Poultry Science 1961;40:368-376.) found that 24 h or 22 h of light per day did not improve egg production in laying chickens. Leighton & Shoffner (1989Leighton AT, Shoffner RN. Effect of light regime and age on reproduction of turkeys. 1. Effect of 15, 24 hour and restricted light treatment. Poultry Science 1989;40:861-870.) reported that exposure to light for more than 16 h per day yielded no further improvements in turkey egg production. It should be mentioned that the optimal daily photoperiod is difficult to measure. Wang et al. (2009) showed that supplementary lighting, with a total daily photoperiod of between 12.0 h and 13.5 h, improved egg production and the peak of lay in geese. Dobie et al. (1946Dobie JB, Carver JS, Roberts J. Poultry lighting for egg production. Washington Experimental Station Bulletin 1946;471:1-27.) concluded that a photoperiod of at least 13 h was needed to maximize egg production in laying chickens, whereas in our study, 16 h maximized egg production in pigeons.
Ciacciariello & Gous (2005Ciacciariello M, Gous RM. A comparison of the effects of feeding treatments and lighting on age at first egg and subsequent laying performance and carcase composition of broiler breeder hens. British Poultry Science 2005;46:246-254.) reported that increasing day length from 8 to 10 h yielded heavier mean egg weight in broiler breeders. Furthermore, Wang et al. (2002Wang SD, Jan DF, Yeh LT, Wu GC, Chen LR. Effect of exposure to long photoperiod during the rearing period on the age at first egg and the subsequent reproductive performance in geese. Animal Reproduction Science 2002;73:227-234.) indicated that shifting the photoperiod back to 10L:14D increased egg weight in geese. Lewis et al. (1997Lewis PD, Perry GC, Morris TR. Effect of size and timing of photoperiod increase on age at first egg and subsequent performance of two breeds of laying hen. British Poultry Science 1997;38:142-150.) found that a longer photoperiod could reduce egg weight in laying hens, whereas daily photoperiod had a minor influence on pigeon egg weight in the present experiment, which is in agreement with the findings of Siopes (2007Siopes TD. Lighting for summer egg production by turkeys: day length and light intensity. Poultry Science 2007;86: 2413-2419.).
Harms et al. (1990Harms RH, Rossi AF, Sloan DR, Miles RD, Christmas RB. A method for estimating shell weight and correcting specific gravity for egg weight in eggshell quality studies. Poultry Science 1990;69:48-52.) suggested that eggshell thickness and egg size are strongly correlated, and Anderson et al. (2004Anderson KE, Tharrington JB, Curtis PA, Jones FT. Shell characteristics of eggs from historic strains of single comb white leghorn chickens and the relationship of egg shape to shell strength. International Journal of Poultry Science 2004;3:17-19.) found that eggshell quality depends on egg weight and egg size. Van Tienhoven & Ostrander (1973Van Tienhoven A, Ostrander CE. The effect of interruption of the dark period at different intervals on egg production and shell breaking strength. Poultry Science 1973;52: 998-1001.) suggested that light regimes had no effect on eggshell breaking strength, which is consistent with our findings. The shapes of pigeon eggs were similar among the different photoperiods, indicating that the ESI was within the normal range (Altuntaş & Şekeroğlu, 2008). Breaking strength is correlated with ESI (Carter, 1976Carter TC. The hen's egg: Shell forces at impact and quasi-static compression. British Poultry Science 1976;17:199-214.), and this partially explains the similar percentage of broken eggs across the various groups in our study.
When Urbanski & Follett (1982Urbanski HF, Follett BK. Sexual differentiation of the photoperiodic response in Japanese quail. The Journal of Endocrinology 1982;92:279-282.) adjusted the photoperiod of Japanese quails from 8L:16D to 16L:8D, LH secretion was significantly increased, which is consistent with our results. Lewis et al. (2007Lewis PD, Gous RM, Ghebremariam WK, Sharp PJ. Broiler breeders do not respond positively to photoperiodic increments given during the laying period. British Poultry Science 2007;48:245-252.) also reported that transferring broilers from 11-h to 16-h photoperiods could increase plasma LH concentration. Dunn & Sharp (1990Dunn IC, Sharp PJ. Photoperiodic requirements for LH release in juvenile broiler and egg-laying strains of domestic chickens fed ad libitum or restricted diets. Journal of Reproduction and Fertility 1990;90:329-335.) concluded that the shortest saturation day length to stimulate LH release in egg-laying birds is between 12.75 h and 15.25 h, which is longer than that required in broilers (Scanes et al., 1980Scanes CG, Van Middelkoop JH, Sharp PJ, Harvey S. Strain differences in the blood concentrations of luteinizing hormone, prolactin, and growth hormone in female chickens. Poultry Science 1980;59:159-163.). This partially explains why LH levels between 12-h and 8-h photoperiods were similar, whereas the 16-h photoperiod significantly increased LH concentration in pigeons. Prolonged exposure to a stimulatory photoperiod was found to reduce plasma FSH concentration (Ciccone et al., 2005Ciccone NA, Sharp PJ, Wilson PW, Dunn IC. Changes in reproductive neuroendocrine mRNAs with decreasing ovarian function in ageing hens. General and Comparative Endocrinology 2005;144:20-27.), differently from the results obtained in the present study. Studies have shown that 3 to 7 d of photostimulation increased E2 circulating levels (Bacon et al., 1980Bacon WL, Brown KI, Musser MA. Changes in plasma calcium, phosphorus, lipids and estrogens in turkey hens with reproductive state. Poultry Science 1980;59:444-452.; Buchanan et al., 2000Buchanan S, Robertson GW, Hocking PM. Development of the reproductive system in turkeys with a high or low susceptibility to prolapse of the oviduct. Poultry Science 2000;79:1491-1498.). Pageaux et al. (1984Pageaux JF, Lagier C, Pal D, Pache H. Development of the oviduct in quail during sexual maturation in relation to plasma concentrations of oestradiol and progesterone. The Journal of Endocrinology 1984;100:167-173.) suggested that E2 and progesterone levels increased during long days compared to short days. In our study, the daily photoperiod increased the concentration of E2; however, Yang et al. (1999Yang J, Long DW, Bacon WL. Ontogeny of luteinizing hormone and estradiol secretion in turkey hens exposed to different photoperiods from hatch to sixty weeks of age. Poultry Science 1999;78:1611-1618.) demonstrated that the concentration of this hormone remained stable in turkey hens exposed to different photoperiods.
Follett & Maung (1978Follett BK, Maung SL. Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial photoperiods and to natural day lengths. The Journal of Endocrinology 1978;78:267-280.) indicated that testicular growth was directly related to plasma FSH level, but not to LH level. Photoperiods evidently affect plasma FSH concentrations in males, but have only a minor effect on LH concentration. Our results showed that photoperiod had no significant effect on plasma T concentration, in contrast to the findings of Tamarkin et al. (1976Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormone in the syrian hamster. Endocrinology 1976;99:1528-1533.) and Jasnow et al. (2000Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Hormones and Behavior 2000;38:102-110.), who reported that short days decreased plasma T concentration in hamsters. The longer photoperiod stimulated LH, FSH, and E2 concentrations in females, indicating it may improve their reproductive performance.
The expressions of LH and FSH in the HPG axis during a 16-h photoperiod was higher than those observed during other daily photoperiods, which is consistent with the trend observed in plasma LH and FSH concentrations. Kobayashi et al. (2004Kobayashi M, Ito T, Ishii S, Wada M. Seasonal change in luteinizing hormone subunit mRNA in Japanese quail and effects of short day length and low temperature. General and Comparative Endocrinology 2004;139:38-47.) reported similar findings. Kubokawa et al. (1994Kubokawa K, Ishii S, Wingfield JC. Effect of day length on luteinizing hormone b subunit mRNA and subsequent gonadal growth in the White-crowned sparrow, Zonotrichia leucophrys Gambelii. General and Comparative Endocrinology 1994;95:42-51.) reported that the photoperiod significantly stimulated pituitary LH mRNA expression in white-crowned sparrows. In addition, Hellqvist et al. (2008Hellqvist A, Schmitz M, Borg B. Effects of castration and androgen-treatment on the expression of FSH-? and LH-? in the three-spine stickleback, gasterosteus aculeatus-Feedback differences mediating the photoperiodic maturation response? General and Comparative Endocrinology 2008;158:178-182.) concluded that the expression of FSHb in the pituitary gland was higher in sham-operated fish kept under a long photoperiod than under a short photoperiod (Hellqvist et al., 2004). In the present study, FSH mRNA was expressed mainly in the pituitary gland, as FSH is secreted by the anterior pituitary (Mashiter et al., 1981Mashiter K, Adams E, Noorden S. Secretion of LH, FSH and PRL shown by cell culture and immunocytochemistry of human functionless pituitary adenomas. Clinical Endocrinology 1981;15:103-112.) and FSH-producing cells are located in distinct areas of the pituitary gland (Proudman et al., 1999Proudman JA, Vandesande F, Berghman LR. Immunohistochemical evidence that follicle-stimulating hormone and luteinizing hormone reside in separate cells in the chicken pituitary. Biology of Reproduction 1999;60:1324-1328.). Gonadal maturation and follicular selection in females are promoted by LH and FSH, and during follicular development, granulosa cells respond to both FSH and LH (Richards et al., 1987Richards JS, Jahnsen T, Hedin L, Lifka J, Ratoosh S, Durica JM, et al. Ovarian follicular development: from physiology to molecular biology. Recent Progress in Hormone Research 1987;43:231-276.). The pituitary gland secretes LH and FSH, both of which play a key role in follicular development that is closely related to egg production in pigeons.
In conclusion, our research shows that egg production in pigeons is improved by a 16-h photoperiod, and that it has minor effects on egg weight and ESI. The 16-h photoperiod also increases plasma LH, FSH, E2, and T concentrations. Furthermore, FSH and LH mRNA expression levels in the HPG axis is affected by differences in the photoperiod. Thus, it is suggested that the 16L:8D photoperiod may maximize the reproductive performance of pigeons.
ACKNOWLEDGMENTS
This study was funded by the National Science Foundation for Young Scientists of China (grant No. 31702155) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD; Jiangsu, China).
REFERENCES
- Altuntas E, Sekeroglu A. Effect of egg shape index on mechanical properties of chicken eggs. Journal of Food Engineering 2008;85:606-612.
- Anderson KE, Tharrington JB, Curtis PA, Jones FT. Shell characteristics of eggs from historic strains of single comb white leghorn chickens and the relationship of egg shape to shell strength. International Journal of Poultry Science 2004;3:17-19.
- Bacon WL, Brown KI, Musser MA. Changes in plasma calcium, phosphorus, lipids and estrogens in turkey hens with reproductive state. Poultry Science 1980;59:444-452.
- Buchanan S, Robertson GW, Hocking PM. Development of the reproductive system in turkeys with a high or low susceptibility to prolapse of the oviduct. Poultry Science 2000;79:1491-1498.
- Cao J, Liu W, Wang Z, Xie D, Jia, Chen Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. Journal of Applied Poultry Research 2008;17:211-218.
- Carter TC. The hen's egg: Shell forces at impact and quasi-static compression. British Poultry Science 1976;17:199-214.
- Ciacciariello M, Gous RM. A comparison of the effects of feeding treatments and lighting on age at first egg and subsequent laying performance and carcase composition of broiler breeder hens. British Poultry Science 2005;46:246-254.
- Ciccone NA, Sharp PJ, Wilson PW, Dunn IC. Changes in reproductive neuroendocrine mRNAs with decreasing ovarian function in ageing hens. General and Comparative Endocrinology 2005;144:20-27.
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. Journal of Biological Rhythms 2001;16:365-380.
- Dittami JP, Goldsmith AR, Follett BK. Seasonal changes in follicle-stimulating hormone in a breeding population of barheaded geese, Anser indicus. General and Comparative Endocrinology 1985;57:195-197.
- Dobie JB, Carver JS, Roberts J. Poultry lighting for egg production. Washington Experimental Station Bulletin 1946;471:1-27.
- Dunn IC, Sharp PJ. Photoperiodic requirements for LH release in juvenile broiler and egg-laying strains of domestic chickens fed ad libitum or restricted diets. Journal of Reproduction and Fertility 1990;90:329-335.
- Dunn IC, Lewis PD, Wilson PW and Sharp PJ. Acceleration of maturation of FSH and LH responses to photostimulation in prepubertal domestic hens by oestrogen. Reproduction 2003;126:217-225.
- Follett BK, Maung SL. Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial photoperiods and to natural day lengths. The Journal of Endocrinology 1978;78:267-280.
- Follett BK. Refractoriness in quail leads to a reduction in the photoperiodic drive on LH secretion. The Journal of Endocrinology 1988;116:363-366.
- Harms RH, Rossi AF, Sloan DR, Miles RD, Christmas RB. A method for estimating shell weight and correcting specific gravity for egg weight in eggshell quality studies. Poultry Science 1990;69:48-52.
- Hellqvist A, Bornestaf C, Borg B, Schmitz M. Cloning and sequencing of the FSH-? and LH ?-subunit in the three-spined stickleback, Gasterosteus aculeatus, and effects of photoperiod and temperature on LH-? and FSH-? mRNA expression. General and Comparative Endocrinology 2004;135:167-174.
- Hellqvist A, Schmitz M, Borg B. Effects of castration and androgen-treatment on the expression of FSH-? and LH-? in the three-spine stickleback, gasterosteus aculeatus-Feedback differences mediating the photoperiodic maturation response? General and Comparative Endocrinology 2008;158:178-182.
- Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Hormones and Behavior 2000;38:102-110.
- Kobayashi M, Ito T, Ishii S, Wada M. Seasonal change in luteinizing hormone subunit mRNA in Japanese quail and effects of short day length and low temperature. General and Comparative Endocrinology 2004;139:38-47.
- Kubokawa K, Ishii S, Wingfield JC. Effect of day length on luteinizing hormone b subunit mRNA and subsequent gonadal growth in the White-crowned sparrow, Zonotrichia leucophrys Gambelii. General and Comparative Endocrinology 1994;95:42-51.
- Livak KJ, Schmittge TD. Analysis of relative gene expression data using real-time quantitative PCR and the2-??CT method. Methods 2001;25:402-408.
- Leighton AT, Shoffner RN. Effect of light regime and age on reproduction of turkeys. 1. Effect of 15, 24 hour and restricted light treatment. Poultry Science 1989;40:861-870.
- Lewis PD, Perry GC, Morris TR. Effect of size and timing of photoperiod increase on age at first egg and subsequent performance of two breeds of laying hen. British Poultry Science 1997;38:142-150.
- Lewis PD, Perry GC, Morris TR, Douthwaite JA. Effect of timing and size of photoperiod change on plasma FSH concentration and the correlation between FSH and age at first egg in pullets. British Poultry Science 1999;40:380-384.
- Lewis PD, Gous RM, Ghebremariam WK, Sharp PJ. Broiler breeders do not respond positively to photoperiodic increments given during the laying period. British Poultry Science 2007;48:245-252.
- Mashiter K, Adams E, Noorden S. Secretion of LH, FSH and PRL shown by cell culture and immunocytochemistry of human functionless pituitary adenomas. Clinical Endocrinology 1981;15:103-112.
- McCartney MG, Sanger VL, Brown KI, Chamberlin VD. Photoperiodism as a factor in the reproduction of the turkey. Poultry Science 1961;40:368-376.
- Noddegaard F, Talbot RT, Sharp PJ. Effect of delayed step-up lighting on plasma luteinizing hormone and reproductive function in broiler breeders. Poultry Science 2000;79:778-783.
- Pageaux JF, Lagier C, Pal D, Pache H. Development of the oviduct in quail during sexual maturation in relation to plasma concentrations of oestradiol and progesterone. The Journal of Endocrinology 1984;100:167-173.
- Proudman JA, Vandesande F, Berghman LR. Immunohistochemical evidence that follicle-stimulating hormone and luteinizing hormone reside in separate cells in the chicken pituitary. Biology of Reproduction 1999;60:1324-1328.
- Richards JS, Jahnsen T, Hedin L, Lifka J, Ratoosh S, Durica JM, et al. Ovarian follicular development: from physiology to molecular biology. Recent Progress in Hormone Research 1987;43:231-276.
- Scanes CG, Van Middelkoop JH, Sharp PJ, Harvey S. Strain differences in the blood concentrations of luteinizing hormone, prolactin, and growth hormone in female chickens. Poultry Science 1980;59:159-163.
- Sharp PJ. Photoperiod control of reproduction in the domestic hen. Poultry Science 1993;72:897-905.
- Siopes TD. Lighting for summer egg production by turkeys: day length and light intensity. Poultry Science 2007;86: 2413-2419.
- Stokkan KA, Sharp PJ. The development of photorefractoriness in castrated willow ptarmigan (Lagopus lagopuslagopus). General and Comparative Endocrinology 1984;54:402-408.
- Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormone in the syrian hamster. Endocrinology 1976;99:1528-1533.
- Urbanski HF, Follett BK. Sexual differentiation of the photoperiodic response in Japanese quail. The Journal of Endocrinology 1982;92:279-282.
- Van Tienhoven A, Ostrander CE. The effect of interruption of the dark period at different intervals on egg production and shell breaking strength. Poultry Science 1973;52: 998-1001.
- Wang SD, Jan DF, Yeh LT, Wu GC, Chen LR. Effect of exposure to long photoperiod during the rearing period on the age at first egg and the subsequent reproductive performance in geese. Animal Reproduction Science 2002;73:227-234.
- Wang CM, Chen LR, Lee SR, Jea YS, Kao JY. Supplementary artificial light to increase egg production of geese under natural lighting conditions. Animal Reproduction Science 2009;113:317-321.
- Yang J, Long DW, Bacon WL. Ontogeny of luteinizing hormone and estradiol secretion in turkey hens exposed to different photoperiods from hatch to sixty weeks of age. Poultry Science 1999;78:1611-1618.
Publication Dates
-
Publication in this collection
20 Dec 2019 -
Date of issue
2019
History
-
Received
25 Dec 2018 -
Accepted
27 June 2019